Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets
Abstract
1. Introduction
2. Results
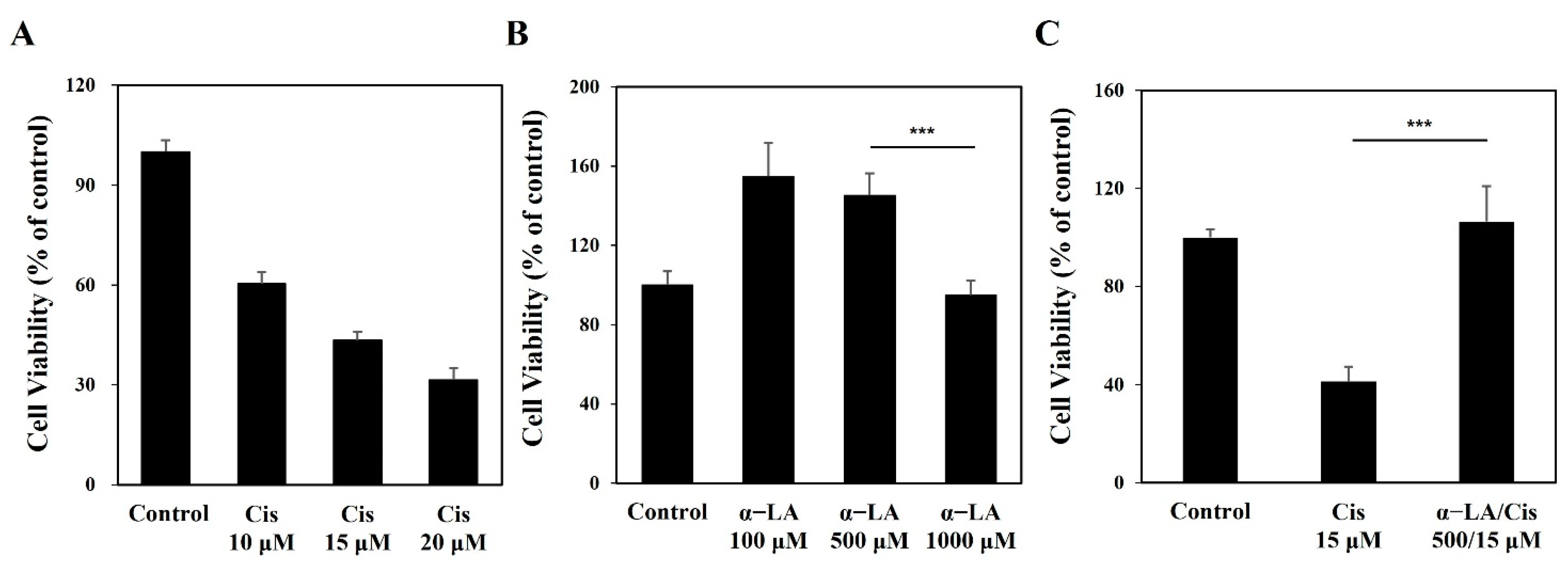
2.1. Cisplatin Induces Cytotoxicity of HEI-OC1 Cells, While α-LA Protects against Cisplatin-Induced Damage to Auditory Hair Cells
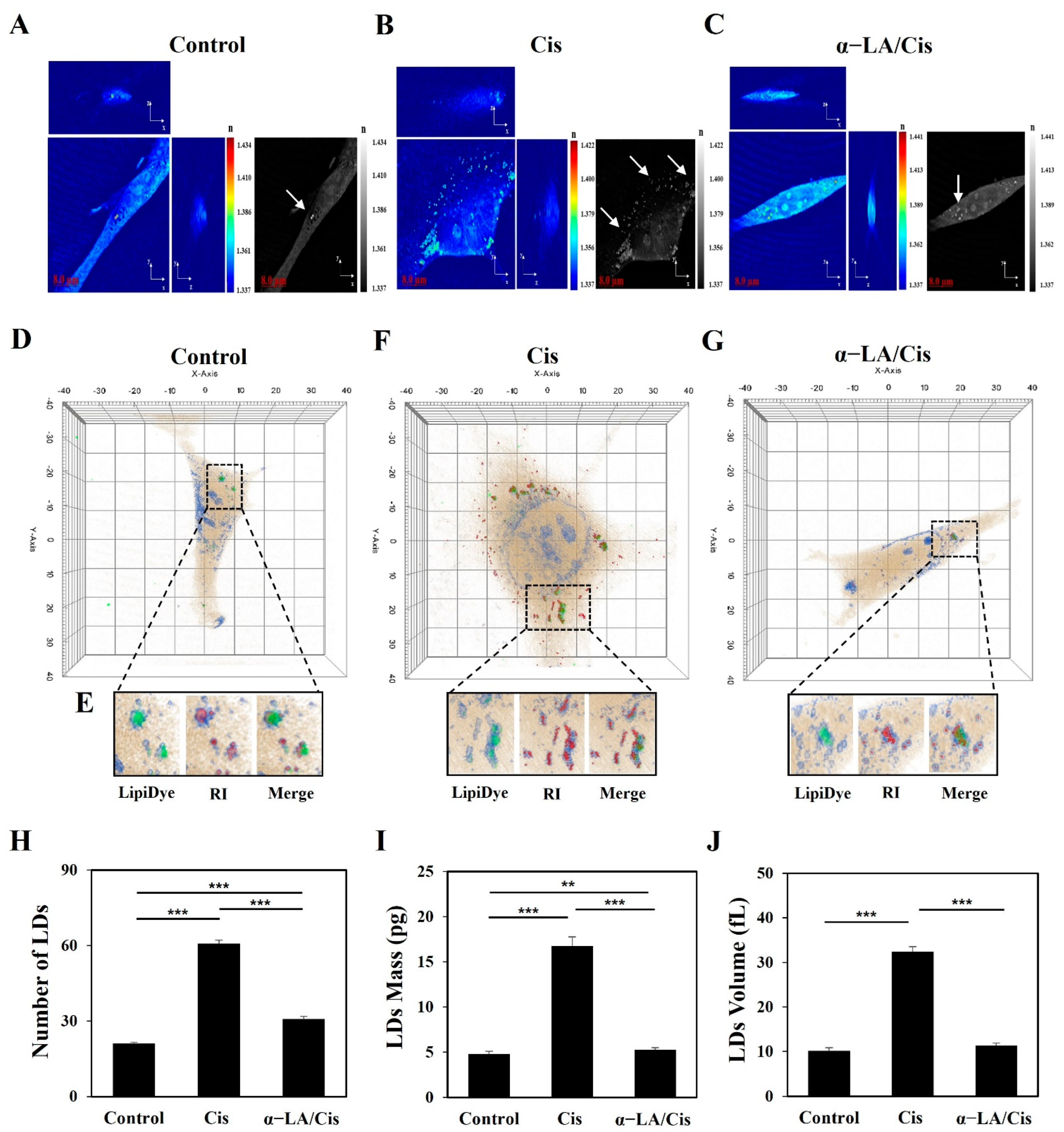
2.2. HEI-OC1 Cell Damage Related to Cisplatin-Induced Ototoxicity Causes Quantitative Changes in LDs
2.3. Cisplatin-Induced Ototoxicity Causes Apoptosis in HEI-OC1 Cells via PARP Cleavage through ROS Increases and Caspase-3 Activation
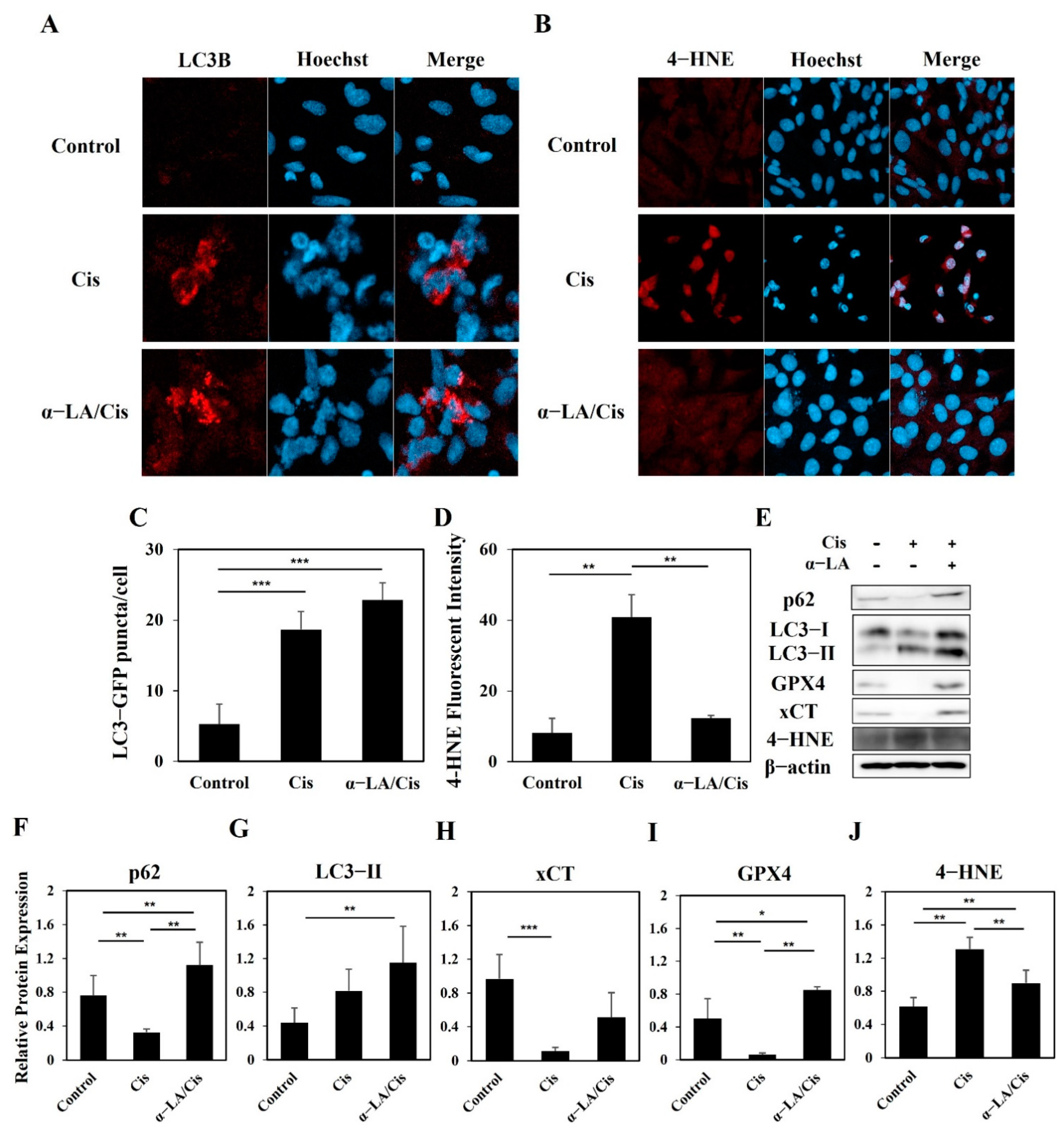
2.4. HEI-OC1 Cells Show Progression toward Ferroptosis Involving Various Cell Death Pathways after Cisplatin Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Counting Kit-8 (CCK-8) Assay of Cell Viability
4.4. Fluorescence Microscopy ROS Assay
4.5. Measurement of Caspase-3 Activity
4.6. Immunofluorescence Staining
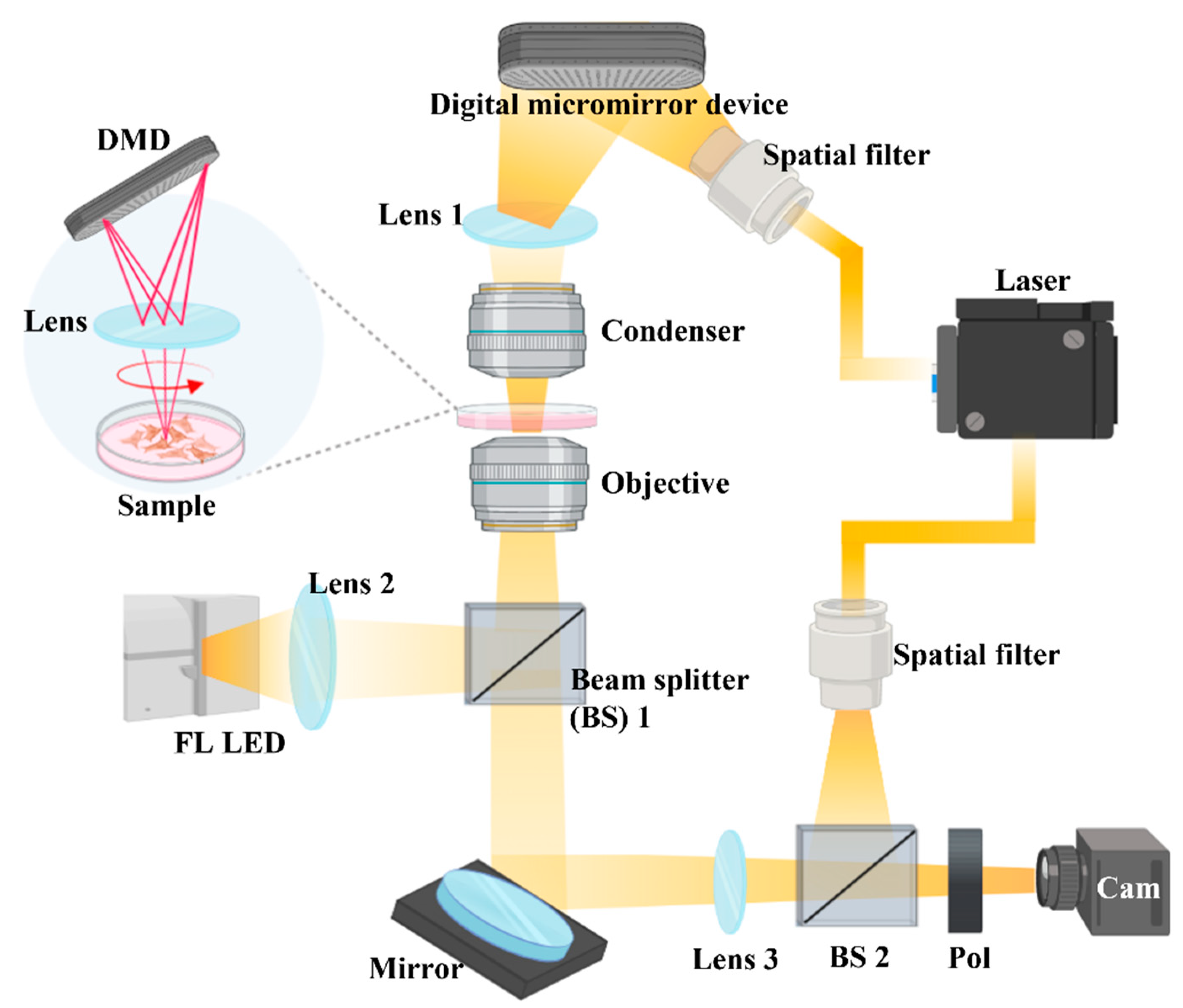
4.7. Holotomography
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.S.; Silveira, A.F.; Teixeira, A.R.; A Hyppolito, M. Mechanisms of cisplatin ototoxicity: Theoretical review. J. Laryngol. Otol. 2013, 127, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 1837–1850. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, A.G.; Stupak, H.; Liu, W.; Kim, A.; Staecker, H.; Lefebvre, P.P.; Malgrange, B.; Kopke, R.; Moonen, G.; et al. Oxidative stress-induced apoptosis of cochlear sensory cells: Otoprotective strategies. Int. J. Dev. Neurosci. 2000, 18, 259–270. [Google Scholar] [CrossRef]
