Inhibition of Borrelia Burgdorferi-Induced TLR2-NFκB Canonical Signaling by Gallic Acid through Targeting the CD14+ Adaptor Protein and p65 Molecule
Abstract
1. Introduction
2. Results
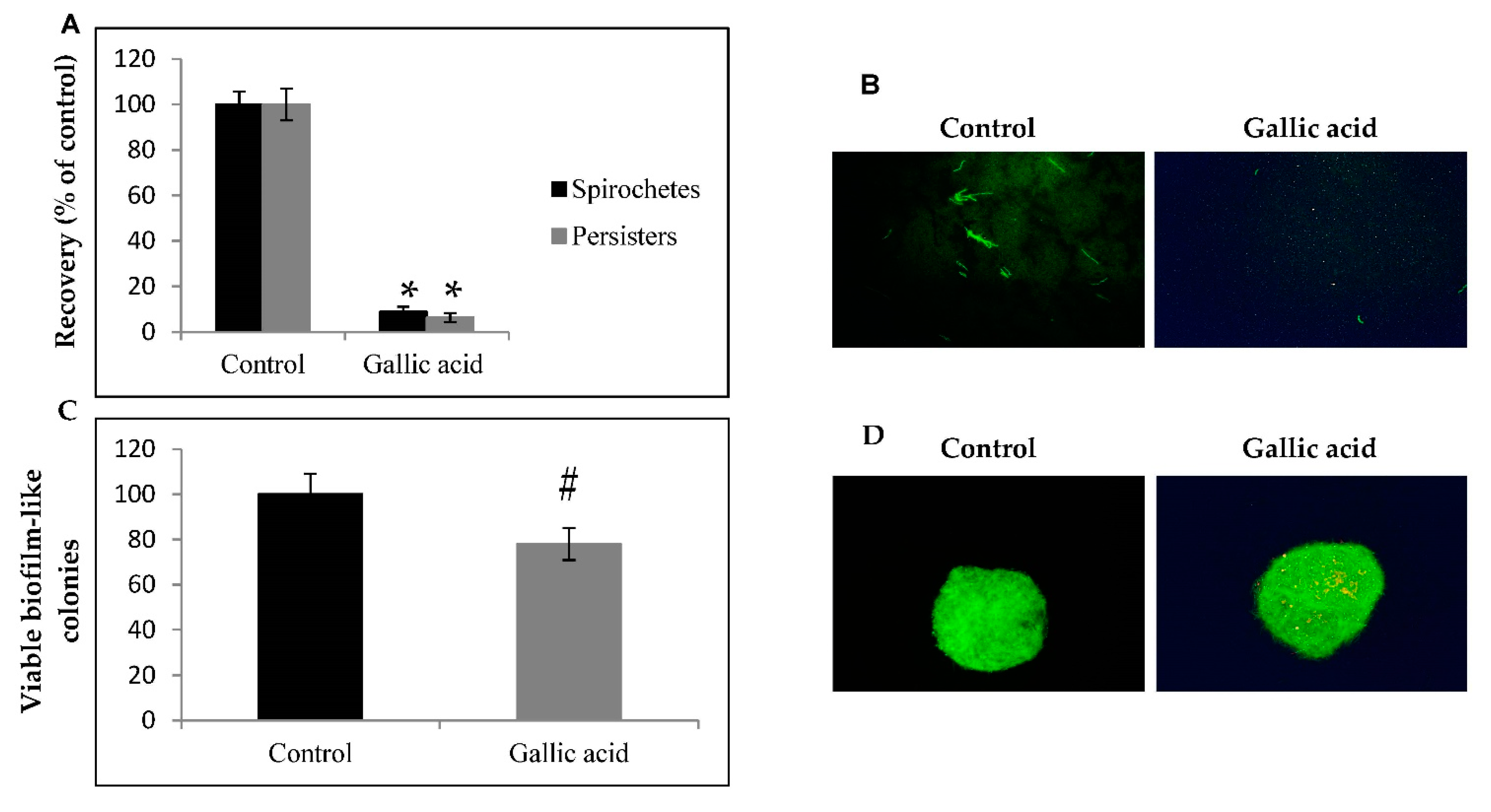
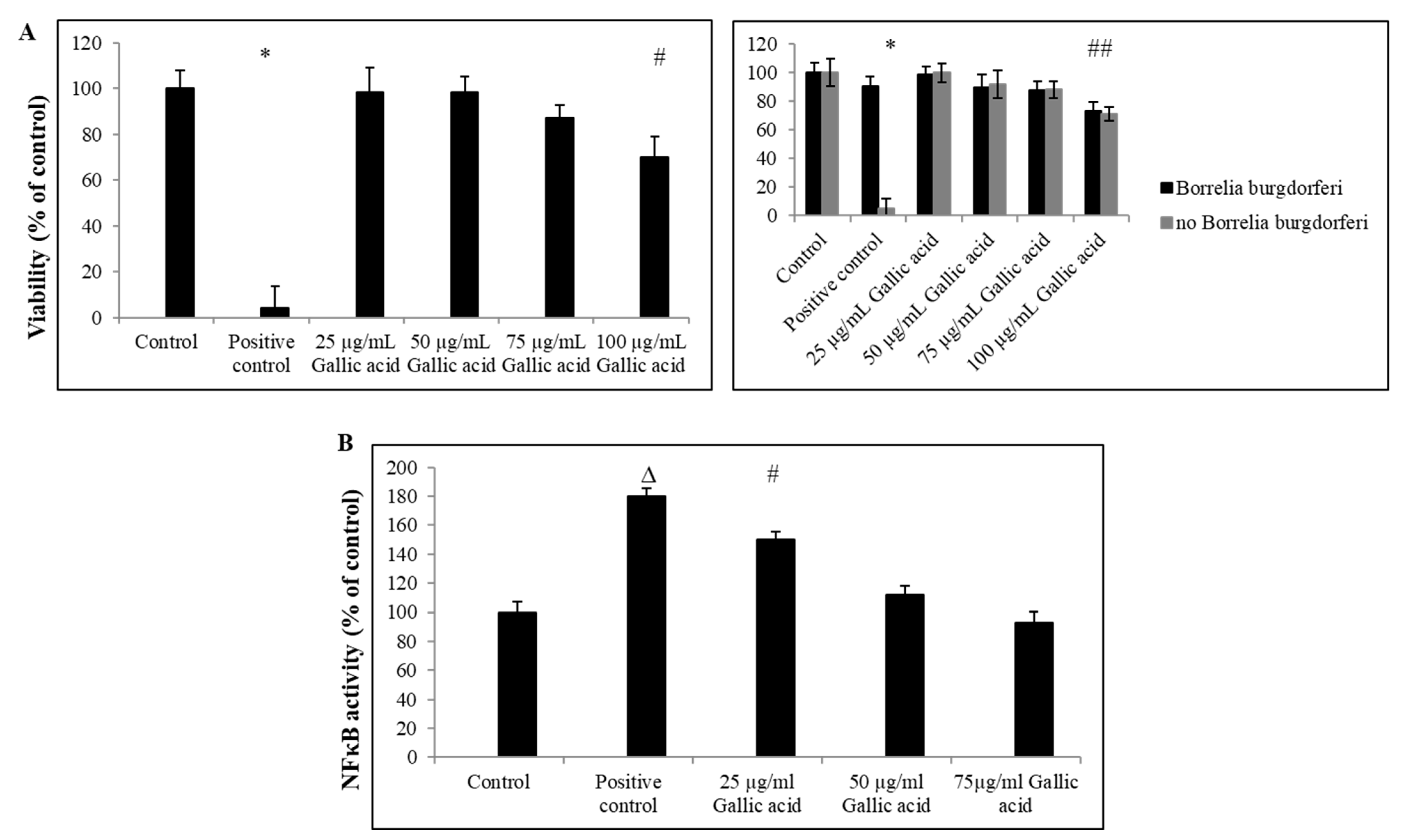
