Role of Essential Amino Acids in Age-Induced Bone Loss
Abstract
1. Introduction
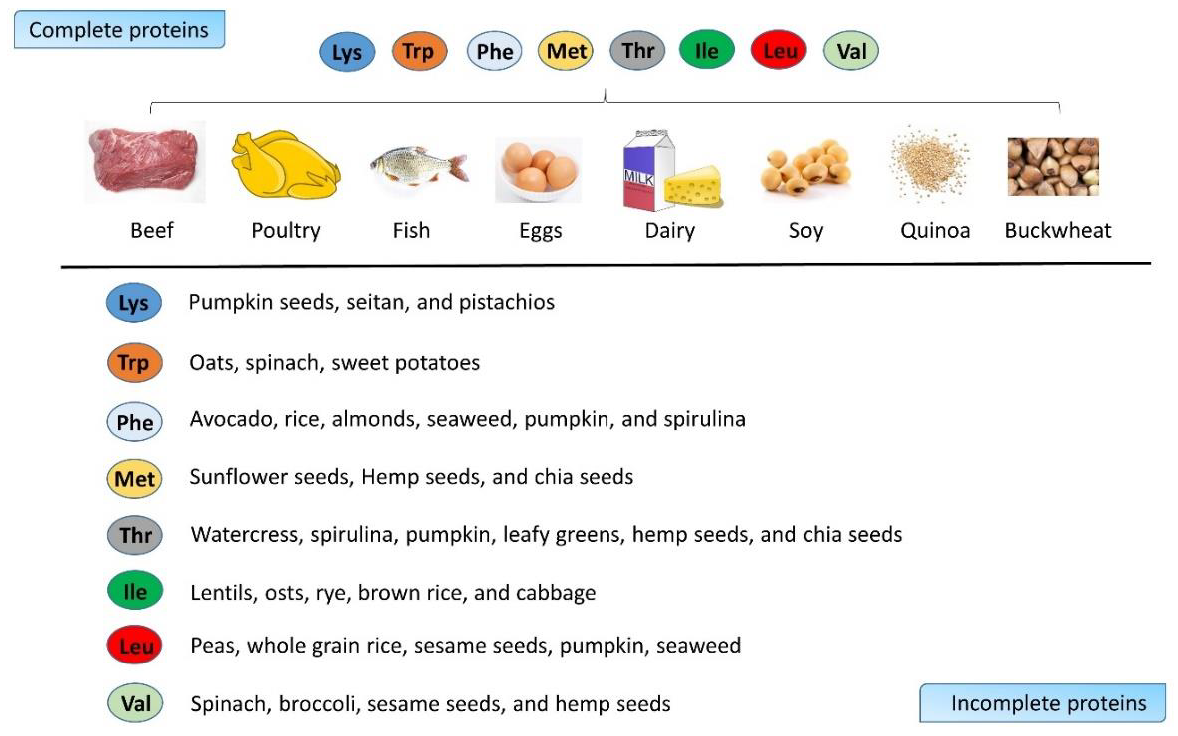
2. Amino Acid as Energy Source and Tissue Builder in Bones
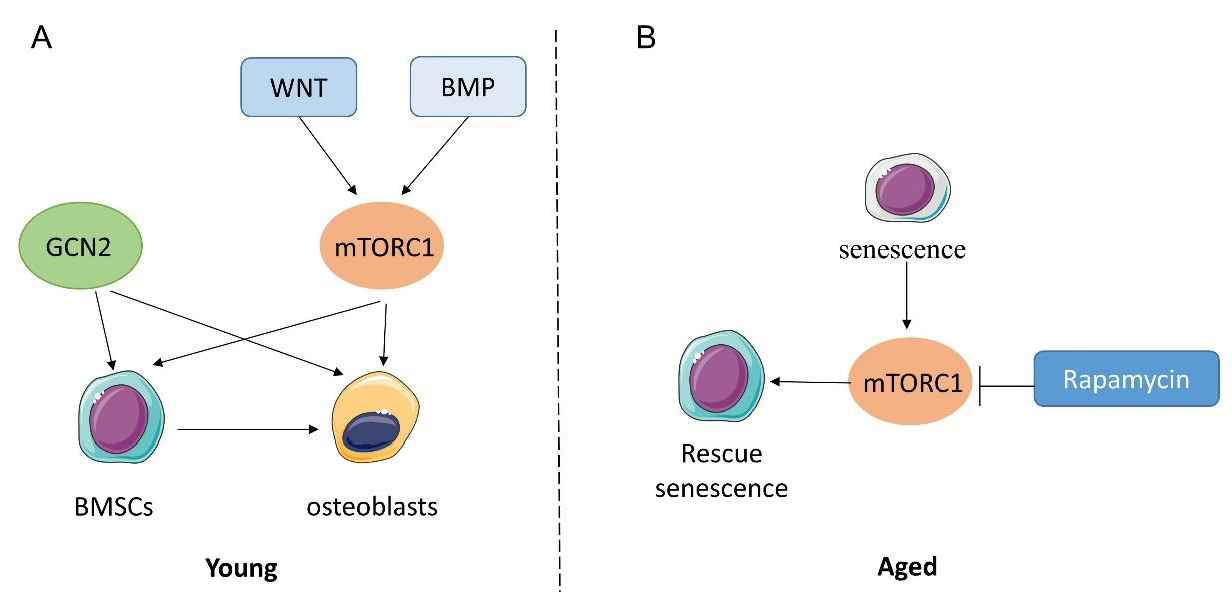
3. Amino Acid Sensing and Bone Metabolism
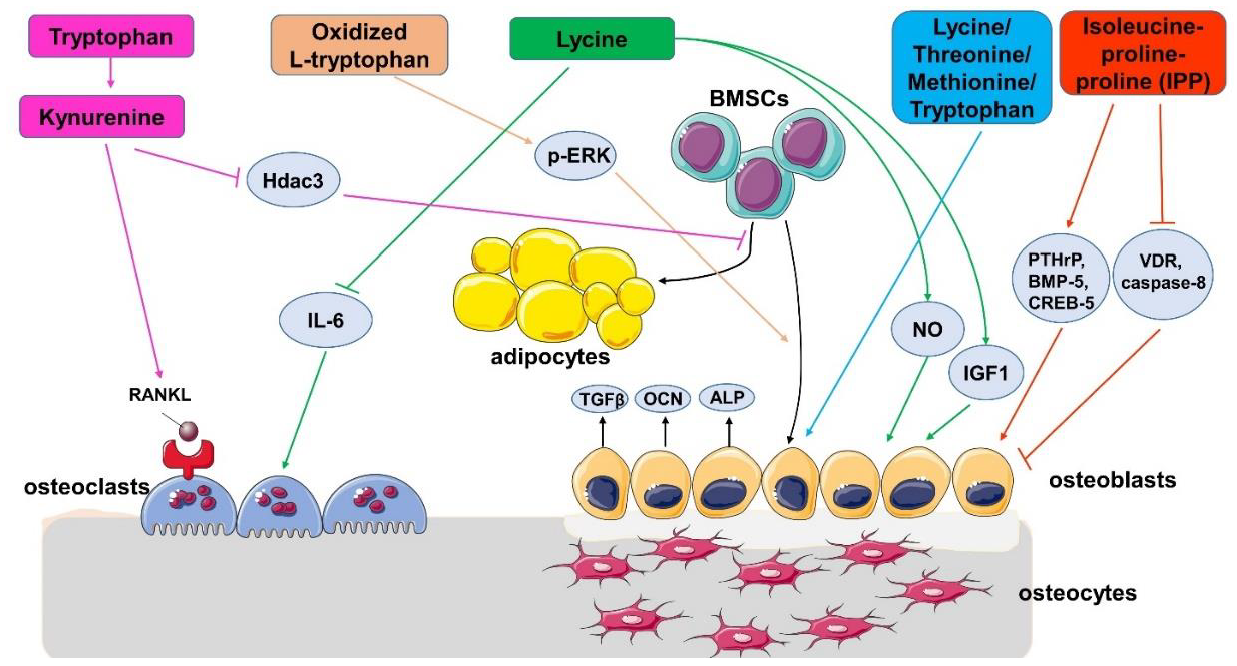
4. The Effect of EAAs on Bone Health in Animals and Cells
5. Relationship between Body EAAs Levels and Bone Quality in Human Research
6. Supplementation of EAAs on Bone Quality in Aging People
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vianna, D.; Resende, G.F.; Torres-Leal, F.L.; Pantaleao, L.C.; Donato, J., Jr.; Tirapegui, J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition 2012, 28, 182–189. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Adachi, J.D.; Schemitsch, E.; Tarride, J.E.; Brown, V.; Bell, A.; Reiner, M.; Oliveira, T.; Motsepe-Ditshego, P.; Burke, N.; et al. Mortality in older adults following a fragility fracture: Real-world retrospective matched-cohort study in Ontario. BMC Musculoskelet. Disord. 2021, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Tangseefa, P.; Martin, S.K.; Chin, P.Y.; Breen, J.; Mah, C.Y.; Baldock, P.A.; Wittert, G.A.; Page, A.J.; Proud, C.G.; Fitter, S.; et al. The mTORC1 complex in pre-osteoblasts regulates whole-body energy metabolism independently of osteocalcin. Bone Res. 2021, 9, 10. [Google Scholar] [CrossRef]
- Chen, J.; Long, F. mTORC1 Signaling Promotes Osteoblast Differentiation from Preosteoblasts. PLoS ONE 2015, 10, e0130627. [Google Scholar] [CrossRef]
- Kim, S.P.; Li, Z.; Zoch, M.L.; Frey, J.L.; Bowman, C.E.; Kushwaha, P.; Ryan, K.A.; Goh, B.C.; Scafidi, S.; Pickett, J.E.; et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight 2017, 2, e92704. [Google Scholar] [CrossRef]
- Barzel, U.S.; Massey, L.K. Excess dietary protein can adversely affect bone. J. Nutr. 1998, 128, 1051–1053. [Google Scholar] [CrossRef]
- Darling, A.L.; Millward, D.J.; Torgerson, D.J.; Hewitt, C.E.; Lanham-New, S.A. Dietary protein and bone health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 90, 1674–1692. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiology. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef]
- Jihyun Im, H.P.a.K.P. Dietary Essential Amino Acid Intake Is Associated with High Muscle Strength in Korean Older Adults. Nutritents 2022, 14, 3104. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Paddon-Jones, D.; Hays, N.P.; Kortebein, P.; Ronsen, O.; Williams, R.H.; McComb, A.; Symons, T.B.; Wolfe, R.R.; Evans, W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. 2010, 29, 18–23. [Google Scholar] [CrossRef]
