Different Acute Kidney Injury Patterns after Renal Ischemia Reperfusion Injury and Extracorporeal Membrane Oxygenation in Mice
Abstract
1. Introduction
2. Results
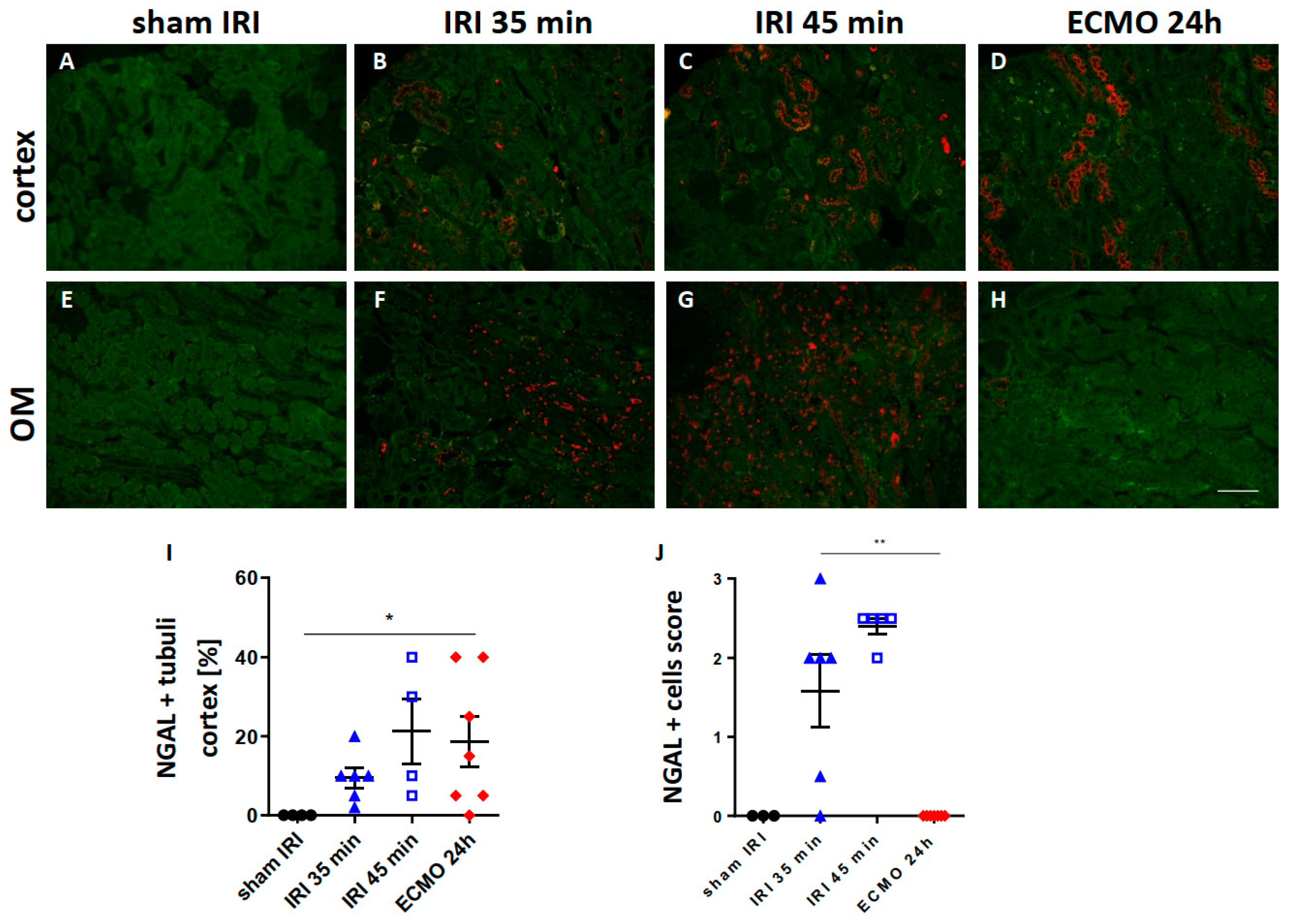
2.1. Renal NGAL Expression
2.2. Kidney Neutrophil Infiltration
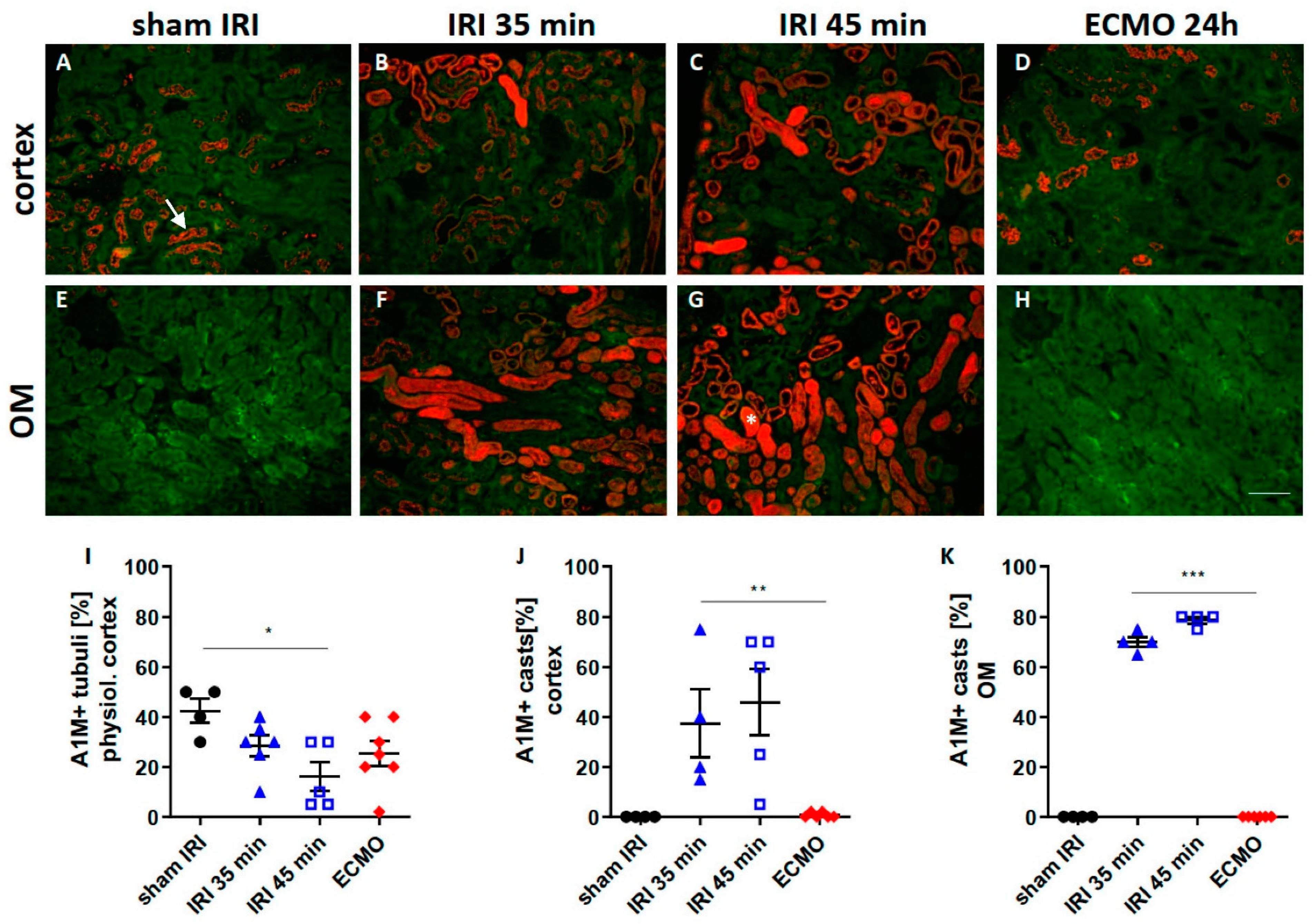
2.3. Tubular Transport Function
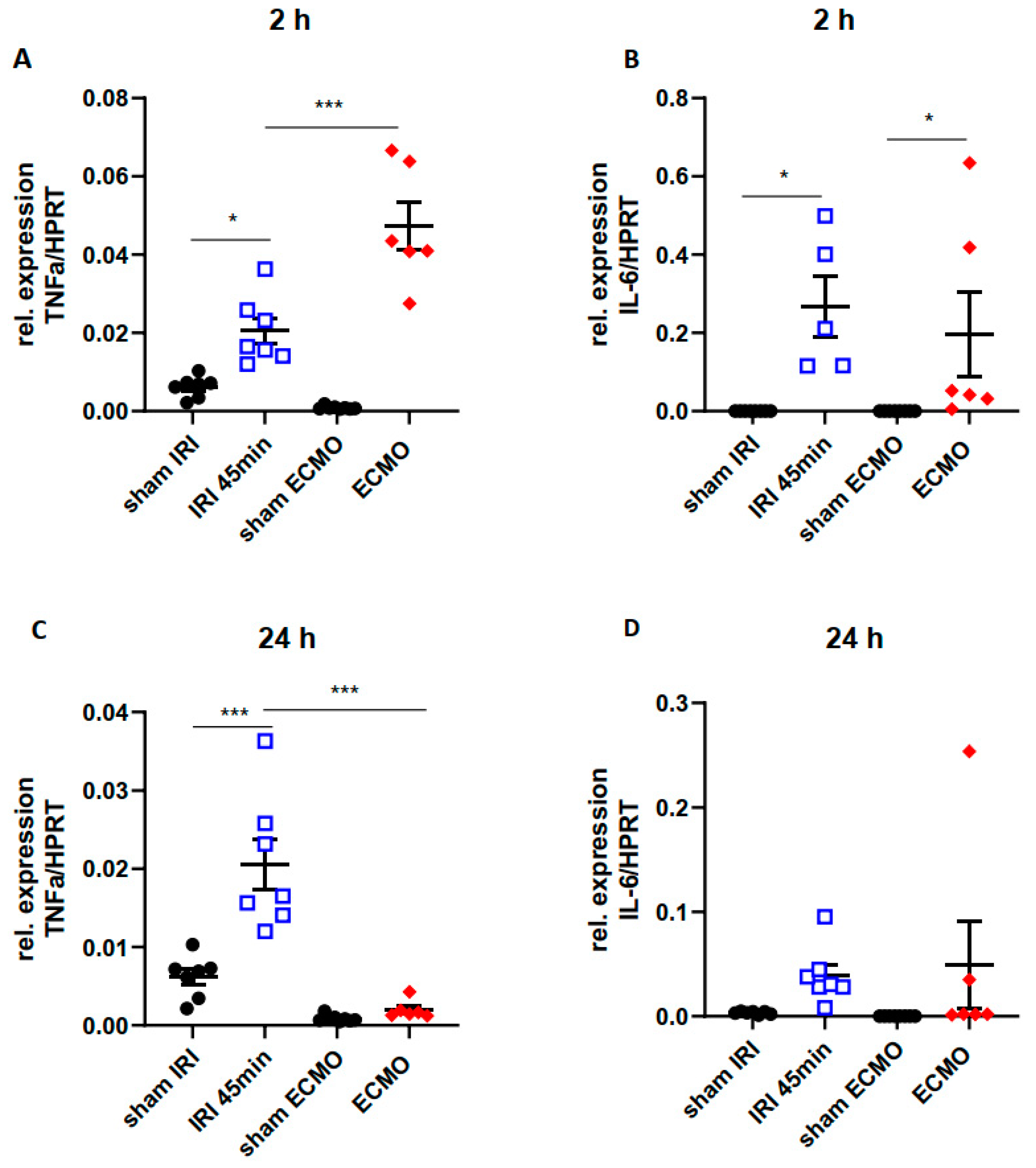
2.4. Time Kinetics of Kidney Tissue Pro-Inflammatory Cytokines
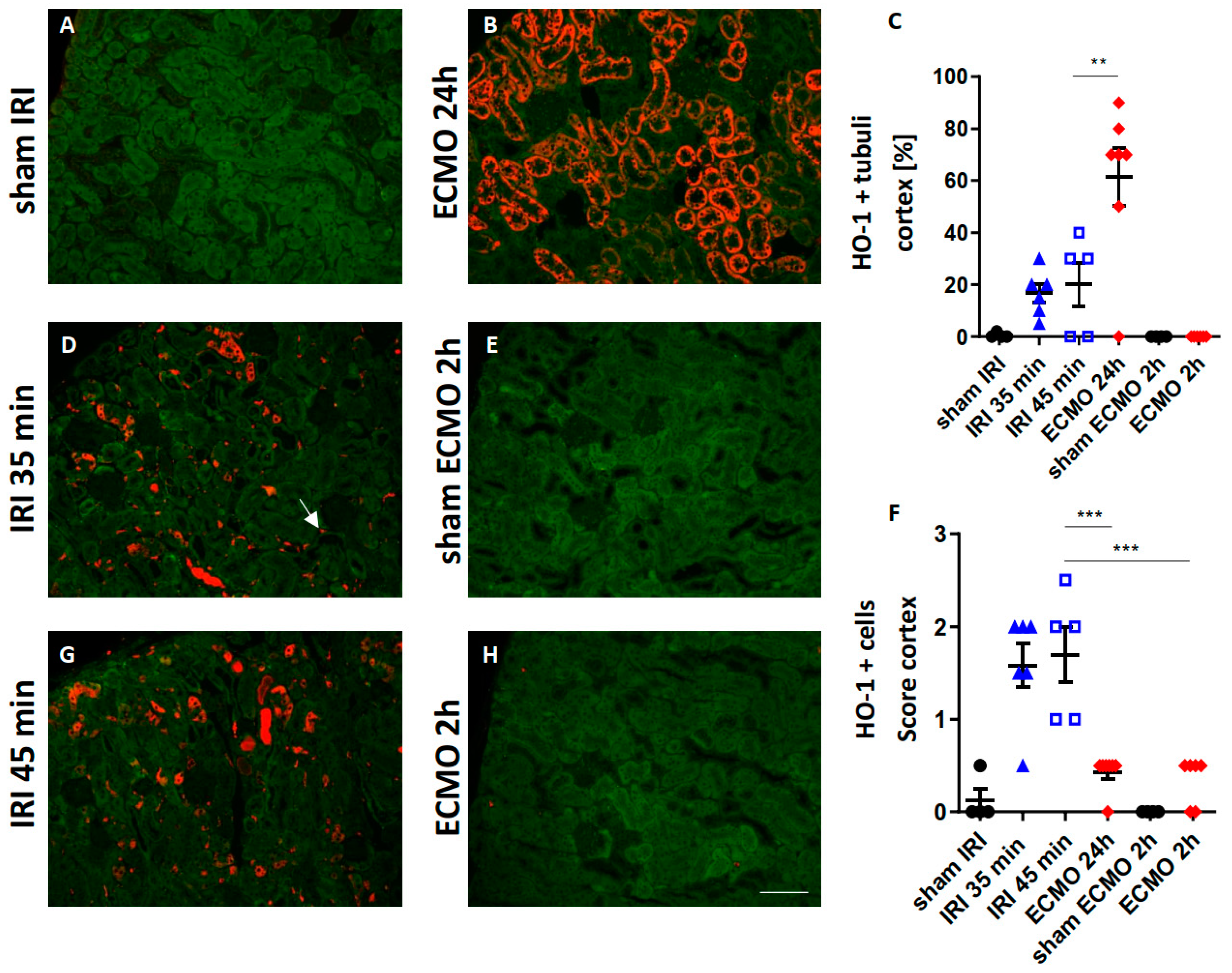
2.5. Renal HO-1 Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Renal Ischemia Reperfusion Injury (IRI)
4.3. vv Extracorporeal Membrane Oxygenation (ECMO)
4.4. Physiological Monitoring during ECMO
4.5. Organ Preservation
4.6. Pro-Inflammatory Cytokine Expression in the Kidney Tissue
4.7. Renal Morphology and Immunofluorescence
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salman, J.; Bernhard, B.A.; Ius, F.; Poyanmehr, R.; Sommer, W.; Aburahma, K.; Alhadidi, H.; Siemeni, T.; Kuehn, C.; Avsar, M.; et al. Intraoperative Extracorporeal Circulatory Support in Lung Transplantation for Pulmonary Fibrosis. Ann. Thorac. Surg. 2021, 111, 1316–1324. [Google Scholar] [CrossRef]
- Ius, F.; Aburahma, K.; Boethig, D.; Salman, J.; Sommer, W.; Draeger, H.; Poyanmehr, R.; Avsar, M.; Siemeni, T.; Bobylev, D.; et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J. Heart Lung Transplant. 2020, 39, 915–925. [Google Scholar] [CrossRef]
- Faccioli, E.; Terzi, S.; Pangoni, A.; Lomangino, I.; Rossi, S.; Lloret, A.; Cannone, G.; Marino, C.; Catelli, C.; Dell’Amore, A. Extracorporeal membrane oxygenation in lung transplantation: Indications, techniques and results. World J. Transplant. 2021, 11, 290–302. [Google Scholar] [CrossRef]