2.1. Anti-Borrelia Efficacy of Gallic Acid
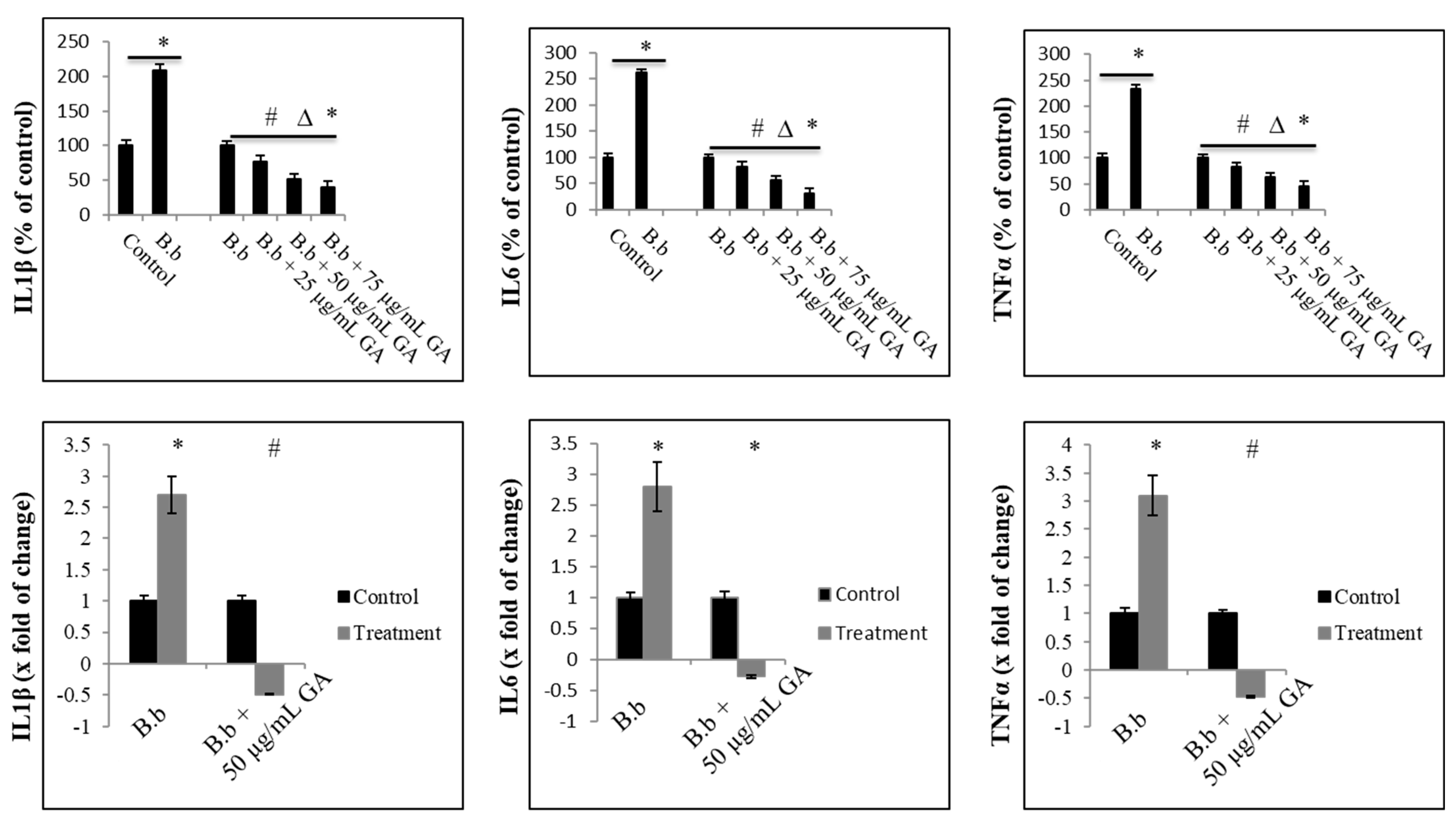
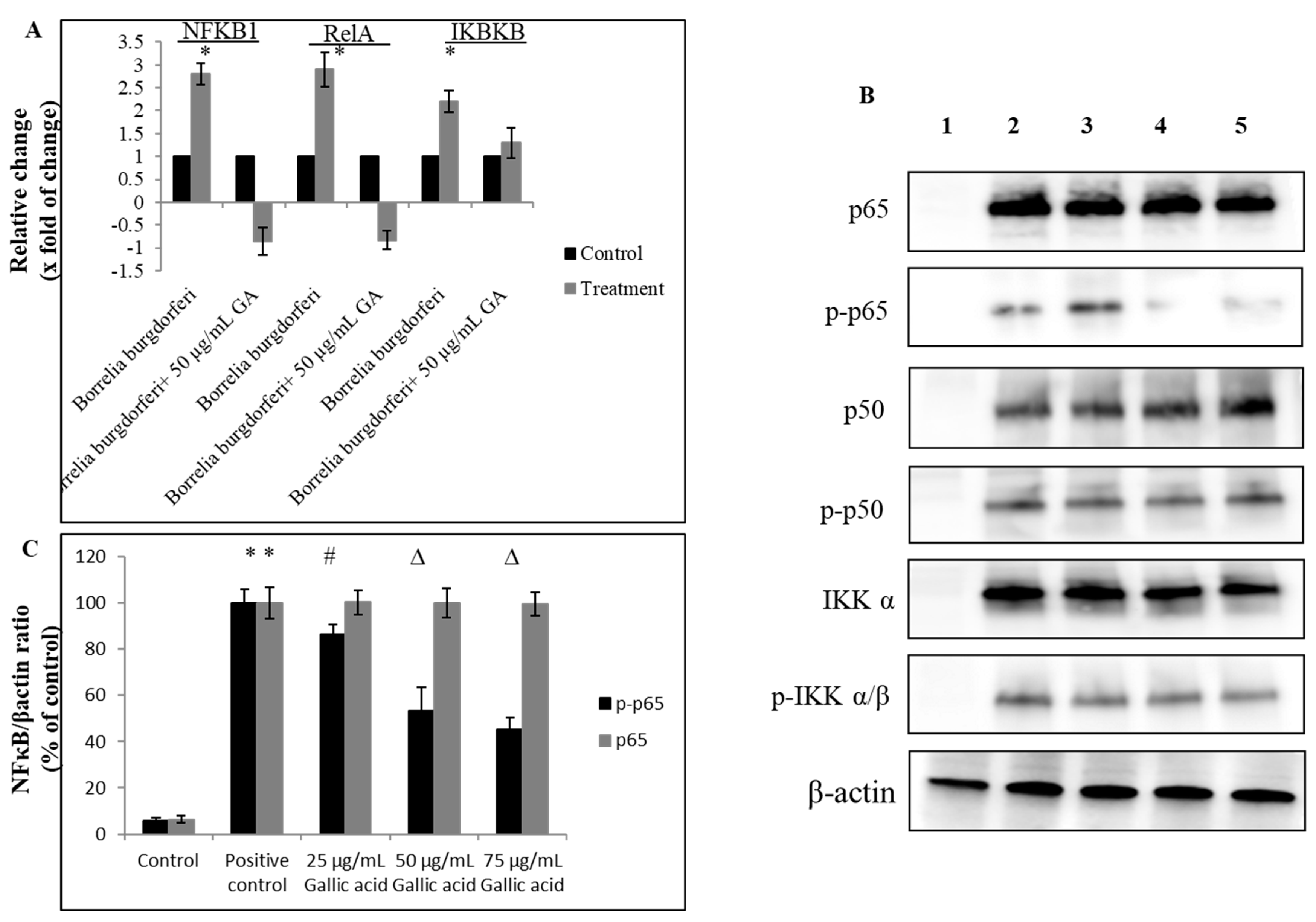
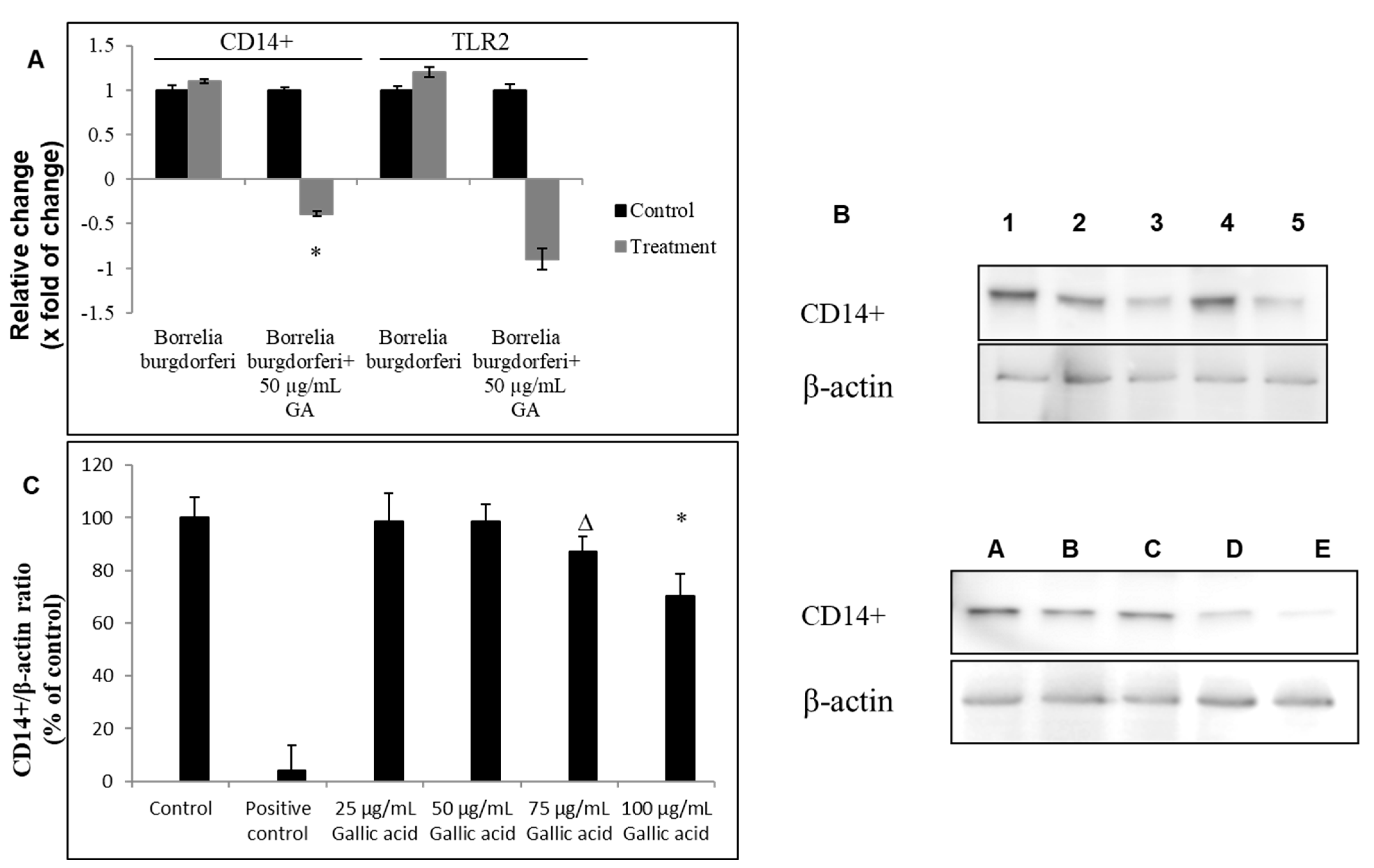
2.2. Anti-Inflammatory Effect of Gallic Acid against Borrelia burgdorferi-Induced In Vitro Infection
3. Discussion
4. Materials and Methods
4.1. Test Compound
4.2. Borrelia burgdorferi Culture
4.3. Evaluation of the Biocidal Efficacy of Gallic Acid against Borrelia burgdorferi
4.4. Analysis of Cytokine Secretion
4.5. NFκB Activity Assay
4.6. RT-qPCR
4.7. Western Blot
4.8. Viability Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, E.D. Lyme disease. N. Engl. J. Med. 2014, 370, 724–1731. [Google Scholar] [CrossRef] [PubMed]
- Lovrich, S.D.; Callister, S.M.; Lim, L.C.; Duchateau, B.K.; Schell, R.F. Seroprotective groups of Lyme borreliosis spirochetes from North America and Europe. J. Infect. Dis. 1994, 170, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Lyme disease website. Available online: http://www.cdc.gov/lyme/ (accessed on 10 May 2022).