- Dickens, F. The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem. J. 1941, 35, 1011–1023. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef]
- Davies, E.; Muller, K.H.; Wong, W.C.; Pickard, C.J.; Reid, D.G.; Skepper, J.N.; Duer, M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 2014, 111, E1354–E1363. [Google Scholar] [CrossRef]
- Delgado-Lopez, J.M.; Bertolotti, F.; Lyngso, J.; Pedersen, J.S.; Cervellino, A.; Masciocchi, N.; Guagliardi, A. The synergic role of collagen and citrate in stabilizing amorphous calcium phosphate precursors with platy morphology. Acta. Biomater. 2017, 49, 555–562. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Dai, H.; Tian, X.; Cui, Z.K.; Chen, Z.; Hu, L.; Song, Q.; Liu, A.; Zhang, Z.; et al. Bone and plasma citrate is reduced in osteoporosis. Bone 2018, 114, 189–197. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J.; et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef]
- Tea, I.; De Luca, A.; Schiphorst, A.M.; Grand, M.; Barille-Nion, S.; Mirallie, E.; Drui, D.; Krempf, M.; Hankard, R.; Tcherkez, G. Stable Isotope Abundance and Fractionation in Human Diseases. Metabolites 2021, 11, 370. [Google Scholar] [CrossRef]
- De Falco, F.G.B.; Carteni, F.; Mazzoleni, S.; Kim, D.-H. Metabolic flux analysis: A comprehensive review on sample preparation, analytical techniques, data analysis, computational modelling, and main application areas. RSC Adv. 2022, 12. [Google Scholar] [CrossRef]
- Long, C.P.; Antoniewicz, M.R. High-resolution (13)C metabolic flux analysis. Nat. Protoc. 2019, 14, 2856–2877. [Google Scholar] [CrossRef]
- Yu, Y.; Newman, H.; Shen, L.; Sharma, D.; Hu, G.; Mirando, A.J.; Zhang, H.; Knudsen, E.; Zhang, G.F.; Hilton, M.J.; et al. Glutamine Metabolism Regulates Proliferation and Lineage Allocation in Skeletal Stem Cells. Cell Metab. 2019, 29, 966–978.e964. [Google Scholar] [CrossRef]
- Srivastava, A.; Lu, J.; Gadalla, D.S.; Hendrich, O.; Gronke, S.; Partridge, L. The Role of GCN2 Kinase in Mediating the Effects of Amino Acids on Longevity and Feeding Behaviour in Drosophila. Front. Aging 2022, 3, 944466. [Google Scholar] [CrossRef]
- Hu, G.; Yu, Y.; Tang, Y.J.; Wu, C.; Long, F.; Karner, C.M. The Amino Acid Sensor Eif2ak4/GCN2 Is Required for Proliferation of Osteoblast Progenitors in Mice. J. Bone Miner. Res. 2020, 35, 2004–2014. [Google Scholar] [CrossRef]
- Karner, C.M.; Esen, E.; Okunade, A.L.; Patterson, B.W.; Long, F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J. Clin. Investig. 2015, 125, 551–562. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Romero-Aleshire, M.J.; Renkema, K.R.; Ventevogel, M.S.; Chew, W.M.; Uhrlaub, J.L.; Smithey, M.J.; Limesand, K.H.; Sempowski, G.D.; Brooks, H.L.; et al. Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell 2015, 14, 130–138. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Selman, C.; Tullet, J.M.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef]