- Warchol, M.E. Cellular mechanisms of aminoglycoside ototoxicity. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, W.; Ni, D.; Zhou, Z.; Gu, J.-H.; Zhang, W.; Sun, H.; Liu, F. Alpha lipoic acid antagonizes cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis-like cell death. J. Nanobiotechnology 2020, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.-J.; Sagong, B.; Kim, S.-J.; Lee, J.-T.; So, H.-S.; Lee, I.-K.; Kim, U.-K.; Lee, K.-Y.; Choo, Y.-S. Alpha-lipoic acid protects against cisplatin-induced ototoxicity via the regulation of MAPKs and proinflammatory cytokines. Biochem. Biophys. Res. Commun. 2014, 449, 183–189. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, B.; Kim, Y.-R.; Kim, M.-A.; Ryu, N.; Jung, D.J.; Kim, U.-K.; Baek, J.-I.; Lee, K.-Y. Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis. 2018, 9, 827. [Google Scholar] [CrossRef]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A novel anti-tumor action for cis-platin. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 445–460. [Google Scholar]
- Liu, Q.; Wang, K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of oste-osarcoma cells to cisplatin. Cell Biol. Int. 2019, 43, 1245–1256. [Google Scholar] [CrossRef]
- Roh, J.-L.; Kim, E.H.; Jang, H.J.; Park, J.Y.; Shin, D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016, 381, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.-L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Zhao, L.; Li, W.; Zheng, Z.; Tang, D.; Lu, X.; He, Y. Inhibition of ferroptosis protects House Ear Institute-Organ of Corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. J. Cell. Mol. Med. 2020, 24, 12065–12081. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; He, C.; Yan, F.; Li, J.