Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics
Abstract
1. Introduction
2. Nutrigenomics and Public Health
Gene–Nutrient Interactions and Their Consequences on Health
3. Dietary Supplements and Fortified Food
4. Nutrients as a Signal in Human Metabolism
4.1. Amino Acid Signalling
4.2. Glucose Sensing and Signalling
4.3. Lipids Signalling
4.4. Fatty Acid Signalling
4.5. Bile Acids and Cholesterol as Signalling Molecules
5. Receptor-Mediated Nutrient Sensing in Metabolism
5.1. Nutrient-Sensing Mechanisms
5.2. Dietary Signals and Sensors
5.3. Nuclear Receptors as Nutrient Sensors
5.4. Metabolism and Nutritional Evolution
5.5. Epigenetic Signalling and Intermediary Metabolism
5.6. Circadian Control of Metabolic Processes
5.7. Modulating the Circadian Clock by Metabolic Systems
6. Nutrient Sensing and Regulation
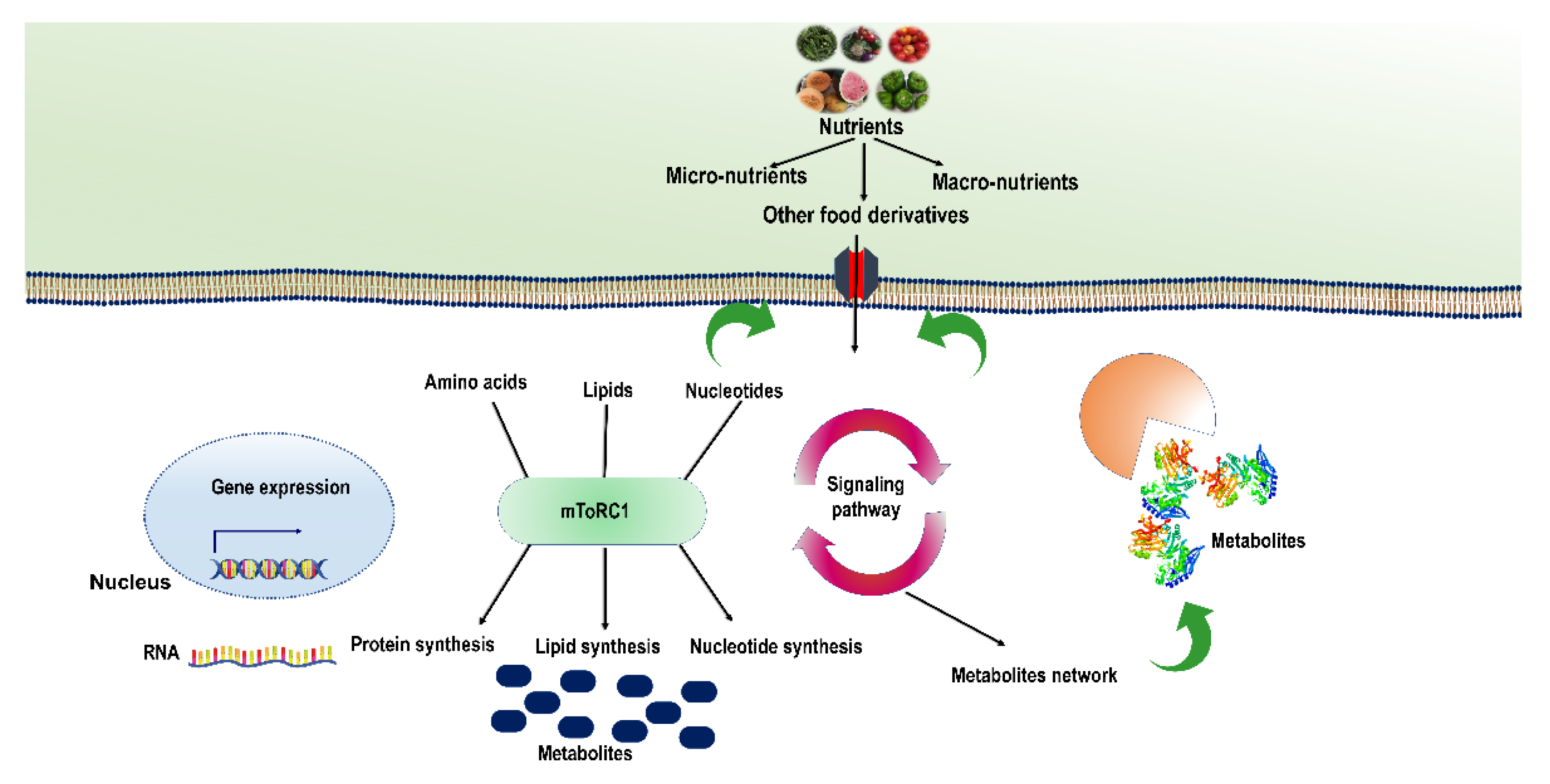
6.1. Role of mTOR Complex in Nutrient Sensing
6.2. Mechanisms Underlying Nutrient Signalling and Regulation
6.2.1. Role of Growth Factor as Signalling Agent for Regulation of mTORC1
6.2.2. Role of Glucose as Signalling Agent for Regulation of mTORC1
6.2.3. Role of Amino Acids as Signalling Agents for Regulation of mTORC1
7. Regulation of Nutrient-Mediated Signalling at the Epigenetic Level
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nolden, A.A.; Feeney, E.L. Genetic differences in taste receptors: Implications for the food industry. Annu. Rev. Food Sci. Technol. 2020, 11, 183–204. [Google Scholar] [CrossRef]
- Tsurugizawa, T. Neuroimaging of gut nutrient perception. Curr. Pharm. Des. 2014, 20, 2738–2744. [Google Scholar] [CrossRef]
- Kaplan, R.E.; Webster, A.K.; Chitrakar, R.; Dent, J.A.; Baugh, L.R. Food perception without ingestion leads to metabolic changes and irreversible developmental arrest in C. elegans. BMC Biol. 2018, 16, 112. [Google Scholar] [CrossRef]
- Riera, C.E.; Dillin, A. Emerging role of sensory perception in aging and metabolism. Trends Endocrinol. Metab. 2016, 27, 294–303. [Google Scholar] [CrossRef]
- Broach, J.R. Nutritional control of growth and development in yeast. Genetics 2012, 192, 73–105. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Lei, Q.-Y. Metabolite sensing and signaling in cell metabolism. Signal Transduct. Target. Ther. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Ozbudak, E.M.; Thattai, M.; Lim, H.N.; Shraiman, B.I.; Van Oudenaarden, A. Multistability in the lactose utilization network of Escherichia coli. Nature 2004, 427, 737–740. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Nudler, E.; Mironov, A.S. The riboswitch control of bacterial metabolism. Trends Biochem. Sci. 2004, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Breaker, R.R. The structural and functional diversity of metabolite-binding riboswitches. Annu. Rev. Biochem. 2009, 78, 305. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Yanes, O.; Ruiz, S.; Kida, Y.S.; Diep, D.; Tautenhahn, R.; Herrerías, A.; Batchelder, E.M.; Plongthongkum, N.; Lutz, M. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012, 22, 168–177. [Google Scholar] [CrossRef]
- Aparicio, S.; Chapman, J.; Stupka, E.; Putnam, N.; Chia, J.-m.; Dehal, P.; Christoffels, A.; Rash, S.; Hoon, S.; Smit, A. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 2002, 297, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A. The complete sequence of a human genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef]
