Abstract
Nitraria sibirica is a shrub that can survive in extreme drought environments. The auxin-response factors (ARFs) are a class of transcription factors that are widely involved in plant growth and development, as well as in the regulation of stress resistance. However, the genome-wide identification of the ARF gene family and its responses to environmental stresses, especially drought stress, in N. sibirica has not yet been reported. Here, we identified a total of 12 ARF genes in the genome of N. sibirica, which were distributed over 10 chromosomes and divided into three clades. Intragenome synteny analysis revealed one collinear gene pair in the ARF gene family, i.e., NsARF9a and NsARF9b. Cis-acting element analysis showed that multiple hormones and stress-responsive cis-acting elements were found in the promoters of NsARFs, suggesting that NsARFs may be involved in multiple biological processes. Quantitative real-time PCR (qRT-PCR) showed that many NsARFs had tissue-specific expression patterns, with the highest expression of NsARF16 in the seedlings of N. sibirica. In addition, most of the NsARFs that were upregulated under drought were independent of endogenous ABA biosynthesis, whereas the response of NsARF5 and NsARF7a to drought was disrupted by the ABA-biosynthesis inhibitor fluridone. These studies provide a basis for further research into how NsARFs in N. sibirica respond to hormonal signaling and environmental stresses.
1. Introduction
Auxin is essential for controlling plant growth and development, as it has been shown to control the processes of cell differentiation, elongation, and division in conjunction with other plant-growth regulators [1]. There are several important auxin-response genes, such as the early responsive auxin-response factor family (ARFs), that play a major role in the regulation of a plants’ auxin responses [2]. The structure of ARF proteins is typically composed of an N-terminal DNA-binding domain (DBD), a variable middle region (MR), and a C-terminal dimerization domain (CTD) [3]. The ARF DNA-binding domain may bind to the ‘TGTCTC’ motif, i.e., auxin-response elements (AuxREs), which are often embedded in the promoters of auxin-response genes, to transcriptionally regulate the expression of these genes that are widely involved in plant growth and development [4,5]. It has been shown in a variety of plants that varying the MR amino acid content can affect the function of ARF proteins either to promote or repress the expression of auxin-response genes. In Arabidopsis, ARF genes rich in glutamine and serine, proline, and glycine are shown to be suppressors or promoters, respectively [3,6]. The ARF DNA-binding domain may bind to TGTCTC auxin-response elements (AuxREs) during plant development; these elements are often associated with genes involved in growth-hormone signaling and regulate their expression [4,5]. Depending on the expression of auxin in plants, the ARF protein responds in various ways. ARF-regulated transcriptional activity can be inhibited by Aux/IAA protein dimers with ARF transcription factors in the presence of low auxin concentrations; however, the extent of the expression suppression is rather modest [7,8]. The 26S proteasome frees interacting ARF proteins from the inhibitory dimeric structure when auxin concentrations are high [9,10,11].
Studying the roles of each member of their family is crucial due to the significant role ARFs play in the control of plant hormone signaling. The ARF gene family has been identified in many plants, and the functional investigation of these members has advanced. In Arabidopsis, 23 members of the ARF family have been identified, with AtARF7 and AtARF9 having a role as activators of lateral root growth [12]; AtARF1, AtARF2, AtARF3, AtARF4, and AtARF9 have been revealed to be auxin repressors, whereas AtARF5, AtARF6, AtARF7, and AtARF8 have been shown to be auxin-promoting factors in carrot protoplasts [3]. Furthermore, ABA’s function in Arabidopsis seed germination is largely dependent on the TIR1/AFB–AUX/IAA–ARF-mediated auxin signaling pathway [13]; for example, AtARF2 is a negative regulator in the pathway of ABA regulating seed germination and primary root growth [14]. In other plant species, ARF’s function has been studied as well: rice has 25 members, of which OsARF12 is connected to phosphate-induced auxin responses [15]; Longan contains 17 members, with the gene DIARF7 being unique to leaves [16,17]. In Cicer, it has been found that the expression of CaARF20 is upregulated under drought conditions [6]. Thus, ARF gene function has been studied in some plant species, but it is yet to be discovered in Nitraria sibirica. Our study is an advancement in the study of growth-hormone-regulation mechanisms in drought-resistant plants. Moreover, this will help researchers to better understand the role of ARFs under drought stress.