-R.; Xu, H.-Z.; Zhuang, J.-F.; Zhou, H.; Peng, Y.-C.; Fu, X.-J. Selective ferroptosis in-hibitor liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neurosci. Bull. 2021, 37, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.-M.; Travain, V.B.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, L.; Han, L.; Jia, Y.; Zhao, Y.; Gao, H.; Kang, R.; Wang, X.; Tang, D.; Dai, E. Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 2019, 508, 997–1003. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, J.; Kang, R.; Yang, M.; Tang, D. Lipid Metabolism in Ferroptosis. Adv. Biol. 2021, 5, 2100396. [Google Scholar] [CrossRef]
- Urrutia, R.A.; Kalinec, F. Biology and pathobiology of lipid droplets and their potential role in the protection of the organ of Corti. Hear. Res. 2015, 330, 26–38. [Google Scholar] [CrossRef]
- Dong, B.; Song, W.; Lu, Y.; Sun, Y.; Lin, W. Revealing the Viscosity Changes in Lipid Droplets during Ferroptosis by the Real-Time and In Situ Near-Infrared Imaging. ACS Sensors 2020, 6, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.I.; Stockert, J.C.; Buzz, L.I.; Blázquez-Castro, A.; Simonetta, S.H. Reliable Screening of Dye Phototoxicity by Using a Caenorhabditis elegans Fast Bioassay. PLoS ONE 2015, 10, e0128898. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Zhou, R.; Mir, M.; Babacan, S.D.; Carney, P.S.; Goddard, L.L.; Popescu, G. White-light diffraction tomography of unlabelled live cells. Nat. Photonics 2014, 8, 256–263. [Google Scholar] [CrossRef]