- Suzuki, T.; Inoki, K. Spatial regulation of the mTORC1 system in amino acids sensing pathway. Acta Biochim. Et. Biophys. Sin. 2011, 43, 671–679. [Google Scholar] [CrossRef]
- Singha, U.K.; Jiang, Y.; Yu, S.; Luo, M.; Lu, Y.; Zhang, J.; Xiao, G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J. Cell. Biochem. 2008, 103, 434–446. [Google Scholar] [CrossRef]
- Karner, C.M.; Lee, S.Y.; Long, F. Bmp Induces Osteoblast Differentiation through both Smad4 and mTORC1 Signaling. Mol. Cell. Biol. 2017, 37. [Google Scholar] [CrossRef]
- Stukenborg, L.; Sell, C.; Potnis, M. MTOR Inhibition Alters miRNA Profile in Cultured Bone Marrow Mesenchymal Stem Cells. Innov. Aging 2021, 5, 667. [Google Scholar] [CrossRef]
- Liu, H.; Huang, B.; Xue, S.; U, K.P.; Tsang, L.L.; Zhang, X.; Li, G.; Jiang, X. Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress-induced senescence of MSCs. Stem Cell Res. Ther. 2020, 11, 279. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Havens, A.M.; Pienta, K.J.; Taichman, R.S. The bone marrow niche: Habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 2008, 22, 941–950. [Google Scholar] [CrossRef]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R. Human osteopenic bone-derived osteoblasts: Essential amino acids treatment effects. Artif. Cells Blood Substit. Immobil. Biotechnol. 2003, 31, 35–46. [Google Scholar] [CrossRef]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R.; Gnudi, S.; Nicolini, A.; Carpi, A. L-arginine and L-lysine stimulation on cultured human osteoblasts. Biomed. Pharmacother. Biomed. Pharmacother. 2002, 56, 492–497. [Google Scholar] [CrossRef]
- Huttunen, M.M.; Pekkinen, M.; Ahlstrom, M.E.; Lamberg-Allardt, C.J. Effects of bioactive peptides isoleucine-proline-proline (IPP), valine-proline-proline (VPP) and leucine-lysine-proline (LKP) on gene expression of osteoblasts differentiated from human mesenchymal stem cells. Br. J. Nutr. 2007, 98, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.H.; Comar, C.L.; Schooley, J.C.; Lengemann, F.W. Interrelated effects of L-lysine and other dietary factors on the gastrointestinal absorption of calcium 45 in the rat and chick. J. Nutr. 1957, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Tommasini, M.; Muratori, E.; Parnigotto, P.P. Essential amino acids increase the growth and alkaline phosphatase activity in osteoblasts cultured in vitro. Farmaco 2001, 56, 755–761. [Google Scholar] [CrossRef]