N. sibirica is a typical drought-tolerant plant that belongs to the genus Nitraria Linn., which resides in the Sapindales family. Although great progress has been achieved regarding N. sibirica cultivation methods, physiological measurements, and resistance-regulation mechanisms, the lack of a reference gene hinders the genome-wide identification of gene families and functional genomics studies.
Abscisic acid (ABA) is a crucial endogenous plant hormone that controls plant development, stress tolerance, and stress induction (drought, high salt, and low temperature) [18]. The function of ABA in drought-resistance activities in wheat [19], maize [20,21], soybeans [22], and other plants has been extensively studied. Here, we identified the ARF gene family of N. sibirica and characterized the relationships between drought, ABA signaling, and NsARF responses.
2. Results
2.1. Genome-Wide Identification of NsARFs in the N. Sibirica Genome
To identify N. sibirica ARF genes, we used HMMER and the ARF gene structural domains as input: the Auxin Resp domain (Pfam 06507), the PB1 domain (Pfam 02309), and the B3 DNA-binding domain (Pfam 02362) were used to search the N. sibirica genome. We then used the SMART database and CDD search tools to determine the NsARF protein sequences manually.
We identified a total of 12 NsARFs in the N. sibirica genome. We named each member according to its Arabidopsis homolog (Table 1). Basic details including the chromosomal location, amino acid counts, and physicochemical characteristics of the proteins were used to identify homologs. The lengths of the NsARF protein sequences varied from 1860 aa (NsARF9b) to 3153 aa (NsARF7b); their molecular weights (MWs), from 619 kDa to 1050 kDa; their pI ranged from 5.51 to 6.71, with an average of 5.96. NsARF homologs are expressed in similar subcellular structures, as seen by the limited difference in pI value fluctuation [23].

Table 1.
Summary of N. sbirica ARF gene family members.
2.2. Phylogenetic Analysis of NsARFs
To investigate the phylogenetic relationship between the NsARF proteins and their homologs from other species, we created phylogenetic trees including the sequences of 24 AtARFs from Arabidopsis, 25 OsARFs from rice, and 12 NsARFs from N. sibirica, using the neighbor-joining (NJ) method. All the ARFs analyzed here were divided into three clades, i.e., I, II, and III. Class I includes six NsARFs, Class II includes five NsARFs, and the single NsARF16 constitutes Class III (Figure 1).
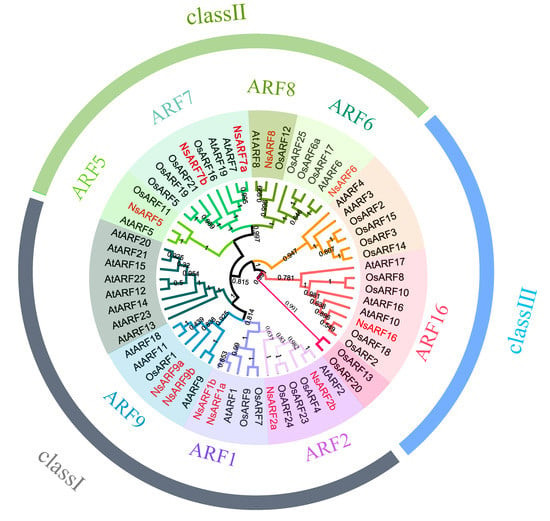
Figure 1.
Phylogenetic relationships of the ARF family genes from Nitraria sibirica (Arabidopsis thaliana, Rice).
2.3. Chromosome Distribution and Synteny Analysis of the NsARF Family
NsARFs are dispersed across 10 out of its 12 chromosomes, with two on CHR5 (NsARF7a and NsARF7b), two on CHR8 (NsARF1a and NsARF1b), and one on each of CHR2, CHR3, CHR4, CHR6, CHR7, CHR10, CHR11, and CHR12 (Figure 2A). Intragenome synteny analysis showed that NsARF9a and NsARF9b are a pair of homologs. From an interspecific covariance analysis between N. sibirica, Arabidopsis, and rice, we identified that two groups, one of seven and one of three NsARF genes, were homologous to nine AtARFs and six OsARFs, respectively (Figure 2B).
