Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
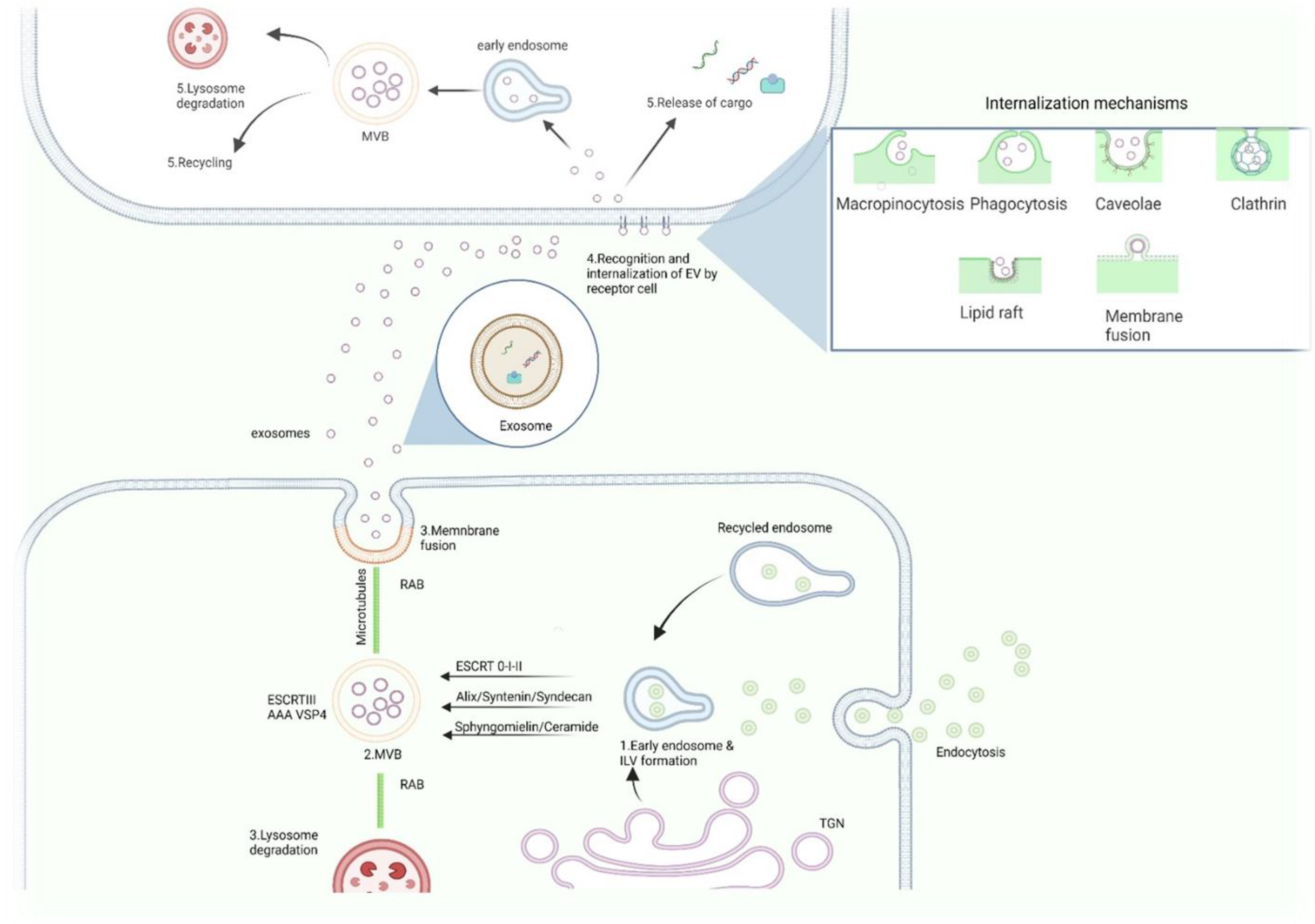
2. Exosomes
2.1. History and Discovery
2.2. Composition of Exosomes
2.3. Biogenesis of Exosomes
2.4. Exosomes–Uptake
3. miRNA Biogenesis
4. miRNAs Sorting into Exosomes
5. Exosomal-miRNAs as Intercellular Communicators
6. Exosome Functions in Respiratory Pathologies
7. Idiopathic Pulmonary Fibrosis (IPF)
8. Exosomes in IPF
9. Exosomal-miRNAs as Intercellular Communicators in IPF
| Sample “Donor Cell” | EVs Source | Recipient-Cell | MiRNA-Cargo in EVs and Function | Target | Major Biologic Effects | Possible Mechanism Associated | EV Isolation | Perspective in IPF Disease | Ref |
|---|---|---|---|---|---|---|---|---|---|
| BMSCs NHLF (CCL210) | BMSCs-CM NHLF-CM | NHLF -TGFβ induced HFLF-TGFβ induced | ↑ miR-630 (anti-fibrotic) | N-cadherin | ↓ Fibroblast differentiation | ↓ profibrotic gene expression α-SMA, Col3a1 | UC | Further studies to know the mechanism of action of miRNAs of BMSC-EVs | [152] |
| AM2Mfs of BLM-fibrotic rat model | AM2Mfs -CM (exosomes) | Interstitial fibroblasts | ↑ miR-328 (profibrotic) | FAM13 | ↑ Proliferation | ↑ profibrotic gene expression α-SMA, Col1a1, Col3a1 | PEG and UC | Exosomal-miR-328 derived of AM2Mfs aggravate PF via FAM13 | [153] |
| WT-BLM fibrosis mouse model and Sdc1−/− BLM-fibrosis mouse model | BALF | LEC re-instilled intratracheally | ↓ miR-503-5p, ↓ miR-34b-5p, ↓ miR-144-3p and ↓ miR-142-3p (anti-fibrotic) | ↑ MUC5b TGFβRI | ↑ Fibroblast proliferation | ↑ Lung fibrosis by activation of TGFβ and WNT/β catenin signaling pathways | UF | Syndecan-1 induces ↑ profibrotic pathways and controls miRNA-cargo | [156] |
| HFLF and NHLF | HFLF-CM NHLF-CM (exosomes) | HBEC | ↑ miR-19a-3p, ↑ miR-23b-3p, ↑ miR-127-3p, ↑ miR-145-5p, ↑ miR-424-5p, ↑ miR-494-3p | ↓ SIRT3 | mitochondrial damage and senescence in epithelial cells | Exosomal ↑ miR-23b-3p and ↑ miR-494-3p ↑ mtROS in epithelial cells | UC | Accelerated epithelial -cell mitochondrial damage and senescence is caused via-exosomal miRNAs | [135] |
| LSC-secretome and MSCs-secretome | Secretome-Exosomes | BLM-fibrotic rat model and Silica-fibrosis mouse model | ↑ miR-99-5p, ↑ 100-5p, ↑ 30a-3p in LSC-Exo and ↑ let-7a-5p, ↑ let-7f-5p in MSC-Exo | ND | ND | ND | UF | LSC-Sec as well LSC-Exo promotes lung repair in pulmonary fibrosis | [158] |
| LL29 HFLF, hBMSCs, BLM-fibrotic mouse model | BMSC-CM-EVs | No transfer assays | ↑ miR-29b-3p (anti-fibrotic) | FZD6, αSMA, Collgen I | EVs inhibit fibroblast proliferation, migration, invasion, and differentiation | ↓ WNT-β catenin signaling pathway | UC | EVs as possible therapeutic agent | [163] |
| BLM-fibrotic mouse model and HELF-TGFβ | Serum (Exosomes) | No transfer assays | ↑ miR-22 | CTGF and alpha SMA | miR-22 inhibits fibroblasts differentiation | Inhibition of ERk1/2 phosphorylation-TGFβ induced | EQ™ | miR-22 as probable therapeutic agent | [164] |
| Sputum and plasma of IPF patients and healthy subjects | THP1-CM (Exosomes) | A549 and MRC5 | ↑ miR-142-3p (anti-fibrotic) | TGFβRI COL1A1 and COL3A1 | Reduce the expression of profibrotic genes and TGFβRI | Repression of fibrotic response TGFβ-induced | UC | New therapeutic strategy | [165] |
| HBEC BMSCs, BEASB-2B, NHDF, HSAEC | HBEC-CM BMSC-CM EVs | NHLF | ↑ miR-26a, ↑ miR-26b, ↑ miR-141a, ↑ miR-200a and ↑ miR-16, ↑ miR-29, ↑ miR-29c and ↑ miR-148a | Wnt-5a WNT10 | Attenuation both myofibroblast differentiation and cellular senescence | Inhibition of TGFβ-WNT signaling pathways | UC | New therapeutic strategy | [166] |
| BLM-mouse model | Serum (Exosomes) | No transfer assays | ↑ miR-16 (anti-fibrotic) | SPARC | Attenuation of hydroxy-proline content in the lungs of BLM-treated mice | Inhibition of mTORC pathway via mTORC2/SPARC axis | EQ™ | New therapeutic strategy | [167] |
| Healthy-BMSCs | BMSCs-CM EVs | HFLF (LL29 cells) and BLM-fibrosis mouse model | ↑ mir-186 (anti-fibrotic) | ↓ αSMA ↓ Col1a1 ↓ SOX4 ↓ DKK1 | ↓ Fibroblast activation, ameliorating of IPF | Inhibition of WNT signaling pathway | UC | New therapeutic strategy | [169] |
| human-uMSCs | uMSCs-CM EVs | NMLF and BLM-fibrosis mouse model | ↑ miR-21-5p ↑ miR-23-3p (anti-fibrotic) | ↓ TGFβII and ↓ TGFβRII | Alleviate PF by ↑ AEC proliferation and ↓ myofibroblast differentiation | Inhibition of TGFβ signaling pathway | UC | New therapeutic strategy | [174] |
| HFLF (LL29 and LL97) HNLF (CCD19) | HFLF-CM NHLF-CM EVs | No transfer assays | ↑ 77 miRNAs and ↓ 68 miRNAs | ND | ND | In vitro approach | UC | In vitro approach | [175] |
10. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bartel, S.; Deshane, J.; Wilkinson, T.; Gabrielsson, S. Extracellular Vesicles as Mediators of Cellular Cross Talk in the Lung Microenvironment. Front. Med. 2020, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. Extracellular Vesicles: Their Emerging Roles in the Pathogenesis of Respiratory Diseases. Respir. Investig. 2021, 59, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Raposo, G. Exosomes--Vesicular Carriers for Intercellular Communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/Microvesicles as a Mechanism of Cell-to-Cell Communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Ramachandran, S.; Palanisamy, V. Horizontal Transfer of RNAs: Exosomes as Mediators of Intercellular Communication. Wiley Interdiscip Rev. RNA 2012, 3, 286–293. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Sze, S.K. Advances in Extracellular Vesicles Analysis. Adv. Clin. Chem. 2020, 97, 73–116. [Google Scholar] [CrossRef]
- Xie, L.; Zeng, Y. Therapeutic Potential of Exosomes in Pulmonary Fibrosis. Front. Pharm. 2020, 11, 590972. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Denzer, K.; van Eijk, M.; Kleijmeer, M.J.; Jakobson, E.; de Groot, C.; Geuze, H.J. Follicular Dendritic Cells Carry MHC Class II-Expressing Microvesicles at Their Surface. J. Immunol. 2000, 165, 1259–1265. [Google Scholar] [CrossRef]
- Purghè, B.; Manfredi, M.; Ragnoli, B.; Baldanzi, G.; Malerba, M. Exosomes in Chronic Respiratory Diseases. Biomed. Pharm. 2021, 144, 112270. [Google Scholar] [CrossRef]
- Levänen, B.; Bhakta, N.R.; Torregrosa Paredes, P.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Sköld, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered MicroRNA Profiles in Bronchoalveolar Lavage Fluid Exosomes in Asthmatic Patients. J. Allergy Clin. Immunol. 2013, 131, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, T.; Yamada, M.; Yoshioka, Y.; Sugiura, H.; Koarai, A.; Chiba, S.; Fujino, N.; Tojo, Y.; Ota, C.; Kubo, H.; et al. Serum Extracellular Vesicular MiR-21-5p Is a Predictor of the Prognosis in Idiopathic Pulmonary Fibrosis. Respir. Res. 2016, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Hao Kwok, Z.; Jin, Y. Extracellular Vesicles: A Novel Opportunity for Precision Medicine in Respiratory Diseases. Front. Med. 2021, 8, 661679. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of Transfer of Functional MicroRNAs between Mouse Dendritic Cells via Exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. The Interplay of the Genetic Architecture, Aging, and Environmental Factors in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 64, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and Immunity in IPF Pathogenesis and Treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Henket, M.; Corhay, J.L.; Moermans, C.; Louis, R. Sputum Biomarkers in IPF: Evidence for Raised Gene Expression and Protein Level of IGFBP-2, IL-8 and MMP-7. PLoS ONE 2017, 12, e0171344. [Google Scholar] [CrossRef] [PubMed]
- Konishi, K.; Gibson, K.F.; Lindell, K.O.; Richards, T.J.; Zhang, Y.; Dhir, R.; Bisceglia, M.; Gilbert, S.; Yousem, S.A.; Song, J.W.; et al. Gene Expression Profiles of Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2009, 180, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef]
- Xue, J.; Kass, D.J.; Bon, J.; Vuga, L.; Tan, J.; Csizmadia, E.; Otterbein, L.; Soejima, M.; Levesque, M.C.; Gibson, K.F.; et al. Plasma B Lymphocyte Stimulator and B Cell Differentiation in Idiopathic Pulmonary Fibrosis Patients. J. Immunol. 2013, 191, 2089–2095. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic Disease and the T(H)1/T(H)2 Paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Tager, A.M.; Borok, Z.; Moore, B.B.; Schwartz, D.A.; Anstrom, K.J.; Bar-Joseph, Z.; Bitterman, P.; Blackburn, M.R.; Bradford, W.; et al. Future Directions in Idiopathic Pulmonary Fibrosis Research. An NHLBI Workshop Report. Am. J. Respir. Crit. Care Med. 2014, 189, 214–222. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Wang, C.-H.; Wang, C.-W.; Chen, C.-J.; Huang, H.-Y.; Chung, F.-T.; Huang, Y.-C.; Lin, C.-W.; Lee, C.-S.; Lin, C.-Y.; et al. Increased Interleukin-17 and Glucocorticoid Receptor-β Expression in Interstitial Lung Diseases and Corticosteroid Insensitivity. Front. Immunol. 2022, 13, 905727. [Google Scholar] [CrossRef]
- Parker, J.M.; Glaspole, I.N.; Lancaster, L.H.; Haddad, T.J.; She, D.; Roseti, S.L.; Fiening, J.P.; Grant, E.P.; Kell, C.M.; Flaherty, K.R. A Phase 2 Randomized Controlled Study of Tralokinumab in Subjects with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 197, 94–103. [Google Scholar] [CrossRef]