- Makdisi, G.; Wang, I.W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 2015, 7, E166–E176. [Google Scholar]
- Xia, Y.; Ragalie, W.; Yang, E.H.; Lluri, G.; Biniwale, R.; Benharash, P.; Gudzenko, V.; Saggar, R.; Sayah, D.; Ardehali, A. Venoarterial versus Venovenous Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation. Ann. Thorac. Surg. 2021, in press. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Lertjitbanjong, P.; Aeddula, N.R.; Bathini, T.; Watthanasuntorn, K.; Srivali, N.; Mao, M.A.; Kashani, K. Incidence and Impact of Acute Kidney Injury in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. J. Clin. Med. 2019, 8, 981. [Google Scholar] [CrossRef]
- Mou, Z.; He, J.; Guan, T.; Chen, L. Acute Kidney Injury during Extracorporeal Membrane Oxygenation: VA ECMO versus VV ECMO. J. Intensive Care Med. 2022, 37, 743–752. [Google Scholar] [CrossRef]
- Gu, M.; Mei, X.L.; Zhao, Y.N. A review on extracorporeal membrane oxygenation and kidney injury. J. Biochem. Mol. Toxicol. 2021, 35, e22679. [Google Scholar] [CrossRef]
- Omar, H.R.; Mirsaeidi, M.; Socias, S.; Sprenker, C.; Caldeira, C.; Camporesi, E.M.; Mangar, D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE 2015, 10, e0124034. [Google Scholar] [CrossRef]
- Martucci, G.; Panarello, G.; Occhipinti, G.; Ferrazza, V.; Tuzzolino, F.; Bellavia, D.; Sanfilippo, F.; Santonocito, C.; Bertani, A.; Vitulo, P.; et al. Anticoagulation and Transfusions Management in Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: Assessment of Factors Associated with Transfusion Requirements and Mortality. J. Intensive Care Med. 2019, 34, 630–639. [Google Scholar] [CrossRef]