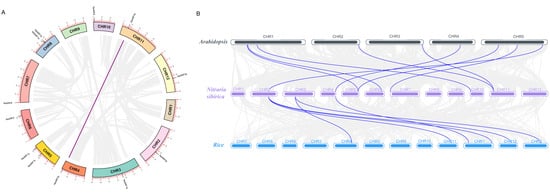
Figure 2.
(A): Chromosome location and interchromosomal relationships of ARFs in Nitraria sibirica. (B): Synteny analyses between the ARFs of Nitraria sibirica, Arabidopsis, and rice.
2.4. Analysis of the NsARF Family Gene Structure
By the domain prediction of proteins encoded by NsARFs, we found that 10 of them contain the classic ARF structural domain (DBD-MR-CTD) (Table 2). Eight NsARFs harbor a glutamine (Q) and leucine (L)-rich middle region, implying that these proteins may be transcriptional activators.

Table 2.
Amino acid content of the NsARF gene family MR domain.
Motif analysis showed that 12 motifs were discovered across the NsARF protein sequences (Figure 3A and Supplemental Figure S1). There are eight motifs conserved across all the NsARFs, while motifs 7, 10, 11, and 12 are not identified in some sequences, of which at least six sequences contain motif 9.
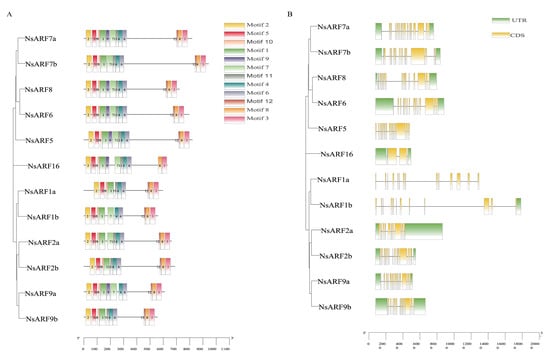
Figure 3.
(A): Conservative motif distribution of NsARF genes. (B): Domain distribution of NsARF genes.
Next, we analyzed the coding DNA sequence (CDS) information and untranslated regions (UTRs) of NsARFs. NsARF1b only has a 3′ UTR, and NsARF1a and NsARF5 have no UTRs, while the remaining nine members have both 5′ and 3′ UTRs. The ARF1 subclade is the only one of which its members do not have a 5′ UTR. The number of CDSs in each member sequence ranges from 3 to 16. The earlier diverging NsARF16 gene only has 3 CDSs, whereas NsARF7a contains 17 CDSs (Figure 3B).
2.5. Prediction of Cis-Acting Elements in NsARF Family Promoter Sequences
We performed analyses to identify cis-acting elements in the NsARF gene promoters. We aimed to identify cis-acting elements of three overarching types: those related to plant hormone signaling, those involved in environmental stress responses, and MYB-binding sites (Figure 4). All the NsARFs contain plant hormone response elements, while seven members have MYB-binding sites linked to drought stress and eleven members have elements linked to responses to light, defense, circadian phenomena, and low temperature. The presence of light-responsive elements in 11 NsARF promoters and abscisic acid-responsive elements in 10 NsARF promoters suggests that the NsARF family is intimately linked to the abscisic acid hormone regulatory network (Table 3). In addition, seven NsARF promoters had the cis-element, which can bind MYBs in response to drought stress, suggesting that they might play a role in drought responses.
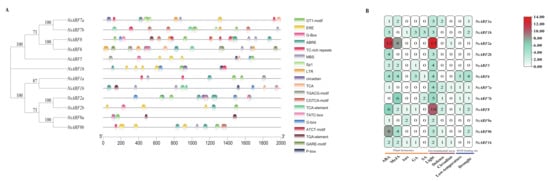
Figure 4.
(A): Cis-acting element analysis of ARF gene in Nitraria sibirica. (B): Summary of cis-acting elements’ number and function of ARF gene in Nitraria sibirica.

Table 3.
NsARF promoter cis-element analysis.