- El Refaey, M.; Watkins, C.P.; Kennedy, E.J.; Chang, A.; Zhong, Q.; Ding, K.H.; Shi, X.M.; Xu, J.; Bollag, W.B.; Hill, W.D.; et al. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol. Cell. Endocrinol. 2015, 410, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Refaey, M.E.; Zhong, Q.; Ding, K.H.; Shi, X.M.; Xu, J.; Bollag, W.B.; Hill, W.D.; Chutkan, N.; Robbins, R.; Nadeau, H.; et al. Impact of dietary aromatic amino acids on osteoclastic activity. Calcif. Tissue Int. 2014, 95, 174–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Refaey, M.E.; McGee-Lawrence, M.E.; Fulzele, S.; Kennedy, E.J.; Bollag, W.B.; Elsalanty, M.; Zhong, Q.; Ding, K.H.; Bendzunas, N.G.; Shi, X.M.; et al. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 2182–2193. [Google Scholar] [CrossRef]
- Cui, Z.; Feng, H.; He, B.; He, J.; Tian, Y. Relationship Between Serum Amino Acid Levels and Bone Mineral Density: A Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 763538. [Google Scholar] [CrossRef] [PubMed]
- Pernow, Y.; Thoren, M.; Saaf, M.; Fernholm, R.; Anderstam, B.; Hauge, E.M.; Hall, K. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone 2010, 47, 959–965. [Google Scholar] [CrossRef]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Chan, R.; Kwok, T. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone 2019, 129, 115082. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Associated With Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence From Discordant Monozygotic Twins. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef]
- Hill, T.R.; Verlaan, S.; Biesheuvel, E.; Eastell, R.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; et al. A Vitamin D, Calcium and Leucine-Enriched Whey Protein Nutritional Supplement Improves Measures of Bone Health in Sarcopenic Non-Malnourished Older Adults: The PROVIDE Study. Calcif. Tissue Int. 2019, 105, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Shih, M.H.; Chen, C.D.; Yeh, S.L. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: An open-label, parallel-group study. Clin. Nutr. 2021, 40, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Isoda, H.; Okura, T. Effect of Citrulline and Leucine Intake with Exercises on Body Composition, Physical Activity, and Amino Acid Concentration in Older Women: A Randomized Double-Blind Placebo-Controlled Study. Foods 2021, 10, 3117. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Mooney, K.; Vogrin, S.; Jackson, M.; Duque, G.; Khaiyat, O.; Amirabdollahian, F. Leucine-enriched whey protein supplementation, resistance-based exercise, and cardiometabolic health in older adults: A randomized controlled trial. J. Cachexia Sarcopenia Muscle 2021, 12, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