- Choi, W.; Sung, Y.; Park, Y.; Fang-Yen, C.; Badizadegan, K.; Dasari, R.R.; Feld, M.S. Quantitative live cell imaging with tomographic phase microscopy. OSA Tech. Dig. 2009, NTuA1. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Yoon, J.; Heo, J.; Choi, C.; Park, Y. Three-dimensional label-free imaging and quantification of lipid droplets in live hepatocytes. Sci. Rep. 2016, 6, 36815. [Google Scholar] [CrossRef]
- Beller, M.; Thiel, K.; Thul, P.J.; Jäckle, H. Lipid droplets: A dynamic organelle moves into focus. FEBS Lett. 2010, 584, 2176–2182. [Google Scholar] [CrossRef]
- Tschapalda, K.; Zhang, Y.-Q.; Liu, L.; Golovnina, K.; Schlemper, T.; Eichmann, T.O.; Lal-Nag, M.; Sreenivasan, U.; McLenithan, J.; Ziegler, S.; et al. A Class of Diacylglycerol Acyltransferase 1 Inhibitors Identified by a Combination of Phenotypic High-throughput Screening, Genomics, and Genetics. eBioMedicine 2016, 8, 49–59. [Google Scholar] [CrossRef]
- Herms, A.; Bosch, M.; Ariotti, N.; Reddy, B.J.; Fajardo, A.; Fernández-Vidal, A.; Alvarez-Guaita, A.; Fernández-Rojo, M.A.; Rentero, C.; Tebar, F.; et al. Cell-to-Cell Heterogeneity in Lipid Droplets Suggests a Mechanism to Reduce Lipotoxicity. Curr. Biol. 2013, 23, 1489–1496. [Google Scholar] [CrossRef]
- Kang, C.; Elledge, S.J. How autophagy both activates and inhibits cellular senescence. Autophagy 2016, 12, 898–899. [Google Scholar] [CrossRef]
- Maejima, Y.; Isobe, M.; Sadoshima, J. Regulation of autophagy by Beclin 1 in the heart. J. Mol. Cell. Cardiol. 2016, 95, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kishi-Itakura, C.; Koyama-Honda, I.; Itakura, E.; Mizushima, N. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 2014, 127, 4089–4102. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, L.; Shu, Y.; Fang, Q.; Zhou, H.; Liu, Y.; Liu, D.; Lu, L.; Zhang, X.; Ding, X.; et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 2017, 13, 1884–1904. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Kang, R.; Tang, D. Autophagy and ferroptosis—What is the connection? Curr. Pathobiol. Rep. 2017, 5, 153–159. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Haberzettl, P.; Hill, B.G. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013, 1, 56–64. [Google Scholar] [CrossRef]
- Hill, B.G.; Haberzettl, P.; Ahmed, Y.; Srivastava, S.; Bhatnagar, A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem. J. 2008, 410, 525–534. [Google Scholar] [CrossRef]
- Krohne, T.U.; Stratmann, N.K.; Kopitz, J.; Holz, F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp. Eye Res. 2010, 90, 465–471. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Fan, L.; Yin, S.; Zhang, E.; Hu, H. Role of p62 in the regulation of cell death induction. Apoptosis 2018, 23, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Iwasaki, S.; Urata, S.; Morishita, H.; Sakamaki, Y.; Fujioka, M.; Kondo, K.; Mizushima, N.; Yamasoba, T. Autophagy is essential for hearing in mice. Cell Death Dis. 2017, 8, e2780. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.; Alferi, D.; De Nicola, S.; De Petrocellis, L.; Finizio, A.; Pierattini, G. Quantitative phase-contrast microscopy by a lateral shear approach to digital holographic image reconstruction. Opt. Lett. 2006, 31, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Kandel, M.E.; Rubessa, M.; Wheeler, M.B.; Popescu, G. Gradient light interference microscopy for 3D imaging of unlabeled specimens. Nat. Commun. 2017, 8, 210. [Google Scholar] [CrossRef]