2.6. Tissue-Specific Expression Analysis of the NsARF Family
To quantify the expression of NsARFs in various tissues, we chose eight NsARFs—NsARF1a, 1b, 5, 7a, 7b, 9a, and 16—and designed customized qRT-PCR primers for each (Table 4). By performing qRT-PCR experiments, we found that NsARFs are widely expressed in all tissues, with high expression in the root and leaf (Figure 5A). The expression of NsARF16 is the highest in all tissues. NsARF5 shows considerable expression in the root, while NsARF5 is little expressed in the leaf. NsARF1a, 7b, 9b, and 16 were significantly differentially expressed in the leaf compared with the other tested tissues. We summarized and determined the relative expression levels of the detected genes in the whole plant, and found that NsARF16 showed the highest expression and NsARF5 the lowest (Figure 5B).

Table 4.
qRT-PCR primers used to quantify NsARF gene expression.
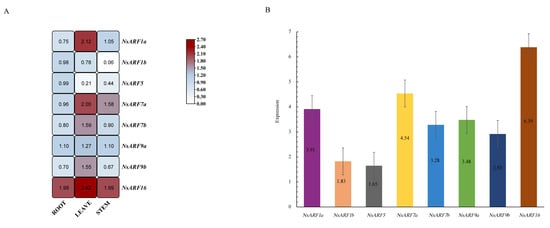
Figure 5.
(A): Expression analysis of NsARF genes in different tissues of Nitraria sibirica. (B): Expression analysis of NsARF genes in one total plant of Nitraria sibirica.
2.7. Expression Analysis of the NsARF Family under Abiotic Stress
N. sibirica demonstrates a high level of drought resilience. In order to investigate the expression pattern of NsARFs under drought stress, we treated the seedlings of N. sibirica with 20% PEG 8000 to simulate conditions of drought. Under drought stress, the expression of NsARF1b, NsARF7b, NsARF9b, and NsARF16 was differentially upregulated, while NsARF7a was the only ARF gene to decrease in expression. The expression levels of NsARF1a, NsARF5, and NsARF9a showed no significant difference (Figure 6).
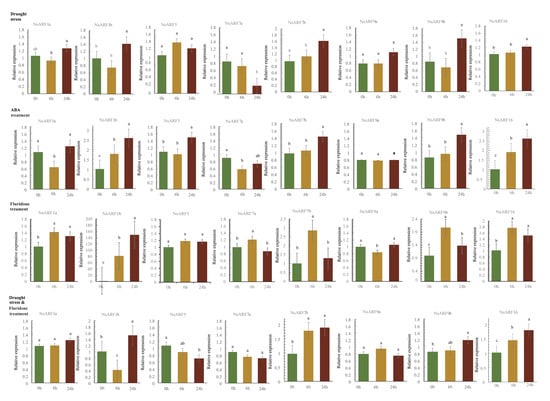
Figure 6.
Expression profiles of NsARFs under abiotic stress. Values with the different letter (a–c) were significantly different when assessed using Duncan’s multiple range test (p < 0.05).
ABA is a phytohormone involved in plant stress responses [18]. In order to understand how ABA influences the expression of NsARFs, we designed three sets of treatments. To N. sibirica seedlings in group A, ABA hormone was directly applied; fluridone, an ABA inhibitor, was administered to group B, and fluridone was also administered to group C, in which the plants were also undergoing drought stress (Figure 6).
The expression patterns of NsARF1a, NsARF1b, NsARF7a, NsARF7b, NsARF9a, NsARF9b, and NsARF16 under ABA treatment are very similar to those under drought stress, with only NsARF5 changing from no significant difference in expression upon drought stress to a significant increase upon ABA treatment. Under fluridone treatment, the expression patterns of NsARF7a and NsARF7b changed significantly compared to those under drought conditions and ABA treatment; the trend for NsARF5 became a downward trend with respect to ABA treatment. Interestingly, the expression of NsARF1b was very high, but the overall trend was not different from that for the other groups. In the presence of both drought and fluridone, the expression dynamics of NsARF1a, NsARF7b, NsARF9a, NsARF9b, and NsARF16 remained unchanged compared to those under drought conditions and ABA treatment. After the application of fluridone, the expression trends of NsARF5 and NsARF7a changed significantly compared to those under only drought conditions. This finding suggests that the response of NsARF5 and NsARF7a to drought was disrupted by the ABA-biosynthesis inhibitor fluridone.
3. Discussion
The ARF gene family regulates growth, hormone responses, and abiotic stress in plants [24,25], making it a critical gene family to study in order to understand plant biology. N. sibirica is a typical desert plant, tolerant of high drought. This is the first investigation into the ARF gene family in N. sibirica.