- Wang, S.; Liang, H.; Wang, H.; Li, L.; Xu, Y.; Liu, Y.; Liu, M.; Wei, J.; Ma, T.; Le, C.; et al. The chromosome-scale genomes of Dipterocarpus turbinatus and Hopea hainanensis (Dipterocarpaceae) provide insights into fragrant oleoresin biosynthesis and hardwood formation. Plant Biotechnol. J. 2022, 20, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sahu, S.K.; Yang, T.; Mu, W.; Wei, J.; Cheng, L.; Yang, J.; Liu, J.; Zhao, Y.; Lisby, M. The Clausena lansium (Wampee) genome reveal new insights into the carbazole alkaloids biosynthesis pathway. Genomics 2021, 113, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sahu, S.K.; Yang, T.; Mu, W.; Wei, J.; Cheng, L.; Yang, J.; Mu, R.; Liu, J.; Zhao, J. Dissecting the genome of star fruit (Averrhoa carambola L.). Hortic. Res. 2020, 7, 94. [Google Scholar] [CrossRef]
- Sahu, S.K.; Liu, M.; Yssel, A.; Kariba, R.; Muthemba, S.; Jiang, S.; Song, B.; Hendre, P.S.; Muchugi, A.; Jamnadass, R. Draft Genomes of two Artocarpus plants, Jackfruit (A. heterophyllus) and Breadfruit (A. altilis). Genes 2020, 11, 27. [Google Scholar] [CrossRef]
- Mead, M.N. Nutrigenomics: The genome–food interface. Environ. Health Perspect. 2007, 115, 12. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Corella, D. Nutritional genomics. Annu. Rev. Genom. Hum. Genet. 2004, 5, 71–118. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the bioactive diet components on the gene expression regulation. Nutrients 2021, 13, 3673. [Google Scholar] [CrossRef] [PubMed]
- Hans, K.B.; Jana, T. Micronutrients in the life cycle: Requirements and sufficient supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Reddy, V.S.; Palika, R.; Ismail, A.; Pullakhandam, R.; Reddy, G.B. Nutrigenomics: Opportunities & challenges for public health nutrition. Indian J. Med. Res. 2018, 148, 632. [Google Scholar]
- Pandita, D.; Pandita, A. Omics Technology for the Promotion of Nutraceuticals and Functional Foods. Front. Physiol. 2022, 13, 680. [Google Scholar] [CrossRef]
- Frosch, D.L.; Mello, P.; Lerman, C. Behavioral consequences of testing for obesity risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1485–1489. [Google Scholar] [CrossRef]
- Panagiotakos, D.; Sitara, M.; Pitsavos, C.; Stefanadis, C. Estimating the 10-year risk of cardiovascular disease and its economic consequences, by the level of adherence to the Mediterranean diet: The ATTICA study. J. Med. Food 2007, 10, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Reyes, T.; Astudillo-López, C.C.; Salgado-Goytia, L.; Muñoz-Valle, J.F.; Salgado-Bernabé, A.B.; Guzmán-Guzmán, I.P.; Castro-Alarcón, N.; Moreno-Godínez, M.E.; Parra-Rojas, I. Interaction of dietary fat intake with APOA2, APOA5 and LEPR polymorphisms and its relationship with obesity and dyslipidemia in young subjects. Lipids Health Dis. 2015, 14, 106. [Google Scholar] [CrossRef]
- Tryndyak, V.P.; Han, T.; Fuscoe, J.C.; Ross, S.A.; Beland, F.A.; Pogribny, I.P. Status of hepatic DNA methylome predetermines and modulates the severity of non-alcoholic fatty liver injury in mice. BMC Genom. 2016, 17, 298. [Google Scholar] [CrossRef]