| Participants Number | Participants Background | Age | Tissue | EAAs | Relationship between EAA Changes and Bone Condition | Publication Year | Reference |
|---|---|---|---|---|---|---|---|
| 502,639 | European | 37–70 | Serum | Isoleucine, valine | Positively associated with total BMD | 2021 | [47] |
| 20–22 | Sweden | 31–66 years | Serum | Threonine | Significant increase in people with osteoporosis | 2010 | [48] |
| 20–22 | Sweden | 31–66 years | Erythrocyte | Lysine, phenylalanine, and tryptophan | Significant decrease in people with osteoporosis | 2010 | [48] |
| 1424 men and 1573 women | Chinese | mean age 72 | Serum | Phenylalanine, tryptophan, methionine, valine leucine, and isoleucine | Significantly lower in osteoporosis subjects | 2019 | [49] |
| Participants Number in Each Group | Participant Background | Age | EAAs Supplement | EAA Treatment Time | Changes after EAA Supplementation | Publication Year | Reference |
|---|---|---|---|---|---|---|---|
| 184–196 | 6 European countries: Belgium, Germany, Ireland, Italy, Sweden, and the UK | Older than 65 years | 6 g of leucine with 40 g of whey protein daily | 13 weeks | BMD increasing in a small but significant range | 2019 | [51] |
| 28 | Taipei | Older than 65 | 1.2 g leucine enriched whey protein supplement | 12 weeks | Significant improvement in walking speed in age 65–74 group | 2021 | [52] |
| 13 | Japanese | 65–80 years | 1.6 g leucine twice daily | 20 weeks | No effect on BMD and bone area | 2021 | [53] |
| 22–31 | Northwest England | 60–90 years, mean age 68.73 ± 5.80 years | 1.50 g/kg BW/day whey protein, plus 0.03 g/kg BW leucine | 16 weeks | No effect on bone mass | 2021 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Shi, W.; Zhang, Q. Role of Essential Amino Acids in Age-Induced Bone Loss. Int. J. Mol. Sci. 2022, 23, 11281. https://doi.org/10.3390/ijms231911281
Lv Z, Shi W, Zhang Q. Role of Essential Amino Acids in Age-Induced Bone Loss. International Journal of Molecular Sciences. 2022; 23(19):11281. https://doi.org/10.3390/ijms231911281
Chicago/Turabian StyleLv, Ziquan, Wenbiao Shi, and Qian Zhang. 2022. "Role of Essential Amino Acids in Age-Induced Bone Loss" International Journal of Molecular Sciences 23, no. 19: 11281. https://doi.org/10.3390/ijms231911281
APA StyleLv, Z., Shi, W., & Zhang, Q. (2022). Role of Essential Amino Acids in Age-Induced Bone Loss. International Journal of Molecular Sciences, 23(19), 11281. https://doi.org/10.3390/ijms231911281