- Vu, T.N.; Chen, X.; Foda, H.D.; Smaldone, G.C.; Hasaneen, N.A. Interferon-γ Enhances the Antifibrotic Effects of Pirfenidone by Attenuating IPF Lung Fibroblast Activation and Differentiation. Respir. Res. 2019, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Egan, A.M.; Shaughnessy, G.F.; Anderson, D.K.; Kottom, T.J.; Dasari, H.; Van Keulen, V.P.; Aubry, M.-C.; Yi, E.S.; Limper, A.H.; et al. Antifibrotics Modify B-Cell-Induced Fibroblast Migration and Activation in Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 64, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A. The Leading Role of Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Cell Signal. 2020, 66, 109482. [Google Scholar] [CrossRef]
- Stancil, I.T.; Michalski, J.E.; Davis-Hall, D.; Chu, H.W.; Park, J.-A.; Magin, C.M.; Yang, I.V.; Smith, B.J.; Dobrinskikh, E.; Schwartz, D.A. Pulmonary Fibrosis Distal Airway Epithelia Are Dynamically and Structurally Dysfunctional. Nat. Commun. 2021, 12, 4566. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Z.-N.; Xu, A.-R.; Fang, Z.-F.; Chen, S.-Y.; Hou, X.-T.; Zhou, Z.-Q.; Lin, H.-M.; Xie, J.-X.; Tang, X.X.; et al. Mucus Hypersecretion and Ciliary Impairment in Conducting Airway Contribute to Alveolar Mucus Plugging in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2021, 9, 810842. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Lunardi, F.; Tauro, V.; Pezzuto, F.; Fortarezza, F.; Vedovelli, L.; Faccioli, E.; Balestro, E.; Schiavon, M.; Esposito, G.; et al. RNA Sequencing of Epithelial Cell/Fibroblastic Foci Sandwich in Idiopathic Pulmonary Fibrosis: New Insights on the Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 3323. [Google Scholar] [CrossRef]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.A.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT-5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef]
- Lacy, S.H.; Woeller, C.F.; Thatcher, T.H.; Pollock, S.J.; Small, E.M.; Sime, P.J.; Phipps, R.P. Activated Human Lung Fibroblasts Produce Extracellular Vesicles with Antifibrotic Prostaglandins. Am. J. Respir. Cell Mol. Biol. 2019, 60, 269–278. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-Mediated Endocytosis of Transferrin and Recycling of the Transferrin Receptor in Rat Reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Endocytosis and Intracellular Processing of Transferrin and Colloidal Gold-Transferrin in Rat Reticulocytes: Demonstration of a Pathway for Receptor Shedding. Eur. J. Cell Biol. 1984, 35, 256–263. [Google Scholar] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Peters, P.J.; Geuze, H.J.; Van der Donk, H.A.; Slot, J.W.; Griffith, J.M.; Stam, N.J.; Clevers, H.C.; Borst, J. Molecules Relevant for T Cell-Target Cell Interaction Are Present in Cytolytic Granules of Human T Lymphocytes. Eur. J. Immunol. 1989, 19, 1469–1475. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Peng, H.; Huyan, T.; Cacalano, N.A. Exosomes: Versatile Nano Mediators of Immune Regulation. Cancers 2019, 11, 1557. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef]
- Pastor, L.; Vera, E.; Marin, J.M.; Sanz-Rubio, D. Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases. Int. J. Mol. Sci. 2021, 22, 583. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Lee, J.; Simpson, R.J.; Lötvall, J.; Gho, Y.S. EVpedia: A Community Web Resource for Prokaryotic and Eukaryotic Extracellular Vesicles Research. Semin. Cell Dev. Biol. 2015, 40, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Zhou, W.; Craft, J.; Ojemann, A.; Bergen, L.; Graner, A.; Gonzales, A.; He, Q.; Kopper, T.; Smith, M.; Graner, M.W.; et al. Glioblastoma Extracellular Vesicle-Specific Peptides Inhibit EV-Induced Neuronal Cytotoxicity. Int. J. Mol. Sci. 2022, 23, 7200. [Google Scholar] [CrossRef]
- Hu, K.; McKay, P.F.; Samnuan, K.; Najer, A.; Blakney, A.K.; Che, J.; O’Driscoll, G.; Cihova, M.; Stevens, M.M.; Shattock, R.J. Presentation of Antigen on Extracellular Vesicles Using Transmembrane Domains from Viral Glycoproteins for Enhanced Immunogenicity. J. Extracell. Vesicles 2022, 11, e12199. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Pesce, E.; Manfrini, N.; Cordiglieri, C.; Santi, S.; Bandera, A.; Gobbini, A.; Gruarin, P.; Favalli, A.; Bombaci, M.; Cuomo, A.; et al. Exosomes Recovered From the Plasma of COVID-19 Patients Expose SARS-CoV-2 Spike-Derived Fragments and Contribute to the Adaptive Immune Response. Front. Immunol. 2021, 12, 785941. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular Transfer of the Oncogenic Receptor EGFRvIII by Microvesicles Derived from Tumour Cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Zhou, S.; Lan, Y.; Li, Y.; Li, Z.; Pu, J.; Wei, L. Hypoxic Tumor-Derived Exosomes Induce M2 Macrophage Polarization via PKM2/AMPK to Promote Lung Cancer Progression. Cell Transpl. 2022, 31, 9636897221106998. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Pan, Y.; Zhang, L.; Shen, C.; Qin, G.; Ashraf, M.; Weintraub, N.; Ma, G.; Tang, Y. Cardiac Progenitor-Derived Exosomes Protect Ischemic Myocardium from Acute Ischemia/Reperfusion Injury. Biochem. Biophys. Res. Commun. 