- Heinsar, S.; Rozencwajg, S.; Suen, J.; Bassi, G.L.; Malfertheiner, M.; Vercaemst, L.; Broman, L.M.; Schmidt, M.; Combes, A.; Ratsep, I.; et al. Heart failure supported by veno-arterial extracorporeal membrane oxygenation (ECMO): A systematic review of pre-clinical models. Intensive Care Med. Exp. 2020, 8, 16. [Google Scholar] [CrossRef]
- Madrahimov, N.; Khalikov, A.; Boyle, E.C.; Natanov, R.; Knoefel, A.K.; Siemeni, T.; Hoeffler, K.; Haverich, A.; Maus, U.; Kuehn, C. Veno-Venous Extracorporeal Membrane Oxygenation in a Mouse. J. Vis. Exp. 2018, 140, e58146. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Cowland, J.B.; Borregaard, N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta 2000, 1482, 272–283. [Google Scholar] [CrossRef]
- Xu, S.; Venge, P. Lipocalins as biochemical markers of disease. Biochim. Biophys. Acta 2000, 1482, 298–307. [Google Scholar] [CrossRef]
- Rund, K.M.; Peng, S.; Greite, R.; Claassen, C.; Nolte, F.; Oger, C.; Galano, J.M.; Balas, L.; Durand, T.; Chen, R.; et al. Dietary omega-3 PUFA improved tubular function after ischemia induced acute kidney injury in mice but did not attenuate impairment of renal function. Prostaglandins Other Lipid Mediat. 2020, 146, 106386. [Google Scholar] [CrossRef]
- Hull, T.D.; Kamal, A.I.; Boddu, R.; Bolisetty, S.; Guo, L.; Tisher, C.C.; Rangarajan, S.; Chen, B.; Curtis, L.M.; George, J.F.; et al. Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI. J. Am. Soc. Nephrol. 2015, 26, 2139–2151. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhang, D.L.; Kovtunovych, G.; Ghosh, M.C.; Ollivierre, H.; Eckhaus, M.A.; Rouault, T.A. Infused wild-type macrophages reside and self-renew in the liver to rescue the hemolysis and anemia of Hmox1-deficient mice. Blood Adv. 2018, 2, 2732–2743. [Google Scholar] [CrossRef]
- Li, Y.; Ma, K.; Han, Z.; Chi, M.; Sai, X.; Zhu, P.; Ding, Z.; Song, L.; Liu, C. Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease. Front. Med. 2021, 8, 708453. [Google Scholar] [CrossRef]
- Jing, L.; Chen, W.; Zhao, L.; Guo, L.; Liang, C.; Chen, J.; Wang, C. Acute kidney injury following adult lung transplantation. Chin. Med. J. 2021, 135, 172–180. [Google Scholar] [CrossRef]
- Nishida, K.; Watanabe, H.; Miyahisa, M.; Hiramoto, Y.; Nosaki, H.; Fujimura, R.; Maeda, H.; Otagiri, M.; Maruyama, T. Systemic and sustained thioredoxin analogue prevents acute kidney injury and its-associated distant organ damage in renal ischemia reperfusion injury mice. Sci. Rep. 2020, 10, 20635. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Gutberlet, M.; Rong, S.; Hartung, D.; Mengel, M.; Lu, X.; Haller, H.; Wacker, F.; Meier, M.; Gueler, F. Acute kidney injury: Arterial spin labeling to monitor renal perfusion impairment in mice-comparison with histopathologic results and renal function. Radiology 2014, 270, 117–124. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Natanov, R.; Khalikov, A.; Gueler, F.; Maus, U.; Boyle, E.C.; Haverich, A.; Kuhn, C.; Madrahimov, N. Four hours of veno-venous extracorporeal membrane oxygenation using bi-caval cannulation affects kidney function and induces moderate lung damage in a mouse model. Intensive Care Med. Exp. 2019, 7, 72. [Google Scholar] [CrossRef]