In this study, we used bioinformatics tools to analyze the unpublished genome of Nitraria sibirica and discovered 12 NsARF genes across 10 chromosomes, which were divided into three phylogenetic clades. Differences in the amino acid content of the MR region affect whether the gene acts as a promoter or a repressor of binding to the AuxRE sequence in the promoters of growth-hormone-regulatory genes [3]. According to the NsARF gene structure analysis, eight proteins may be transcriptional activators. The specific functional validation of these putative transcriptional activators has not yet been established. NsARF members are expressed in close subcellular structures, as shown by the little difference in pI value fluctuation; this will be further investigated by subcellular localization in the future.
The number of motifs in the NsARF gene sequences ranged from 8 to 12, indicating that the NsARFs are highly conserved. NsARF16, which is an early-diverging member within the ARF phylogenetic tree, has 3 CDSs, whereas NsARF7a has 17 CDSs. The interspecific examination of covariance revealed that eight NsARFs were homologous in Arabidopsis or rice. The coding region of the gene has remained stable and preserved as a result of replicative evolution that the ARF gene family may have undergone during speciation. By examining their cis-acting elements, it was discovered that every member of the NsARFs contained hormone-responsive elements. Up to 10 of these members contain ABA-response elements, and 7 NsARFs are predicted to bind with MYBs in response to drought stress, suggesting that they might play a role in drought response.
The function of unknown genes in a species is frequently predicted using the tissue-specific expression of genes. Therefore, the roots, stems, and leaves of N. sibirica were examined using qRT-RCR technology to test the NsARF expression levels. The results show that eight homologs have high levels of expression in the leaf and root, and low expression in the stems. Fluorescence quantitative PCR showed that NsARF1a, 7b, 9b, and 16 had tissue-specific expression patterns, with the highest expression of NsARF16 in the total seedlings of N. sibirica. In the root, NsARF5 shows significantly increased expression, while AtARF5 is similar to NsARF5 in the phylogenetic tree, and it has been confirmed in Arabidopsis that AtARF5 is very important in the formation of the radicle [26]. It is suspected that NsARF5 is crucial in the control of the root growth and development in N. sibirica. NsARF1a, 7a, 7b, 9b, and 16 were highly expressed in the leaf. AtARF7 has been reported to play a role in regulating leaf expansion [26], indicating that NsARF7a and NsARF7b may play roles in leaf development. Significantly, auxin responses in plants have very strong cell-type specificity, and the expression of the ARF gene is a part of the transduction of auxin signaling [27]. Many studies have confirmed that distinct tissues and cell types have different responses to auxin, and the specific responses of tissues or cells play an important role in hormone-mediated plant growth and development [28,29]. For example, the epidermal cell’s specific responses to auxin in the root and stem are key to those aspects of development [30,31]. At present, there is no relevant research about cell-type-specific auxin responses in N. sibirica. In the future, these may be revealed by the transcriptomic analysis of the responses to auxin within different tissues. On this basis, we can further explore the role of NsARFs in the growth and development of N. sibirica.