2013, 431, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Davidson, S.M. Exosomes: Nanoparticles Involved in Cardioprotection? Circ. Res. 2014, 114, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, J.; Miao, Y.; Zhang, Q. MicroRNA in Extracellular Vesicles Regulates Inflammation through Macrophages under Hypoxia. Cell Death Discov. 2021, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, Ó.; Sanz-Rubio, D.; Hernaiz, A.; Betancor, M.; Otero, A.; Castilla, J.; Andréoletti, O.; Badiola, J.J.; Zaragoza, P.; Bolea, R.; et al. Cerebrospinal Fluid and Plasma Small Extracellular Vesicles and MiRNAs as Biomarkers for Prion Diseases. Int. J. Mol. Sci. 2021, 22, 6822. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.-M. Extracellular Vesicles as Mediators of Neuron-Glia Communication. Front. Cell Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, L.; Du, X.; Zhou, K.; Qin, L.; Wang, L.; Yang, M.; Wu, M.; Zheng, Z.; Xiang, Y.; et al. Involvement of Epithelia-Derived Exosomes in Chronic Respiratory Diseases. Biomed. Pharm. 2021, 143, 112189. [Google Scholar] [CrossRef]
- Kaur, G.; Maremanda, K.P.; Campos, M.; Chand, H.S.; Li, F.; Hirani, N.; Haseeb, M.A.; Li, D.; Rahman, I. Distinct Exosomal MiRNA Profiles from BALF and Lung Tissue of COPD and IPF Patients. Int. J. Mol. Sci. 2021, 22, 11830. [Google Scholar] [CrossRef]
- Hao, Y.; Song, H.; Zhou, Z.; Chen, X.; Li, H.; Zhang, Y.; Wang, J.; Ren, X.; Wang, X. Promotion or Inhibition of Extracellular Vesicle Release: Emerging Therapeutic Opportunities. J. Control. Release 2021, 340, 136–148. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Strous, G.J.; Geuze, H.J.; Oorschot, V.; Schwartz, A.L. Late Endosomes Derive from Early Endosomes by Maturation. Cell 1991, 65, 417–427. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome Maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current Knowledge on Exosome Biogenesis and Release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Hanson, P.I.; Cashikar, A. Multivesicular Body Morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. ESCRT Complexes and the Biogenesis of Multivesicular Bodies. Curr. Opin. Cell Biol. 2008, 20, 4–11. [Google Scholar] [CrossRef]
- Migliano, S.M.; Teis, D. ESCRT and Membrane Protein Ubiquitination. Prog. Mol. Subcell. Biol. 2018, 57, 107–135. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Matanis, T.; Akhmanova, A.; Wulf, P.; Del Nery, E.; Weide, T.; Stepanova, T.; Galjart, N.; Grosveld, F.; Goud, B.; De Zeeuw, C.I.; et al. Bicaudal-D Regulates COPI-Independent Golgi-ER Transport by Recruiting the Dynein-Dynactin Motor Complex. Nat. Cell Biol. 2002, 4, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V.; Orci, L.; Glick, B.S.; Block, M.R.; Rothman, J.E. Role of an N-Ethylmaleimide-Sensitive Transport Component in Promoting Fusion of Transport Vesicles with Cisternae of the Golgi Stack. Cell 1988, 54, 221–227. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs--Engines for Membrane Fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-Dependent Cellular Uptake of Exosomes. Nanomedicine 2017, 13, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular Uptake of Extracellular Vesicles Is Mediated by Clathrin-Independent Endocytosis and Macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Buschow, S.I.; Nolte-’t Hoen, E.N.M.; van Niel, G.; Pols, M.S.; ten Broeke, T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in Dendritic Cells Is Targeted to Lysosomes or T Cell-Induced Exosomes via Distinct Multivesicular Body Pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A Common Pathway for a Specialized Function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef]
- Moran, Y.; Agron, M.; Praher, D.; Technau, U. The Evolutionary Origin of Plant and Animal MicroRNAs. Nat. Ecol. Evol. 2017, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Bofill-De Ros, X.; Kasprzak, W.K.; Bhandari, Y.; Fan, L.; Cavanaugh, Q.; Jiang, M.; Dai, L.; Yang, A.; Shao, T.-J.; Shapiro, B.A.; et al. Structural Differences between Pri-MiRNA Paralogs Promote Alternative Drosha Cleavage and Expand Target Repertoires. Cell Rep. 2019, 26, 447–459.e4. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Iwai, N.; Naraba, H. Polymorphisms in Human Pre-MiRNAs. Biochem. Biophys. Res. Commun. 2005, 331, 1439–1444. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of MicroRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 Mediates the Nuclear Export of Pre-MicroRNAs and Short Hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Pan, P. Extracellular Vesicle MicroRNA Transfer in Lung Diseases. Front. Physiol. 2017, 8, 1028. [Google Scholar] [CrossRef]