- Tryndyak, V.P.; Marrone, A.K.; Latendresse, J.R.; Muskhelishvili, L.; Beland, F.A.; Pogribny, I.P. MicroRNA changes, activation of progenitor cells and severity of liver injury in mice induced by choline and folate deficiency. J. Nutr. Biochem. 2016, 28, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Haro, D.; Marrero, P.F.; Relat, J. Nutritional regulation of gene expression: Carbohydrate-, fat-and amino acid-dependent modulation of transcriptional activity. Int. J. Mol. Sci. 2019, 20, 1386. [Google Scholar] [CrossRef]
- Sohi, G.; Marchand, K.; Revesz, A.; Arany, E.; Hardy, D.B. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7 α-hydroxylase promoter. Mol. Endocrinol. 2011, 25, 785–798. [Google Scholar] [CrossRef]
- Barry, R.L.; Byun, N.E.; Williams, J.M.; Siuta, M.A.; Tantawy, M.N.; Speed, N.K.; Saunders, C.; Galli, A.; Niswender, K.D.; Avison, M.J. Brief exposure to obesogenic diet disrupts brain dopamine networks. PLoS ONE 2018, 13, e0191299. [Google Scholar] [CrossRef]
- Gracia, A.; Elcoroaristizabal, X.; Fernández-Quintela, A.; Miranda, J.; Bediaga, N.G.; De Pancorbo, M.M.; Rimando, A.M.; Portillo, M.P. Fatty acid synthase methylation levels in adipose tissue: Effects of an obesogenic diet and phenol compounds. Genes Nutr. 2014, 9, 411. [Google Scholar] [CrossRef]
- Peña-Romero, A.C.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. The future of nutrition: Nutrigenomics and nutrigenetics in obesity and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 3030–3041. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, L.-Z.; Hong, L.; Shan, C.; Shi, W.; Cai, W. Alteration in methylation pattern of GATA-4 promoter region in vitamin A-deficient offspring’s heart. J. Nutr. Biochem. 2013, 24, 1373–1380. [Google Scholar] [CrossRef]
- Takaya, J.; Iharada, A.; Okihana, H.; Kaneko, K. Magnesium deficiency in pregnant rats alters methylation of specific cytosines in the hepatic hydroxysteroid dehydrogenase-2 promoter of the offspring. Epigenetics 2011, 6, 573–578. [Google Scholar] [CrossRef]
- Takaya, J.; Iharada, A.; Okihana, H.; Kaneko, K. A calcium-deficient diet in pregnant, nursing rats induces hypomethylation of specific cytosines in the 11β-hydroxysteroid dehydrogenase-1 promoter in pup liver. Nutr. Res. 2013, 33, 961–970. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, Y.; Dong, X.; Duan, Z.; Niu, X.; Wei, J. TLR2-ICAM1-Gadd45α axis mediates the epigenetic effect of selenium on DNA methylation and gene expression in Keshan disease. Biol. Trace Elem. Res. 2014, 159, 69–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, Z.; Qi, C.; Wang, T. Dietary chromium restriction of pregnant mice changes the methylation status of hepatic genes involved with insulin signaling in adult male offspring. PLoS ONE 2017, 12, e0169889. [Google Scholar]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Bañuelos, Ó.; Soria, A.C.; Rodríguez-Sánchez, S.; Martínez, J.A.; Campión, J. Prevention of diet-induced obesity by apple polyphenols in W istar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef]
- Liew, S.-C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Yu, X. Correlation between the 677C> T polymorphism in the methylene tetrahydrofolate reductase gene and serum homocysteine levels in coronary heart disease. Genet. Mol. Res. 2016, 15, 1–6. [Google Scholar] [CrossRef]
- Groziak, S.M.; Miller, G. Natural bioactive substances in milk and colostrum: Effects on the arterial blood pressure system. Br. J. Nutr. 2000, 84, 119–125. [Google Scholar] [CrossRef]