- Govender, K.; Cabrales, P. Extracorporeal circulation impairs microcirculation perfusion and organ function. J. Appl. Physiol. 2022, 132, 794–810. [Google Scholar] [CrossRef]
- Villa, G.; Katz, N.; Ronco, C. Extracorporeal Membrane Oxygenation and the Kidney. Cardiorenal Med. 2015, 6, 50–60. [Google Scholar] [CrossRef]
- Nemoto, M. Experimental evaluation of the influence of complete artificial circulation on renal circulation and tissue metabolism -comparative study of pulsatile vs nonpulsatile circulation. Ann. Thorac. Cardiovasc. Surg. 2003, 9, 355–364. [Google Scholar]
- Undar, A.; Masai, T.; Yang, S.Q.; Goddard-Finegold, J.; Frazier, O.H.; Fraser, C.D., Jr. Effects of perfusion mode on regional and global organ blood flow in a neonatal piglet model. Ann. Thorac. Surg. 1999, 68, 1336–1342; discussion 1342–1343. [Google Scholar] [CrossRef]
- Szabo-Biczok, A.; Varga, G.; Varga, Z.; Bari, G.; Vigyikan, G.; Gajda, A.; Vida, N.; Hodoniczki, A.; Rutai, A.; Juhasz, L.; et al. Veno-Venous Extracorporeal Membrane Oxygenation in Minipigs as a Robust Tool to Model Acute Kidney Injury: Technical Notes and Characteristics. Front. Med. 2022, 9, 866667. [Google Scholar] [CrossRef]
- Redfors, B.; Bragadottir, G.; Sellgren, J.; Sward, K.; Ricksten, S.E. Effects of norepinephrine on renal perfusion, filtration and oxygenation in vasodilatory shock and acute kidney injury. Intensive Care Med. 2011, 37, 60–67. [Google Scholar] [CrossRef]