- Kawamata, T.; Tomari, Y. Making RISC. Trends Biochem. Sci. 2010, 35, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Redfern, A.D.; Colley, S.M.; Beveridge, D.J.; Ikeda, N.; Epis, M.R.; Li, X.; Foulds, C.E.; Stuart, L.M.; Barker, A.; Russell, V.J.; et al. RNA-Induced Silencing Complex (RISC) Proteins PACT, TRBP, and Dicer Are SRA Binding Nuclear Receptor Coregulators. Proc. Natl. Acad. Sci. USA 2013, 110, 6536–6541. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated HnRNPA2B1 Controls the Sorting of MiRNAs into Exosomes through Binding to Specific Motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef]
- Koppers-Lalic, D.; Hackenberg, M.; Bijnsdorp, I.V.; van Eijndhoven, M.A.J.; Sadek, P.; Sie, D.; Zini, N.; Middeldorp, J.M.; Ylstra, B.; de Menezes, R.X.; et al. Nontemplated Nucleotide Additions Distinguish the Small RNA Composition in Cells from Exosomes. Cell Rep. 2014, 8, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.J.; Hoshino, D.; Hong, N.H.; Cha, D.J.; Franklin, J.L.; Coffey, R.J.; Patton, J.G.; Weaver, A.M. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016, 15, 978–987. [Google Scholar] [CrossRef]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Karfilis, K.V.; Ri, S.; Schekman, R. Y-Box Protein 1 Is Required to Sort MicroRNAs into Exosomes in Cells and in a Cell-Free Reaction. Elife 2016, 5, e19276. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sánchez-Madrid, F. Intercellular Communication: Diverse Structures for Exchange of Genetic Information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-Cell Communication: MicroRNAs as Hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA Signatures of Tumor-Derived Exosomes as Diagnostic Biomarkers of Ovarian Cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional Delivery of Viral MiRNAs via Exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage Microvesicles Induce Macrophage Differentiation and MiR-223 Transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef]
- Selman, M.; López-Otín, C.; Pardo, A. Age-Driven Developmental Drift in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2016, 48, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Königshoff, M.; Kramer, M.; Balsara, N.; Wilhelm, J.; Amarie, O.V.; Jahn, A.; Rose, F.; Fink, L.; Seeger, W.; Schaefer, L.; et al. WNT1-Inducible Signaling Protein–1 Mediates Pulmonary Fibrosis in Mice and Is Upregulated in Humans with Idiopathic Pulmonary Fibrosis. J. Clin. Investig. 2009, 119, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Barkauskas, C.E.; Cronce, M.J.; Xue, Y.; Harris, J.R.; Liang, J.; Noble, P.W.; Hogan, B.L.M. Multiple Stromal Populations Contribute to Pulmonary Fibrosis without Evidence for Epithelial to Mesenchymal Transition. Proc. Natl. Acad. Sci. USA 2011, 108, E1475–E1483. [Google Scholar] [CrossRef]
- Kadota, T.; Fujita, Y.; Araya, J.; Ochiya, T.; Kuwano, K. Extracellular Vesicle-Mediated Cellular Crosstalk in Lung Repair, Remodelling and Regeneration. Eur. Respir. Rev. 2022, 31, 210106. [Google Scholar] [CrossRef]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like Vesicles Are Present in Human Blood Plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and Proteomic Profiling of Exosomes in Human Urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenbäck, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with Major Histocompatibility Complex Class II and Co-Stimulatory Molecules Are Present in Human BAL Fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H. Extracellular Vesicles in Respiratory Disease. Adv. Clin. Chem. 2022, 108, 105–127. [Google Scholar] [CrossRef]
- Cha, S.; Seo, E.-H.; Lee, S.H.; Kim, K.S.; Oh, C.-S.; Moon, J.-S.; Kim, J.K. MicroRNA Expression in Extracellular Vesicles from Nasal Lavage Fluid in Chronic Rhinosinusitis. Biomedicines 2021, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and Transcriptomic Analyses of Human Saliva Derived Exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.-G.; Tang, L.; Gong, S.-G.; Sun, Y.-Y.; Wang, L.; Jiang, R.; Wu, W.-H.; Luo, C.-J.; Zhang, J.; et al. Plasma Exosomal MiR-596: A Novel Biomarker Predicts Survival in Patients with Idiopathic Pulmonary Artery Hypertension. J. Int. Med. Res. 2021, 49, 3000605211002379. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Long, Y.; Chen, Y. Emerging Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Chronic Respiratory Diseases: An Overview of Recent Progress. Front. Bioeng. Biotechnol. 2022, 10, 845042. [Google Scholar] [CrossRef]
- Abreu, S.C.; Weiss, D.J.; Rocco, P.R.M. Extracellular Vesicles Derived from Mesenchymal Stromal Cells: A Therapeutic Option in Respiratory Diseases? Stem. Cell Res. Ther. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Zargar, M.J.; Kaviani, S.; Vasei, M.; Soufi Zomorrod, M.; Heidari Keshel, S.; Soleimani, M. Therapeutic Role of Mesenchymal Stem Cell-Derived Exosomes in Respiratory Disease. Stem. Cell Res. Ther. 2022, 13, 194. [Google Scholar] [CrossRef]