- Baudin, B. Angiotensin I-Converting Enzyme Gene Polymorphism and Drug Response. Clin. Chem. Lab. Med. 2000, 38, 853–885. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Gulin, J.P.; Felson, D.T. Glucosamine and chondroitin for treatment of osteoarthritis: A systematic quality assessment and meta-analysis. Jama 2000, 283, 1469–1475. [Google Scholar] [CrossRef]
- Chard, J.; Dieppe, P. Glucosamine for osteoarthritis: Magic, hype, or confusion? It’s probably safe—but there’s no good evidence that it works. BMJ 2001, 322, 1439–1440. [Google Scholar] [CrossRef]
- Towheed, T.; Maxwell, L.; Anastassiades, T.P.; Shea, B.; Houpt, J.; Welch, V.; Hochberg, M.C.; Wells, G.A. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst. Rev. 2005, 2009, CD002946. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005, 59, 407–450. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Jørgensen, S.B.; Nielsen, J.N.; Birk, J.B.; Olsen, G.S.; Viollet, B.; Andreelli, F.; Schjerling, P.; Vaulont, S.; Hardie, D.G.; Hansen, B.F. The α2–5′ AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 2004, 53, 3074–3081. [Google Scholar] [CrossRef]
- Sahu, S.K.; Hannun, Y.A.; Yao, N. Emergence of membrane sphingolipids as a potential therapeutic target. Biochimie 2019, 158, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Hasegawa, S.; Kasubuchi, M.; Ichimura, A.; Nakajima, A.; Kimura, I. Nutritional signaling via free fatty acid receptors. Int. J. Mol. Sci. 2016, 17, 450. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hirasawa, A.; Hara, T.; Tsujimoto, G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009, 89, 82–88. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191. [Google Scholar] [PubMed]
- Radhakrishnan, A.; Sun, L.-P.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-sensing domain. Mol. Cell 2004, 15, 259–268. [Google Scholar] [CrossRef]
- Lee, M.J.; Yaffe, M.B. Protein regulation in signal transduction. Cold Spring Harb. Perspect. Biol. 2016, 8, a005918. [Google Scholar] [CrossRef]
- Sharari, S.; Abou-Alloul, M.; Hussain, K.; Ahmad Khan, F. Fanconi–bickel syndrome: A review of the mechanisms that lead to dysglycaemia. Int. J. Mol. Sci. 2020, 21, 6286. [Google Scholar] [CrossRef]
- Sternisha, S.M.; Miller, B.G. Molecular and cellular regulation of human glucokinase. Arch. Biochem. Biophys. 2019, 663, 199–213. [Google Scholar] [CrossRef]
- Müller, M.; Kersten, S. Nutrigenomics: Goals and strategies. Nat. Rev. Genet. 2003, 4, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mangelsdorf, D.J. LuXuRies of lipid homeostasis: The unity of nuclear hormone receptors, transcription regulation, and cholesterol sensing. Mol. Interv. 2002, 2, 78. [Google Scholar] [CrossRef]
- Mandard, S.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor α target genes. Cell. Mol. Life Sci. CMLS 2004, 61, 393–416. [Google Scholar] [CrossRef]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef]
- Patsouris, D.; Mandard, S.; Voshol, P.J.; Escher, P.; Tan, N.S.; Havekes, L.M.; Koenig, W.; März, W.; Tafuri, S.; Wahli, W. PPARα governs glycerol metabolism. J. Clin. Investig. 2004, 114, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, R.; Tarr, P.T.; Wu, P.-H.; Handschin, C.; Li, S.; Yang, W.; Pei, L.; Uldry, M.; Tontonoz, P. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 2005, 120, 261–273. [Google Scholar] [CrossRef]
- Sever, R.; Glass, C.K. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef]