- Natanov, R.; Gueler, F.; Falk, C.S.; Kuhn, C.; Maus, U.; Boyle, E.C.; Siemeni, T.; Knoefel, A.K.; Cebotari, S.; Haverich, A.; et al. Blood cytokine expression correlates with early multi-organ damage in a mouse model of moderate hypothermia with circulatory arrest using cardiopulmonary bypass. PLoS ONE 2018, 13, e0205437. [Google Scholar] [CrossRef] [PubMed]
- Heise, D.; Rentsch, K.; Braeuer, A.; Friedrich, M.; Quintel, M. Comparison of urinary neutrophil glucosaminidase-associated lipocalin, cystatin C, and alpha1-microglobulin for early detection of acute renal injury after cardiac surgery. Eur. J. Cardiothorac. Surg. 2011, 39, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care. 2016, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, F.; Chen, Y.; Chen, Y.; Shushakova, N.; Yao, Y.; Zeng, R.; Li, J.; Lu, X.; Chen, R.; et al. Pre-ischemic renal lavage protects against renal ischemia-reperfusion injury by attenuation of local and systemic inflammatory responses. FASEB J. 2020, 34, 16307–16318. [Google Scholar] [CrossRef] [PubMed]
- Mc, I.R.B.; Timpa, J.G.; Kurundkar, A.R.; Holt, D.W.; Kelly, D.R.; Hartman, Y.E.; Neel, M.L.; Karnatak, R.K.; Schelonka, R.L.; Anantharamaiah, G.M.; et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab. Investig. 2010, 90, 128–139. [Google Scholar]
- Adrian, K.; Mellgren, K.; Skogby, M.; Friberg, L.G.; Mellgren, G.; Wadenvik, H. Cytokine release during long-term extracorporeal circulation in an experimental model. Artif. Organs 1998, 22, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, Q.; Yu, W.; Shen, J.; Gong, J.; He, C.; Hu, Y.; Zhang, J.; Gao, T.; Xi, F.; et al. Continuous renal replacement therapy reduces the systemic and