Plants under drought stress will express ABA-synthesis-related genes, which will result in ABA production mainly from root and leaf tissue [32]. The ability to synthesize is significantly higher in leaves than in roots, and ABA from the root can be transferred to the shoot. Fluridone, an inhibitor of ABA synthesis, has been used in many plants to inhibit the accumulation of ABA [33]. Deducting from the cis-acting elements that we identified in NsARF promoters, NsARFs may be intimately linked with the abscisic acid hormone regulatory network. To explore whether ABA is involved in the response of NsARFs to drought stress, we examined the expression changes of NsARFs under four sets of treatment, i.e., 20% PEG, ABA, the ABA inhibitor fluridone, and 20% PEG in combination with fluridone. In seedlings treated with ABA and subjected to drought, the ABA content increased, with a generally consistent pattern for the majority of NsARFs. Under fluridone treatment, the synthesis of ABA was inhibited. Interestingly, the expression of some members increased in a short time (6 h), and the expression of NsARF1b changed greatly, but the regulation mechanism is not clear. These phenomena are worth further exploring. The change trend of the expression of NsARF5 was opposite to that of ABA treatment, and the decreasing trend of the expression of NsARF7a under drought conditions and ABA treatment was suppressed under treatment with fluridone. Different drought-related evaluation system pathways were activated in plants during drought stress and fluridone treatment; however, ABA synthesis was blocked. NsARF1a expression began to vary at 6 h and tended to decline in the presence of increasing ABA content and vice versa, without noticeably different results. This suggests that an early buildup of ABA has an inhibitory influence on NsARF1a expression. The rising trend of NsARF1b under the treatment of directly applied ABA or fluridone appeared at 6 h and further increased at 24 h. The expression of NsARF1b under drought stress combined with fluridone treatment clearly first decreased and then increased again after 24 h. It is likely that other relevant response factors in the drought regulatory pathway also significantly influence the expression of NsARF1b. In contrast to that under drought and ABA treatment, the expression of NsARF5 showed a declining trend after the administration of fluridone and the decrease in NsARF7a expression was absent, suggesting that the expression of NsARF5 and NsARF7a is linked with ABA levels. Under both drought and ABA treatment, NsARF7b had the same trend, and in just 24 h, the expression greatly increased. Fluridone treatment caused the expression of NsARF7b to considerably increase in 6 h, and it was higher than in the other two treatment groups without fluridone. ABA may suppress the expression of NsARF7b, which was not controlled directly by the ABA-response pathway. NsARF9a, NsARF9b, and NsARF16 did not exhibit any appreciable differences in expression between treatments. In addition, most of the NsARFs that were upregulated under drought were independent of endogenous ABA biosynthesis, whereas the response of NsARF5 and NsARF7a to drought was disrupted by the ABA-biosynthesis inhibitor fluridone. The auxin responses in plants were very cell-type specific and regulated epigenetically.
4. Materials and Methods
4.1. Plant Materials and Abiotic Stress Treatment
The seeds used for this investigation originated from the Inner Mongolia municipality, Dengkou County, China. For more than 60 days, the seeds were vernalized at 4 °C, covered in moist sand. The seeds were germinated at 23 °C and grew for over a month in a 4:1 soil combination of nutritious soil and perlite, until they reached a height of around 20 cm. Healthy N. sibirica seedlings were separated into root, stem, and leaf sections in order to gather plant components for tissue-specific analysis. Following that, plant tissues were gathered, frozen in liquid nitrogen, and kept at −80 °C. Then, the seedlings were treated at the same developmental stage with 20% PEG to simulate drought stress, 50 mg/L ABA, 50 µM fluridone, and 20% PEG with 50 µM fluridone [34,35]. We collected plant tissues after 0, 6, and 24 h. All the tissues were immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction. The dates of sample collection were 20 May to 30 May.
4.2. Identification of ARF Genes in N. sibirica
The Pfam codes of 3 structural domains—the Auxin Resp domain (Pfam 06507), the PB1 domain (Pfam 02309), and the B3 DNA-binding domain (Pfam 02362)—were used to extract a total of 23 sequences of AtARFs from the Arabidopsis genome (TAIR.10) [16]. According to the China Rice Date Center, 25 OsARFs were recovered from the rice genome (China Rice Date Center).
Using the HMMER software (v3.0), we searched NsARFs based on the genome of N. sibirica (unpublished). Then, the blastp software (v2.90), SMART (http://smart.embl-heidelberg.de/, accessed on 1 May 2022), and the CDD search tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi, accessed on 1 May 2022) were used to reconfirm the sequence correctness. Naming methods were adopted to identify homology with AtARFs. The protein’s pI and MWs (molecular weights) were discovered using a UniProt (https://www.uniprot.org/, accessed on 1 May 2022) search query.
4.3. Phylogenetic Analysis of ARF Proteins in N. sibirica
MEGA v10.1.8 (Temple, Philadelphia, PA, USA) was used to examine ARFs from N. sibirica, rice, and Arabidopsis to determine their phylogenetic relationship. We used MUSCLE implemented in MEGA to align the amino acid sequences and neighbor-joining algorithm to create phylogenetic trees with a bootstrap value of 1000. DANMAN v9.0 (Lynnon Corporation, San Ramon, CA, USA) was used for the multi-fragment alignment of amino acid sequences.