- Cheng, Z. Nutritional Signaling Pathway Activities in Obesity and Diabetes; Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Chen, Y.; Michalak, M.; Agellon, L.B. Focus: Nutrition and food science: Importance of nutrients and nutrient metabolism on human health. Yale J. Biol. Med. 2018, 91, 95. [Google Scholar]
- Meddens, S.F.W.; de Vlaming, R.; Bowers, P.; Burik, C.A.; Linnér, R.K.; Lee, C.; Okbay, A.; Turley, P.; Rietveld, C.A.; Fontana, M.A. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol. Psychiatry 2021, 26, 2056–2069. [Google Scholar] [CrossRef]
- James, W.; Johnson, R.; Speakman, J.; Wallace, D.; Frühbeck, G.; Iversen, P.; Stover, P. Nutrition and its role in human evolution. J. Intern. Med. 2019, 285, 533–549. [Google Scholar] [CrossRef]
- Dauncey, M.; Astley, S. Genômica nutricional: Novos estudos sobre as interações entre nutrição eo genoma humano. Nutr. Pauta 2006, 77, 4–9. [Google Scholar]
- Dauncey, M. Recent advances in nutrition, genes and brain health. Proc. Nutr. Soc. 2012, 71, 581–591. [Google Scholar] [CrossRef]
- De Godoy, M.; Swanson, K. Companion animals symposium: Nutrigenomics: Using gene expression and molecular biology data to understand pet obesity. J. Anim. Sci. 2013, 91, 2949–2964. [Google Scholar] [CrossRef] [PubMed]
- Dauncey, M. Nutrition, environment and gene expression: Impact on health, welfare and production. Proc. FACTA Avian Nutr. Course 2014, 27–28. [Google Scholar]
- Ekmay, R.D.; Salas, C.; England, J.; Cerrate, S.; Coon, C.N. The effects of age, energy and protein intake on protein turnover and the expression of proteolysis-related genes in the broiler breeder hen. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 164, 38–43. [Google Scholar] [CrossRef]
- Brennan, K.; Graugnard, D.; Xiao, R.; Spry, M.; Pierce, J.; Lumpkins, B.; Mathis, G. Comparison of gene expression profiles of the jejunum of broilers supplemented with a yeast cell wall-derived mannan oligosaccharide versus bacitractin methylene disalicylate. Br. Poult. Sci. 2013, 54, 238–246. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Druyan, S.; Uni, Z.; Ashwell, C.; Ferket, P. Metabolic profiling of late-term turkey embryos by microarrays. Poult. Sci. 2013, 92, 1011–1028. [Google Scholar] [CrossRef]
- Dauncey, M.J. Genomic and epigenomic insights into nutrition and brain disorders. Nutrients 2013, 5, 887–914. [Google Scholar] [CrossRef]
- Liu, G.E. Recent applications of DNA sequencing technologies in food, nutrition and agriculture. Recent Pat. Food Nutr. Agric. 2011, 3, 187–195. [Google Scholar] [CrossRef]
- Kilpinen, H.; Barrett, J.C. How next-generation sequencing is transforming complex disease genetics. Trends Genet. 2013, 29, 23–30. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB J. 2009, 23, 1572–1583. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Gardiner, L.-J.; Rusholme-Pilcher, R.; Colmer, J.; Rees, H.; Crescente, J.M.; Carrieri, A.P.; Duncan, S.; Pyzer-Knapp, E.O.; Krishna, R.; Hall, A. Interpreting machine learning models to investigate circadian regulation and facilitate exploration of clock function. Proc. Natl. Acad. Sci. USA 2021, 118, e2103070118. [Google Scholar] [CrossRef]
- Albrecht, U. The circadian clock, metabolism and obesity. Obes. Rev. 2017, 18, 25–33. [Google Scholar] [CrossRef]