- Merola, F.; Memmolo, P.; Miccio, L.; Savoia, R.; Mugnano, M.; Fontana, A.; D’Ippolito, G.; Sardo, A.D.S.; Iolascon, A.; Gambale, A.; et al. Tomographic flow cytometry by digital holography. Light. Sci. Appl. 2017, 6, e16241. [Google Scholar] [CrossRef]
- Kim, S.K.; Im, G.J.; An, Y.S.; Lee, S.H.; Jung, H.H.; Park, S.Y. The effects of the antioxidant α-tocopherol succinate on cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 9–14. [Google Scholar] [CrossRef]
- Beuthan, J.; Minet, O.; Helfmann, J.; Herrig, M.; Müller, G. The spatial variation of the refractive index in biological cells. Phys. Med. Biol. 1996, 41, 369. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Hong, S.J.; Kang, S.H.; Park, Y.; Kim, S.K. Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets. Int. J. Mol. Sci. 2022, 23, 10981. https://doi.org/10.3390/ijms231810981
Cho S, Hong SJ, Kang SH, Park Y, Kim SK. Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets. International Journal of Molecular Sciences. 2022; 23(18):10981. https://doi.org/10.3390/ijms231810981
Chicago/Turabian StyleCho, Sam, Seok Jin Hong, Sung Hun Kang, YongKeun Park, and Sung Kyun Kim. 2022. "Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets" International Journal of Molecular Sciences 23, no. 18: 10981. https://doi.org/10.3390/ijms231810981
APA StyleCho, S., Hong, S. J., Kang, S. H., Park, Y., & Kim, S. K. (2022). Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets. International Journal of Molecular Sciences, 23(18), 10981. https://doi.org/10.3390/ijms231810981