4.4. Gene Structure, Cis-Acting Element Prediction, and Chromosomal Localization Analysis of NsARFs
The MEME software (https://meme-suite.org/meme/tools/meme, accessed on 1 May 2022) was used for conserved motif structure analysis, while the Pfam sequence search algorithm (http://pfam.xfam.org/, accessed on 1 May 2022) was used to identify the location information of the protein kinase domain and NAF/FLSL domain of candidate genes. The GSDS (http://gsds.cbi.pku.edu.cn/, accessed on 1 May 2022) online analysis tool was used to analyze the structural domains of candidate genes in order to determine the chromosomal position of NsARFs and to visualize the findings of previous research. Cis-acting elements within the NsARF gene promoters were analyzed using the Plant CARE online tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 1 May 2022). TBtools [36] was used to synthesize and visualize these results.
4.5. Expression Analysis of ARF Genes in N. sibirica
RNA was extracted using an RNA-extraction kit (Promega, Shanghai, China); the RNA was then reverse transcribed into single-stranded cDNA using a reverse-transcription kit (Vazyme, Nanjing, China). Quantitative real-time PCR (qRT-PCR) was performed using a LightCycler 480II (Roche, Basel, Switzerland) with the AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China). Specific qRT-PCR primers were designed in NCBI (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 1 May 2022) to detect expression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231911122/s1.
Author Contributions
Conceptualization, Y.L. (Yuxin Liu) and L.Z.; methodology, Y.L. (Yuxin Liu); validation, J.Z., Z.L. and H.F.; formal analysis, Z.H.; investigation, L.L.; resources, Y.L. (Ye Lu); data curation, X.L.; writing—original draft preparation, Y.L. (Yuxin Liu); writing—review and editing, L.Z. and Z.H.; visualization, Y.L. (Yuxin Liu); supervision, J.S.; project administration, J.C.; funding acquisition, T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Youth Foundation of Nature Science Foundation of Jiangsu Province (BK20210614) and the Nature Science Foundation of China (31770715), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Information Files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abel, S.P.G.E.; Theologis, A. Early genes and auxin action. Plant Physiol. 1996, 111, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.; Zhang, J. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Shiv, B.; Hagen, G.; Guilfoyle, T. The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription. Plant Cell 2003, 15, 533–543. Available online: http://www.jstor.org/stable/3871883 (accessed on 23 June 2016).
- Liscum, E.; Reed, J.W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef]
- Woodward, A.W. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Die, J.V.; Gil, J.; Millan, T. Genome-wide identification of the auxin response factor gene family in Cicer arietinum. BMC Genom. 2018, 19, 301. [Google Scholar] [CrossRef]
- Vernoux, T.; Brunoud, G.; Farcot, E.; Morin, V.; Van den Daele, H.; Legrand, J.; Oliva, M.; Das, P.; Larrieu, A.; Wells, D.; et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol. Syst. Biol. 2011, 7, 508. [Google Scholar] [CrossRef]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. Activation and Repression of Transcription by Auxin-Response Factors. Proc. Natl. Acad. Sci. USA 1999, 96, 5844–5849. [Google Scholar] [CrossRef]
- Finet, C.; Berne-Dedieu, A.; Scutt, C.P.; Marlétaz, F. Evolution of the ARF Gene Family in Land Plants: Old Domains, New Tricks. Mol. Biol. Evol. 2013, 30, 45–56. [Google Scholar] [CrossRef]
- Weijers, D.; Benkova, E.; Jager, K.E.; Schlereth, A.; Hamann, T.; Kientz, M.; Wilmoth, J.C.; Reed, J.W.; Jurgens, G. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 2005, 24, 1874–1885. [Google Scholar] [CrossRef]
- De Smet, I.; Lau, S.; Voß, U.; Vanneste, S.; Benjamins, R.; Rademacher, E.H.; Schlereth, A.; De Rybel, B.; Vassileva, V.; Grunewald, W.; et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Taniguchi, N.; Tasaka, M. PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant J. 2006, 48, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.; Luan, S.; Li, J.; He, Z. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediatedABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin Response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet. 2011, 7, e1002172. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef]