- Carlberg, C.; Ulven, S.M.; Molnár, F. Nutrigenomics: How science Works; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef]
- Robitaille, A.M.; Christen, S.; Shimobayashi, M.; Cornu, M.; Fava, L.L.; Moes, S.; Prescianotto-Baschong, C.; Sauer, U.; Jenoe, P.; Hall, M.N. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 2013, 339, 1320–1323. [Google Scholar] [CrossRef]
- Jewell, J.L.; Guan, K.-L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef]
- Yecies, J.L.; Manning, B.D. mTOR links oncogenic signaling to tumor cell metabolism. J. Mol. Med. 2011, 89, 221–228. [Google Scholar] [CrossRef]
- Cornu, M.; Albert, V.; Hall, M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013, 23, 53–62. [Google Scholar] [CrossRef]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Lee, K.-H.; Kim, Y.-M.; Kim, D.-H.; Oh, B.-H.; Kim, Y.-G. Crystal structure of the Gtr1pGTP-Gtr2pGDP protein complex reveals large structural rearrangements triggered by GTP-to-GDP conversion. J. Biol. Chem. 2012, 287, 29648–29653. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.-L. Amino acid signaling in TOR activation. Annu. Rev. Biochem. 2011, 80, 1001–1032. [Google Scholar] [CrossRef]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.-i.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Oh, W.J.; Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 2011, 10, 2305–2316. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Yang, Q.; Inoki, K.; Ikenoue, T.; Guan, K.-L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006, 20, 2820–2832. [Google Scholar] [CrossRef]
- Woo, S.-Y.; Kim, D.-H.; Jun, C.-B.; Kim, Y.-M.; Vander Haar, E.; Lee, S.-i.; Hegg, J.W.; Bandhakavi, S.; Griffin, T.J.; Kim, D.-H. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor β expression and signaling. J. Biol. Chem. 2007, 282, 25604–25612. [Google Scholar] [CrossRef]
- Zeng, Z.; Sarbassov, D.D.; Samudio, I.J.; Yee, K.W.; Munsell, M.F.; Ellen Jackson, C.; Giles, F.J.; Sabatini, D.M.; Andreeff, M.; Konopleva, M. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 2007, 109, 3509–3512. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef]
- Ballif, B.A.; Roux, P.P.; Gerber, S.A.; MacKeigan, J.P.; Blenis, J.; Gygi, S.P. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc. Natl. Acad. Sci. USA 2005, 102, 667–672. [Google Scholar] [CrossRef]
- Dibble, C.C.; Elis, W.; Menon, S.; Qin, W.; Klekota, J.; Asara, J.M.; Finan, P.M.; Kwiatkowski, D.J.; Murphy, L.O.; Manning, B.D. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol. Cell 2012, 47, 535–546. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Lizcano, J.M.; Göransson, O.; Toth, R.; Deak, M.; Morrice, N.A.; Boudeau, J.; Hawley, S.A.; Udd, L.; Mäkelä, T.P.; Hardie, D.G. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004, 23, 833–843. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2–mTOR signaling and tumor suppression through REDD1-mediated 14–3–3 shuttling. Genes Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Ochocki, J.D.; Simon, M.C. Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol. 2013, 203, 23–33. [Google Scholar] [CrossRef]
- Barbet, N.C.; Schneider, U.; Helliwell, S.B.; Stansfield, I.; Tuite, M.F.; Hall, M.N. TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 1996, 7, 25–42. [Google Scholar] [CrossRef]