- Brorson, Ø.; Brorson, S.H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 1998, 26, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Murgia, R.; Cinco, M. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS 2004, 112, 57–62. [Google Scholar] [CrossRef]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.; Pham, T.V.; et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS ONE 2012, 7, e48277. [Google Scholar]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.; et al. Evidence of in vivo existence of Borrelia biofilm in borrelial lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Oosting, M.; Hofstede, H.; Sturm, P.; Adema, G.J.; Kullberg, B.J.; van der Meer, J.W.M.; Netea, M.G.; Joosten, L.A.B. TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes. PLoS ONE 2011, 6, e25998. [Google Scholar] [CrossRef]
- Coburn, J.; Garcia, B.; Hu, L.T.; Jewett, M.W.; Kraiczy, P.; Norris, S.J.; Skare, J. Lyme Disease pathogenesis. Curr. Issues Mol. Biol. 2021, 42, 473–518. [Google Scholar]
- Benjamin, S.J.; Hawley, K.L.; Vera-Licona, P.; La Vake, C.J.; Cervantes, J.L.; Ruan, Y.; Radolf, J.D.; Salazar, J.C. Macrophage mediated recognition and clearance of Borrelia burgdorferi elicits MyD88-dependent and -independent phagosomal signals that contribute to phagocytosis and inflammation. BMC Immunol. 2021, 22, 32. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell. Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Greene, W.C. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell. Biol. 2004, 5, 392–401. [Google Scholar] [CrossRef]
- Barger, S.W.; Moerman, A.M.; Mao, X. Molecular mechanisms of cytokine-induced neuroprotection: NFkappaB and neuroplasticity. Curr. Pharm. Des. 2005, 11, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappaB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y.; Shishodia, S.; Gutierrez, A.M.; Oommen, O.V.; Ichikawa, H.; Baba, Y.; Kumar, A. Nuclear transcription factor NF-kappa B: Role in biology and medicine. Indian J. Exp. Biol. 2004, 42, 341–353. [Google Scholar]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 195–224. [Google Scholar] [CrossRef]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-kappa B system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef]
- Baud, V.; Jacque, E. The alternative NF-kB activation pathway and cancer: Friend or foe? Med. Sci. 2008, 24, 1083–1088. [Google Scholar]
- Israel, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and gallic acid attenuate LPS-induced inflammation and oxidative stress via MAPK/NF-κB and Akt/AMPK/Nrf2 Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hou, L.; Xue, H.; Wang, C. Gallic acid inhibits inflammatory response of RAW264.7 macrophages by blocking the activation of TLR4/NF-κB induced by LPS. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 1610–1614. [Google Scholar]
- Sapi, E.; Kaur, N.; Anyanwu, S.; Luecke, D.F.; Datar, A.; Patel, S.; Rossi, M.; Stricker, R.B. Evaluation of in vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 2011, 4, 97–113. [Google Scholar]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emer. Microbes Infect. 2014, 3, e49. [Google Scholar]
- Feng, J.; Zhang, S.; Shi, W.; Zhang, Y. Ceftriaxone Pulse Dosing Fails to Eradicate Biofilm-Like microcolony Borrelia burgdorferi persisters which are sterilized by daptomycin/doxycycline/cefuroxime without pulse dosing. Front. Microbiol. 2016, 7, 1744–1752. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. In vitro evaluation of antibacterial activity of phytochemicals and micronutrients against Borrelia burgdorferi and Borrelia garinii. J. Appl. Microbiol. 2015, 119, 1561–1572. [Google Scholar] [CrossRef]