pulmonary inflammation induced by venovenous extracorporeal membrane oxygenation in a porcine model. Artif. Organs 2014, 38, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Risnes, I.; Wagner, K.; Ueland, T.; Mollnes, T.; Aukrust, P.; Svennevig, J. Interleukin-6 may predict survival in extracorporeal membrane oxygenation treatment. Perfusion 2008, 23, 173–178. [Google Scholar] [CrossRef]
- Yimin, H.; Wenkui, Y.; Jialiang, S.; Qiyi, C.; Juanhong, S.; Zhiliang, L.; Changsheng, H.; Ning, L.; Jieshou, L. Effects of continuous renal replacement therapy on renal inflammatory cytokines during extracorporeal membrane oxygenation in a porcine model. J. Cardiothorac. Surg. 2013, 8, 113. [Google Scholar] [CrossRef]
- Chintagari, N.R.; Nguyen, J.; Belcher, J.D.; Vercellotti, G.M.; Alayash, A.I. Haptoglobin attenuates hemoglobin-induced heme oxygenase-1 in renal proximal tubule cells and kidneys of a mouse model of sickle cell disease. Blood Cells Mol. Dis. 2015, 54, 302–306. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Gouveia, Z.; Soares, M.P.; Gozzelino, R. Heme cytotoxicity and the pathogenesis of immune-mediated inflammatory diseases. Front. Pharmacol. 2012, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Roumenina, L.T.; Rayes, J.; Lacroix-Desmazes, S.; Dimitrov, J.D. Heme: Modulator of Plasma Systems in Hemolytic Diseases. Trends Mol. Med. 2016, 22, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Immenschuh, S.; Vijayan, V.; Janciauskiene, S.; Gueler, F. Heme as a Target for Therapeutic Interventions. Front. Pharmacol. 2017, 8, 146. [Google Scholar] [CrossRef]