- Oldham, S.; Montagne, J.; Radimerski, T.; Thomas, G.; Hafen, E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000, 14, 2689–2694. [Google Scholar] [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Li, Y.; Tollefsbol, T.O. Prenatal epigenetics diets play protective roles against environmental pollution. Clin. Epigenetics 2019, 11, 82. [Google Scholar] [CrossRef]
- Tiffon, C. The impact of nutrition and environmental epigenetics on human health and disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Davis, C.D.; Ross, S.A. Dietary components impact histone modifications and cancer risk. Nutr. Rev. 2007, 65, 88–94. [Google Scholar] [CrossRef]
- Park, L.K.; Friso, S.; Choi, S.-W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012, 71, 75–83. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.; Li, X.; Wang, M.; Cai, D.; Li, X.; Zhao, R. In Ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks. PLoS ONE 2015, 10, e0122643. [Google Scholar] [CrossRef]
- Choi, S.-W.; Friso, S. Epigenetics: A new bridge between nutrition and health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef]
- Zhang, N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Xiao, Y.; Zhang, X.; Cui, S.; Huang, G. Folic acid inhibits tau phosphorylation through regulation of PP2A methylation in SH-SY5Y cells. J. Nutr. Health Aging 2015, 19, 123–129. [Google Scholar] [CrossRef]
- Feil, R.; Fraga, M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-De La Vega, H.; Hernández de la Cruz, O.N.; López-Camarillo, C. Dietary compounds as epigenetic modulating agents in cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef]
- Sedley, L. Advances in Nutritional Epigenetics—A Fresh Perspective for an Old Idea. Lessons Learned, Limitations, and Future Directions. Epigenetics Insights 2020, 13, 2516865720981924. [Google Scholar] [CrossRef]
- Remely, M.; Stefanska, B.; Lovrecic, L.; Magnet, U.; Haslberger, A.G. Nutriepigenomics: The role of nutrition in epigenetic control of human diseases. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 328–333. [Google Scholar] [CrossRef]
- Farkas, S.A.; Befekadu, R.; Hahn-Strömberg, V.; Nilsson, T.K. DNA methylation and expression of the folate transporter genes in colorectal cancer. Tumor Biol. 2015, 36, 5581–5590. [Google Scholar] [CrossRef]
- Wong, C.; Qian, Y.; Yu, J. Interplay between epigenetics and metabolism in oncogenesis: Mechanisms and therapeutic approaches. Oncogene 2017, 36, 3359–3374. [Google Scholar] [CrossRef]
- Mukherjee, N.; Kumar, A.P.; Ghosh, R. DNA methylation and flavonoids in genitourinary cancers. Curr. Pharmacol. Rep. 2015, 1, 112–120. [Google Scholar] [CrossRef]

| Gene | Nutrient | Related Diseases | References |
|---|---|---|---|
| NAT2 | High protein, Vitamin A, folic acid | Gastric cancer | [26] |
| GSTM1, ADH | Alcohol intake | Colorectal | [26] |
| Aflatoxins (polluted grains) | CYP2E1 | Liver | [26] |
| CYP2E1 | Nitrosamines (fried potatoes) | Nasopharyngeal, stomach | [26] |
| APOA2 | Fat (high intake) | Obesity, dyslipidaemia | [27] |
| APOA5 | Fat (high intake) | Obesity, dyslipidaemia | [27] |
| APOE | Vit. B9, choline (deficit) | Non-alcoholic fatty liver | [28,29] |
| ChREBP | Sugar (high intake) | Insulin resistance | [30] |
| CYP7A1 | Protein (low intake) | Dyslipidaemia | [31] |
| DAT | Fat (high intake) | Obesity | [32] |
| FASN | Sugar and fat (high intake) | Non-alcoholic fatty liver, obesity | [33] |