- Tieu, A.; Hu, K.; Gnyra, C.; Montroy, J.; Fergusson, D.A.; Allan, D.S.; Stewart, D.J.; Thébaud, B.; Lalu, M.M. Mesenchymal Stromal Cell Extracellular Vesicles as Therapy for Acute and Chronic Respiratory Diseases: A Meta-Analysis. J. Extracell. Vesicles 2021, 10, e12141. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic Pulmonary Fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef]
- Sauleda, J.; Núñez, B.; Sala, E.; Soriano, J.B. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med. Sci. 2018, 6, 110. [Google Scholar] [CrossRef]
- Ryerson, C.J.; Kolb, M. The Increasing Mortality of Idiopathic Pulmonary Fibrosis: Fact or Fallacy? Eur. Respir. J. 2018, 51, 1702420. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Selman, M. Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 5), S417–S421. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Suárez, A.R.; Hernández-Hernández, H.A.; Alvarado-Vásquez, N.; Rangel-Escareño, C.; Sommer, B.; Negrete-García, M.C. Role of MicroRNAs in Signaling Pathways Associated with the Pathogenesis of Idiopathic Pulmonary Fibrosis: A Focus on Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2022, 23, 6613. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Yue, R.; Peng, X.; Yu, H.; Huang, X. Exosomes Derived from Hypoxia-Induced Alveolar Epithelial Cells Stimulate Interstitial Pulmonary Fibrosis through a HOTAIRM1-Dependent Mechanism. Lab. Investig. 2022, 102, 935–944. [Google Scholar] [CrossRef]
- Kadota, T.; Yoshioka, Y.; Fujita, Y.; Araya, J.; Minagawa, S.; Hara, H.; Miyamoto, A.; Suzuki, S.; Fujimori, S.; Kohno, T.; et al. Extracellular Vesicles from Fibroblasts Induce Epithelial Cell Senescence in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 63, 623–636. [Google Scholar] [CrossRef]
- Chanda, D.; Otoupalova, E.; Hough, K.P.; Locy, M.L.; Bernard, K.; Deshane, J.S.; Sanderson, R.D.; Mobley, J.A.; Thannickal, V.J. Fibronectin on the Surface of Extracellular Vesicles Mediates Fibroblast Invasion. Am. J. Respir. Cell Mol. Biol. 2019, 60, 279–288. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-Derived Exosomal MiRNAs Promote Metastasis of Lung Cancer Cells via STAT3-Induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Soccio, P.; Bergantini, L.; Cameli, P.; Scioscia, G.; Foschino Barbaro, M.P.; Lacedonia, D.; Bargagli, E. Extracellular Vesicle Surface Signatures in IPF Patients: A Multiplex Bead-Based Flow Cytometry Approach. Cells 2021, 10, 1045. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in Bodily Fluids Are a Highly Stable Resource of Disease Biomarkers. Proteomics Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, T.; Hu, X.; Liu, Z.; Zhao, L.; Liu, H.; Liu, Z.; Ma, L. Downregulation of MicroRNA-30a in Bronchoalveolar Lavage Fluid from Idiopathic Pulmonary Fibrosis Patients. Mol. Med. Rep. 2018, 18, 5799–5806. [Google Scholar] [CrossRef]
- Njock, M.-S.; Guiot, J.; Henket, M.A.; Nivelles, O.; Thiry, M.; Dequiedt, F.; Corhay, J.-L.; Louis, R.E.; Struman, I. Sputum Exosomes: Promising Biomarkers for Idiopathic Pulmonary Fibrosis. Thorax 2019, 74, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Lacedonia, D.; Scioscia, G.; Soccio, P.; Conese, M.; Catucci, L.; Palladino, G.P.; Simone, F.; Quarato, C.M.I.; Di Gioia, S.; Rana, R.; et al. Downregulation of Exosomal Let-7d and MiR-16 in Idiopathic Pulmonary Fibrosis. BMC Pulm. Med. 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-J.; Pan, Q.; Li, D.-G.; Sun, H.; Liu, B.-W. MiR-15b and MiR-16 Are Implicated in Activation of the Rat Hepatic Stellate Cell: An Essential Role for Apoptosis. J. Hepatol. 2009, 50, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, S.; Chen, Y.; Bai, X.; Liu, L.; Dong, Y.; Hu, M.; Su, X.; Chen, Y.; Huangfu, L.; et al. MiR-26a Suppresses EMT by Disrupting the Lin28B/Let-7d Axis: Potential Cross-Talks among MiRNAs in IPF. J. Mol. Med. 2016, 94, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Shan, H.; Liang, H. MicroRNAs in Idiopathic Pulmonary Fibrosis: Involvement in Pathogenesis and Potential Use in Diagnosis and Therapeutics. Acta Pharm. Sin. B 2016, 6, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Enríquez, J.M.; Santos-Álvarez, J.C.; Ramírez-Hernández, A.A.; Reyes-Jiménez, E.; López-Martínez, A.; Pina-Canseco, S.; Aguilar-Ruiz, S.R.; Romero-Tlalolini, M.d.l.Á.; Castro-Sánchez, L.; Arellanes-Robledo, J.; et al. Proteomic Analysis Reveals Key Proteins in Extracellular Vesicles Cargo Associated with Idiopathic Pulmonary Fibrosis In Vitro. Biomedicines 2021, 9, 1058. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and Stoichiometric Analysis of the MicroRNA Content of Exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Fricke, F.; Nitschke, K.; Worst, T.; Erb, U.; Karremann, M.; Buschmann, D.; Elvers-Hornung, S.; Tucher, C.; Schiller, M.; et al. Inter-Laboratory Comparison of Extracellular Vesicle Isolation Based on Ultracentrifugation. Transfus. Med. Hemother. 2021, 48, 48–59. [Google Scholar] [CrossRef]