- Theophilus, P.A.; Victoria, M.J.; Socarras, K.M.; Filush, K.R.; Gupta, K.; Luecke, D.F.; Sapi, E. Effectiveness of Stevia Rebaudiana whole leaf extract against the various morphological forms of Borrelia Burgdorferi in vitro. Eur. J. Microbiol. Immunol. 2015, 5, 268–280. [Google Scholar] [CrossRef]
- Goc, A.; Rath, M. The anti-borreliae efficacy of phytochemicals and micronutrients: An update. Therap. Adv. Infect. Dis. 2016, 3, 75–82. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Synergistic Anti-Borreliae Efficacy of a Composition of Naturally-occurring Compounds: An In vitro Study. J. Nutri. Biol. 2019, 5, 350–363. [Google Scholar] [CrossRef]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug combinations against Borrelia burgdorferi persisters in vitro: Eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Levin, E.S.; Schweitzer, P.J.; Hardesty, D. Inflammation and central nervous system Lyme disease. Neurobiol. Dis. 2010, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.C.; Duhnam-Ems, S.; La Vake, C.; Cruz, A.R.; Moore, M.W.; Caimano, M.J.; Velez-Climent, L.; Shupe, J.; Krueger, W.; Radolf, J.D. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-β. PLoS Pathog. 2009, 5, e1000444. [Google Scholar] [CrossRef]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [CrossRef]
- Klempner, M.S.; Baker, P.J.; Shapiro, E.D.; Marques, A.; Dattwyler, R.J.; Halperin, J.J.; Wormser, G.P. Treatment trials for post-Lyme disease symptoms revisited. Am. J. Med. 2013, 126, 665–669. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Giambartolomei, G.H.; Dennis, V.D.; Lasater, B.L.; Phillip, M.T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immunol. 1999, 67, 140–147. [Google Scholar] [CrossRef]
- Sellati, T.J.; Bouis, D.A.; Caimano, M.J.; Feulner, J.A.; Ayers, C.; Lien, E.; Radolf, J.D. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 1999, 163, 2049–2056. [Google Scholar] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Benhnia, M.R.E.I.; Wroblewski, D.; Akhtar, M.N.; Patel, R.A.; Lavezzi, W.; Gangloff, S.C.; Goyert, S.M.; Caimano, M.J.; Radolf, J.D.; Sellati, T.J. Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. J. Immunol. 2005, 174, 1539–1548. [Google Scholar] [CrossRef]
- Cruz, A.R.; Moore, M.W.; La Vake, C.J.; Eggers, C.H.; Salazar, J.C.; Radolf, J.D. Phagocytosis of Borrelia burgdorferi, the Lyme Disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect. Immunol. 2008, 76, 56–70. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goc, A.; Rath, M.; Niedzwiecki, A. Inhibition of Borrelia Burgdorferi-Induced TLR2-NFκB Canonical Signaling by Gallic Acid through Targeting the CD14+ Adaptor Protein and p65 Molecule. Int. J. Mol. Sci. 2022, 23, 10987. https://doi.org/10.3390/ijms231910987
Goc A, Rath M, Niedzwiecki A. Inhibition of Borrelia Burgdorferi-Induced TLR2-NFκB Canonical Signaling by Gallic Acid through Targeting the CD14+ Adaptor Protein and p65 Molecule. International Journal of Molecular Sciences. 2022; 23(19):10987. https://doi.org/10.3390/ijms231910987
Chicago/Turabian StyleGoc, Anna, Matthias Rath, and Aleksandra Niedzwiecki. 2022. "Inhibition of Borrelia Burgdorferi-Induced TLR2-NFκB Canonical Signaling by Gallic Acid through Targeting the CD14+ Adaptor Protein and p65 Molecule" International Journal of Molecular Sciences 23, no. 19: 10987. https://doi.org/10.3390/ijms231910987
APA StyleGoc, A., Rath, M., & Niedzwiecki, A. (2022). Inhibition of Borrelia Burgdorferi-Induced TLR2-NFκB Canonical Signaling by Gallic Acid through Targeting the CD14+ Adaptor Protein and p65 Molecule. International Journal of Molecular Sciences, 23(19), 10987. https://doi.org/10.3390/ijms231910987