| FOXA1 | Vit. B9 and choline (deficit) | Non-alcoholic fatty liver | [28,29] |
| FOXA2 | Vit. B9 and choline (deficit) | Non-alcoholic fatty liver | [28,29] |
| FTO | Protein (high intake) | Obesity | [34] |
| GATA4 | Vit. A (deficit) | Cardiovascular diseases | [35] |
| HSD11B1 | Calcium (deficit) | Diabetes mellitus (Type 2) | [36] |
| HSD11B2 | Magnesium (deficit) | Diabetes mellitus (Type 2) | [37] |
| ICAM1 | Selenium (deficit) | Cardiovascular diseases | [38] |
| Insulin signalling genes | Chromium (deficit) | Diabetes mellitus (Type 2) | [39] |
| LEP | Sugar and fat (high intake) | Obesity | [40] |
| MTHFR | Vit. B9 (low intake) | Cardiovascular diseases, cancer | [41,42] |
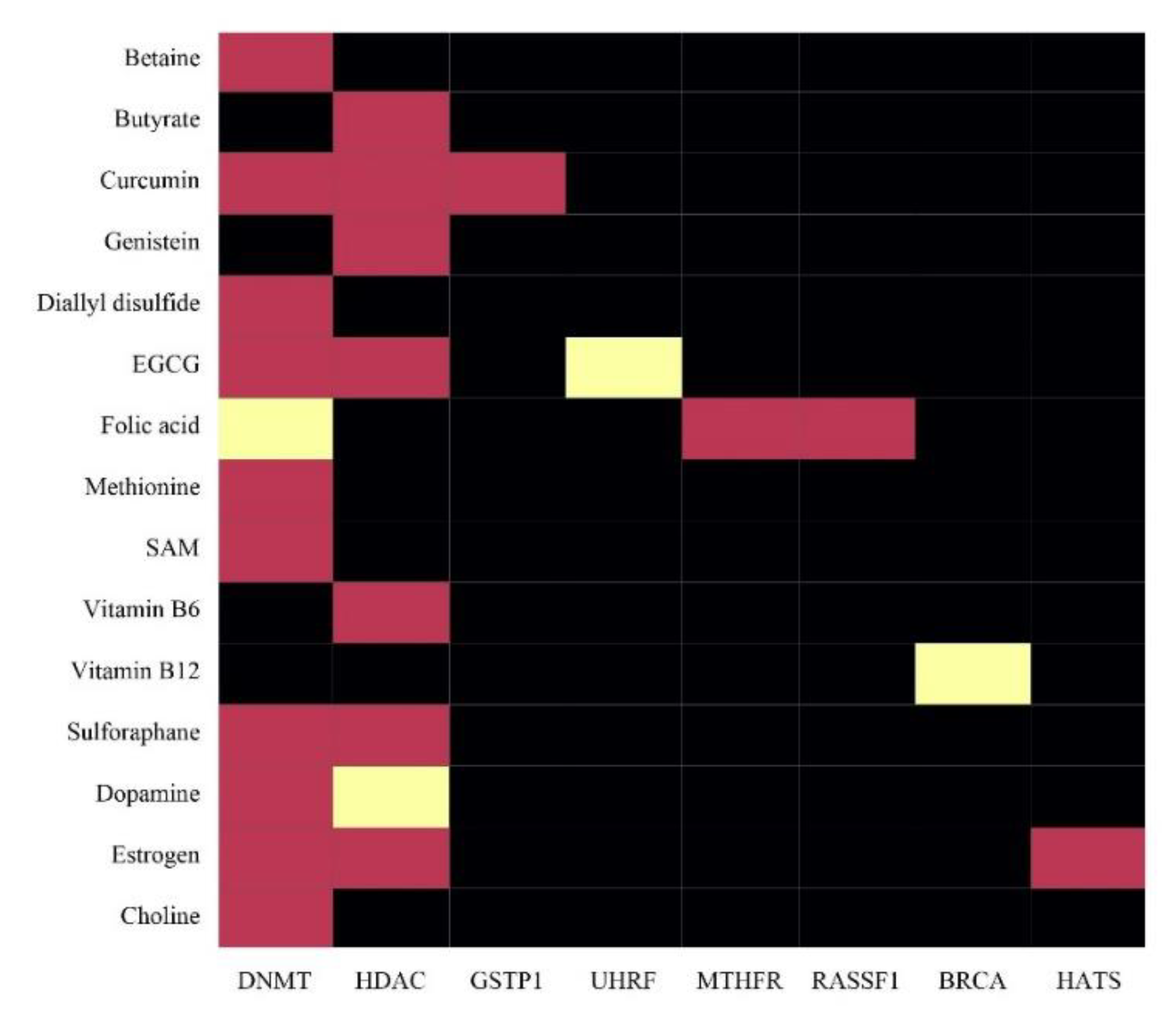
| Nutrient | Food Origin | Epigenetic Role | References |
|---|---|---|---|
| Betaine | Wheat, spinach, sugar beets | Break down the toxic by-products of SAM synthesis | [123] |
| Butyrate | An intestinal compound | Increased histone acetylation turning on “protective” genes | [124] |
| Choline | Egg yolks, cooked beef, chicken | Methyl donor to SAM | [125] |
| Curcumin | Curcuma longa | Regulation of DNMT and SAM synthesis | [122] |
| Diallyl sulphide | Garlic | Increased histone acetylation turning on anticancer genes | [118] |
| EGCG | Green tea polyphenol | DNMT1 inhibition | [126] |
| Genistein | Soybean | SAM synthesis, increased methylation | [126] |
| Folic Acid | Leafy vegetables, sunflower seeds, baker’s yeast | Methionine synthesis | [127] |
| Methionine | Sesame seeds, Brazil nuts, peppers, spinach | SAM synthesis | [128] |
| SAM-e (SAM) | Popular dietary supplement pill | Enzymes transfer methyl groups from SAM to the DNA | [120] |
| Vitamin B6 | Meats, whole grain products, vegetables | Methionine synthesis | [128] |
| Vitamin B12 | Meat, liver, shellfish, milk | Methionine synthesis | [128] |
| Sulforaphane | Broccoli | Increased histone acetylation turning on anticancer genes | [129] |
| Dopamine | Amino acid tyrosine | Role in reward and movement regulation | [130] |
| Oestrogen | Dairy, nuts and seeds, legumes | Epigenetic transcription factor JAK2 | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lal, M.K.; Sharma, E.; Tiwari, R.K.; Devi, R.; Mishra, U.N.; Thakur, R.; Gupta, R.; Dey, A.; Lal, P.; Kumar, A.; et al. Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics. Int. J. Mol. Sci. 2022, 23, 11305. https://doi.org/10.3390/ijms231911305
Lal MK, Sharma E, Tiwari RK, Devi R, Mishra UN, Thakur R, Gupta R, Dey A, Lal P, Kumar A, et al. Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics. International Journal of Molecular Sciences. 2022; 23(19):11305. https://doi.org/10.3390/ijms231911305
Chicago/Turabian StyleLal, Milan Kumar, Eshita Sharma, Rahul Kumar Tiwari, Rajni Devi, Udit Nandan Mishra, Richa Thakur, Rucku Gupta, Abhijit Dey, Priyanka Lal, Awadhesh Kumar, and et al. 2022. "Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics" International Journal of Molecular Sciences 23, no. 19: 11305. https://doi.org/10.3390/ijms231911305
APA StyleLal, M. K., Sharma, E., Tiwari, R. K., Devi, R., Mishra, U. N., Thakur, R., Gupta, R., Dey, A., Lal, P., Kumar, A., Altaf, M. A., Sahu, D. N., Kumar, R., Singh, B., & Sahu, S. K. (2022). Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics. International Journal of Molecular Sciences, 23(19), 11305. https://doi.org/10.3390/ijms231911305