- Zhang, X.; Borg, E.G.F.; Liaci, A.M.; Vos, H.R.; Stoorvogel, W. A Novel Three Step Protocol to Isolate Extracellular Vesicles from Plasma or Cell Culture Medium with Both High Yield and Purity. J. Extracell. Vesicles 2020, 9, 1791450. [Google Scholar] [CrossRef]
- Zhou, Y.; Hagood, J.S.; Lu, B.; Merryman, W.D.; Murphy-Ullrich, J.E. Thy-1-Integrin Alphav Beta5 Interactions Inhibit Lung Fibroblast Contraction-Induced Latent Transforming Growth Factor-Beta1 Activation and Myofibroblast Differentiation. J. Biol. Chem. 2010, 285, 22382–22393. [Google Scholar] [CrossRef]
- Fiore, V.F.; Strane, P.W.; Bryksin, A.V.; White, E.S.; Hagood, J.S.; Barker, T.H. Conformational Coupling of Integrin and Thy-1 Regulates Fyn Priming and Fibroblast Mechanotransduction. J. Cell Biol. 2015, 211, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Shentu, T.-P.; Huang, T.-S.; Cernelc-Kohan, M.; Chan, J.; Wong, S.S.; Espinoza, C.R.; Tan, C.; Gramaglia, I.; van der Heyde, H.; Chien, S.; et al. Thy-1 Dependent Uptake of Mesenchymal Stem Cell-Derived Extracellular Vesicles Blocks Myofibroblastic Differentiation. Sci. Rep. 2017, 7, 18052. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.-Y.; Zhang, W.-H.; Ma, W.-T.; Liu, Q.-H.; Xing, L.-H.; Zhao, G.-F. MicroRNA-328 in Exosomes Derived from M2 Macrophages Exerts a Promotive Effect on the Progression of Pulmonary Fibrosis via FAM13A in a Rat Model. Exp. Mol. Med. 2019, 51, 63. [Google Scholar] [CrossRef] [PubMed]
- Parimon, T.; Brauer, R.; Schlesinger, S.Y.; Xie, T.; Jiang, D.; Ge, L.; Huang, Y.; Birkland, T.P.; Parks, W.C.; Habiel, D.M.; et al. Syndecan-1 Controls Lung Tumorigenesis by Regulating MiRNAs Packaged in Exosomes. Am. J. Pathol. 2018, 188, 1094–1103. [Google Scholar] [CrossRef]
- Altemeier, W.A.; Schlesinger, S.Y.; Buell, C.A.; Brauer, R.; Rapraeger, A.C.; Parks, W.C.; Chen, P. Transmembrane and Extracellular Domains of Syndecan-1 Have Distinct Functions in Regulating Lung Epithelial Migration and Adhesion*. J. Biol. Chem. 2012, 287, 34927–34935. [Google Scholar] [CrossRef]
- Parimon, T.; Yao, C.; Habiel, D.M.; Ge, L.; Bora, S.A.; Brauer, R.; Evans, C.M.; Xie, T.; Alonso-Valenteen, F.; Medina-Kauwe, L.K.; et al. Syndecan-1 Promotes Lung Fibrosis by Regulating Epithelial Reprogramming through Extracellular Vesicles. JCI Insight 2019, 5, e129359. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal Role of Paracrine Effects in Stem Cell Therapies in Regenerative Medicine: Can We Translate Stem Cell-Secreted Paracrine Factors and Microvesicles into Better Therapeutic Strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of Lung Spheroid Cell Secretome and Exosomes Promotes Lung Repair in Pulmonary Fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem. Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef]
- Guo, H.; Su, Y.; Deng, F. Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Future Perspectives. Stem. Cell Rev. Rep. 2020, 17, 440–458. [Google Scholar] [CrossRef]
- Álvarez-Viejo, M. Mesenchymal Stem Cells from Different Sources and Their Derived Exosomes: A Pre-Clinical Perspective. World J. Stem. Cells 2020, 12, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Chen, S.; Fang, Y.; Zuo, W.; Cui, J.; Xie, S. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppress the Fibroblast Proliferation by Downregulating FZD6 Expression in Fibroblasts via MicrRNA-29b-3p in Idiopathic Pulmonary Fibrosis. J. Cell Physiol. 2020, 235, 8613–8625. [Google Scholar] [CrossRef]
- Kuse, N.; Kamio, K.; Azuma, A.; Matsuda, K.; Inomata, M.; Usuki, J.; Morinaga, A.; Tanaka, T.; Kashiwada, T.; Atsumi, K.; et al. Exosome-Derived MicroRNA-22 Ameliorates Pulmonary Fibrosis by Regulating Fibroblast-to-Myofibroblast Differentiation Both in Vitro and in Vivo. J. Nippon Med. Sch. 2019, 87, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Cambier, M.; Boeckx, A.; Henket, M.; Nivelles, O.; Gester, F.; Louis, E.; Malaise, M.; Dequiedt, F.; Louis, R.; et al. Macrophage-Derived Exosomes Attenuate Fibrosis in Airway Epithelial Cells through Delivery of Antifibrotic MiR-142-3p. Thorax 2020, 75, 870–881. [Google Scholar] [CrossRef]
- Kadota, T.; Fujita, Y.; Araya, J.; Watanabe, N.; Fujimoto, S.; Kawamoto, H.; Minagawa, S.; Hara, H.; Ohtsuka, T.; Yamamoto, Y.; et al. Human Bronchial Epithelial Cell-derived Extracellular Vesicle Therapy for Pulmonary Fibrosis via Inhibition of TGF-β-WNT Crosstalk. J. Extracell. Vesicles 2021, 10, e12124m. [Google Scholar] [CrossRef]