- Peng, Y.; Fang, T.; Zhang, Y.; Zhang, M.; Zeng, L. Genome-Wide Identification and Expression Analysis of Auxin Response Factor (ARF) Gene Family in Longan (Dimocarpus longan L.). Plants 2020, 9, 221. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Chen, W.; Qian, Y.; Ma, Q.; Cheng, B.; Zhu, S. Genome-wide analysis of the auxin response factor (ARF) gene family in maize (Zea mays). Plant Growth Regul. 2011, 63, 225–234. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic Acid and Abiotic Stress Signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Travaglia, C.; Cohen, A.C.; Reinoso, H.; Castillo, C.; Bottini, R. Exogenous Abscisic Acid Increases Carbohydrate Accumulation and Redistribution to the Grains in Wheat Grown Under Field Conditions of Soil Water Restriction. J. Plant Growth Regul. 2007, 26, 285–289. [Google Scholar] [CrossRef]
- Kizis, D.; Pagès, M. Maize DRE-binding proteins DBF1 and DBF2 are involved in rab17 regulation through the drought-responsive element in an ABA-dependent pathway. Plant J. Cell Mol. Biol. 2002, 30, 679–689. [Google Scholar] [CrossRef]
- Battal, P.; Erez, M.E.; Turker, M.; Berber, I. Molecular and Physiological Changes in Maize (Zea mays) Induced by Exogenous NAA, ABA and MeJa during Cold Stress. Ann. Bot. Fenn. 2008, 45, 173–185. [Google Scholar] [CrossRef]
- Luo, X.; Bai, X.; Sun, X.; Zhu, D.; Liu, B.; Ji, W.; Cai, H.; Cao, L.; Wu, J.; Hu, M.; et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, B. Adaptations of proteins to cellular and subcellular pH. J. Biol. 2009, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, X.; Liu, K.; Wang, J.; Zhang, J.; Feng, Y.; Wang, Y.; Lin, L.; Feng, J.; Li, C. Genome-wide identification and expression profiling of the auxin response factor (ARF) gene family in physic nut. PLoS ONE 2018, 13, e201024. [Google Scholar] [CrossRef]
- Jain, M.; Khurana, J.P. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009, 276, 3148–3162. [Google Scholar] [CrossRef]
- Li, S.; Xie, Z.; Hu, C.; Zhang, J. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef]
- Chapman, E.J.; Estelle, M. Mechanism of Auxin-Regulated Gene Expression in Plants. Annu. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef]
- Bargmann, B.O.R.; Vanneste, S.; Krouk, G.; Nawy, T.; Efroni, I.; Shani, E.; Choe, G.; Friml, J.; Bergmann, D.C.; Estelle, M.; et al. A map of cell type-specific auxin responses. Mol. Syst. Biol. 2013, 9, 688. [Google Scholar] [CrossRef]
- Ding, T.; Zhang, F.; Wang, J.; Wang, F.; Liu, J.; Xie, C.; Hu, Y.; Shani, E.; Kong, X.; Ding, Z.; et al. Cell-type action specificity of auxin on Arabidopsis root growth. Plant J. 2021, 106, 928–941. [Google Scholar] [CrossRef]
- Procko, C.; Burko, Y.; Jaillais, Y.; Ljung, K.; Long, J.A.; Chory, J. The epidermis coordinates auxin-induced stem growth in response to shade. Genes Dev. 2016, 30, 1529–1541. [Google Scholar] [CrossRef]
- Swarup, R.; Kramer, E.M.; Perry, P.; Knox, K.; Leyser, H.M.O.; Haseloff, J.; Beemster, G.T.S.; Bhalerao, R.; Bennett, M.J. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 2005, 7, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A. The Role of Abscisic Acid in Drought Stress: How ABA Helps Plants to Cope with Drought Stress; Springer International Publishing: Cham, Switerland, 2016; pp. 123–151. [Google Scholar]
- Yoshioka, T.T.U.S.; Endo, T.; Satoh, S. Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol. 1998, 39, 307–312. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Mangu, V.R.; Zandkarimi, H.; Prasad, M.; Baisakh, N. Structure, organization and evolution of ADP-ribosylation factors in rice and foxtail millet and their expression in rice. Sci. Rep. 2016, 6, 24008. [Google Scholar] [CrossRef] [PubMed]
- Gamble, P.E.; Mullet, J.E. Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur. J. Biochem. 1986, 160, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).