- Madrahimov, N.; Boyle, E.C.; Gueler, F.; Goecke, T.; Knofel, A.K.; Irkha, V.; Maegel, L.; Hoffler, K.; Natanov, R.; Ismail, I.; et al. Novel mouse model of cardiopulmonary bypass. Eur. J. Cardiothorac. Surg. 2018, 53, 186–193. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greite, R.; Störmer, J.; Gueler, F.; Khalikov, R.; Haverich, A.; Kühn, C.; Madrahimov, N.; Natanov, R. Different Acute Kidney Injury Patterns after Renal Ischemia Reperfusion Injury and Extracorporeal Membrane Oxygenation in Mice. Int. J. Mol. Sci. 2022, 23, 11000. https://doi.org/10.3390/ijms231911000
Greite R, Störmer J, Gueler F, Khalikov R, Haverich A, Kühn C, Madrahimov N, Natanov R. Different Acute Kidney Injury Patterns after Renal Ischemia Reperfusion Injury and Extracorporeal Membrane Oxygenation in Mice. International Journal of Molecular Sciences. 2022; 23(19):11000. https://doi.org/10.3390/ijms231911000
Chicago/Turabian StyleGreite, Robert, Johanna Störmer, Faikah Gueler, Rasul Khalikov, Axel Haverich, Christian Kühn, Nodir Madrahimov, and Ruslan Natanov. 2022. "Different Acute Kidney Injury Patterns after Renal Ischemia Reperfusion Injury and Extracorporeal Membrane Oxygenation in Mice" International Journal of Molecular Sciences 23, no. 19: 11000. https://doi.org/10.3390/ijms231911000
APA StyleGreite, R., Störmer, J., Gueler, F., Khalikov, R., Haverich, A., Kühn, C., Madrahimov, N., & Natanov, R. (2022). Different Acute Kidney Injury Patterns after Renal Ischemia Reperfusion Injury and Extracorporeal Membrane Oxygenation in Mice. International Journal of Molecular Sciences, 23(19), 11000. https://doi.org/10.3390/ijms231911000