- Inomata, M.; Kamio, K.; Azuma, A.; Matsuda, K.; Usuki, J.; Morinaga, A.; Tanaka, T.; Kashiwada, T.; Atsumi, K.; Hayashi, H.; et al. Rictor-Targeting Exosomal MicroRNA-16 Ameliorates Lung Fibrosis by Inhibiting the MTORC2-SPARC Axis. Exp. Cell Res. 2021, 398, 112416. [Google Scholar] [CrossRef]
- Kamio, K.; Azuma, A.; Usuki, J.; Matsuda, K.; Inomata, M.; Nishijima, N.; Itakura, S.; Hayashi, H.; Kashiwada, T.; Kokuho, N.; et al. XPLN Is Modulated by HDAC Inhibitors and Negatively Regulates SPARC Expression by Targeting MTORC2 in Human Lung Fibroblasts. Pulm. Pharmacol. Ther. 2017, 44, 61–69. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Y.; Kang, X.; Liu, Z.; Zhang, W.; Xu, F. MicroRNA-186 in Extracellular Vesicles from Bone Marrow Mesenchymal Stem Cells Alleviates Idiopathic Pulmonary Fibrosis via Interaction with SOX4 and DKK1. Stem. Cell. Res. Ther. 2021, 12, 96. [Google Scholar] [CrossRef]
- Lei, G.-S.; Kline, H.L.; Lee, C.-H.; Wilkes, D.S.; Zhang, C. Regulation of Collagen V Expression and Epithelial-Mesenchymal Transition by MiR-185 and MiR-186 during Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2016, 186, 2310–2316. [Google Scholar] [CrossRef]
- Huang, K.Y.; Petretto, E. Cross-Species Integration of Single-Cell RNA-Seq Resolved Alveolar-Epithelial Transitional States in Idiopathic Pulmonary Fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L491–L506. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, J.; Li, Q.; Hou, L.; Wang, Y.; Li, S.; Jiang, F.; Zhu, Z.; Tian, L. Exosomes Derived from Three-Dimensional Cultured Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Pulmonary Fibrosis in a Mouse Silicosis Model. Stem. Cell Res. Ther. 2020, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; He, N.; Zhu, L.; Zhou, M.; Zhang, K.; Wang, C.; Huang, H.; Chen, S.; Li, Y.; Liu, Q.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via MiRNA-214-3p. Antioxid Redox Signal. 2021, 35, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ren, J.; Li, J.; Wang, D.; Wang, Y.; Qin, T.; Li, X.; Zhang, G.; Li, C.; Wang, Y. Extracellular Vesicles Derived from Umbilical Cord Mesenchymal Stromal Cells Alleviate Pulmonary Fibrosis by Means of Transforming Growth Factor-β Signaling Inhibition. Stem. Cell Res. Ther. 2021, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Santos-Álvarez, J.C.; Velázquez-Enríquez, J.M.; García-Carrillo, R.; Rodríguez-Beas, C.; Ramírez-Hernández, A.A.; Reyes-Jiménez, E.; González-García, K.; López-Martínez, A.; Pérez-Campos Mayoral, L.; Aguilar-Ruiz, S.R.; et al. MiRNAs Contained in Extracellular Vesicles Cargo Contribute to the Progression of Idiopathic Pulmonary Fibrosis: An In Vitro Aproach. Cells 2022, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, W.; Yang, R. MiR-1246 Is Responsible for Lung Cancer Cells-Derived Exosomes-Mediated Promoting Effects on Lung Cancer Stemness via Targeting TRIM17. Env. Toxicol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, D.; Katare, R. Exosomal MicroRNAs in Diabetic Heart Disease. Cardiovasc. Diabetol. 2022, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; James, V.; Onion, D.; Fairclough, L.C. Extracellular Vesicles and Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. Respir. Res. 2022, 23, 82. [Google Scholar] [CrossRef]
- Gambardella, J.; Kansakar, U.; Sardu, C.; Messina, V.; Jankauskas, S.S.; Marfella, R.; Maggi, P.; Wang, X.; Mone, P.; Paolisso, G.; et al. Exosomal MiR-145 and MiR-885 Regulate Thrombosis in COVID-19. J. Pharmacol. Exp. Ther. 2022, JPET-AR-2022-001209. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrete-García, M.C.; de Jesús Ramos-Abundis, J.; Alvarado-Vasquez, N.; Montes-Martínez, E.; Montaño, M.; Ramos, C.; Sommer, B. Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 11047. https://doi.org/10.3390/ijms231911047
Negrete-García MC, de Jesús Ramos-Abundis J, Alvarado-Vasquez N, Montes-Martínez E, Montaño M, Ramos C, Sommer B. Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences. 2022; 23(19):11047. https://doi.org/10.3390/ijms231911047
Chicago/Turabian StyleNegrete-García, María Cristina, Javier de Jesús Ramos-Abundis, Noé Alvarado-Vasquez, Eduardo Montes-Martínez, Martha Montaño, Carlos Ramos, and Bettina Sommer. 2022. "Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis" International Journal of Molecular Sciences 23, no. 19: 11047. https://doi.org/10.3390/ijms231911047
APA StyleNegrete-García, M. C., de Jesús Ramos-Abundis, J., Alvarado-Vasquez, N., Montes-Martínez, E., Montaño, M., Ramos, C., & Sommer, B. (2022). Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences, 23(19), 11047. https://doi.org/10.3390/ijms231911047





