Abstract
Esophageal cancer (EC), an aggressive and poorly understood disease, is one of the top causes of cancer-related fatalities. GLOBOCAN 2020 reports that there are 544,076 deaths and 604,100 new cases expected worldwide. Even though there are various advancements in treatment procedures, this cancer has been reported as one of the most difficult cancers to cure, and to increase patient survival; treatment targets still need to be established. Nuclear receptors (NRs) are a type of transcription factor, which has a key role in several biological processes such as reproduction, development, cellular differentiation, stress response, immunity, metabolism, lipids, and drugs, and are essential regulators of several diseases, including cancer. Numerous studies have demonstrated the importance of NRs in tumor immunology and proved the well-known roles of multiple NRs in modulating proliferation, differentiation, and apoptosis. There are surplus of studies conducted on NRs and their implications in EC, but only a few studies have demonstrated the diagnostic and prognostic potential of NRs. Therefore, there is still a paucity of the role of NRs and different ways to target them in EC cells to stop them from spreading malignancy. This review emphasizes the significance of NRs in EC by discussing their diverse agonists as well as antagonists and their response to tumor progression. Additionally, we emphasize NRs’ potential to serve as a novel therapeutic target and their capacity to treat and prevent EC.
1. Introduction
Esophageal cancer (EC) is an aggressive and poorly understood disease that remains one of the leading causes of cancer-related deaths around the world [1,2]. This cancer is fundamentally resistant to systemic therapy due to morphological, molecular, and etiological heterogeneity [3]. Even though there are various advancements in treatment procedures, this cancer has been reported as one of the most difficult cancer to cure, and a favorable prognosis is only possible in the pilot stages [4]. EC is one of the most common types of cancer in people; GLOBOCAN 2020 estimated 604,100 new cases and 544,076 fatal cases worldwide [5]. Individuals with EC have dismal 5-year overall survival (OS) rates [3]. The incident rate of EC varies a lot depending on their location [1,2,6,7]. Squamous cell carcinoma (SCC), adenocarcinoma (AC), and other subtypes of EC are histologically classified, and more than 95 percent of esophageal malignancies are squamous cell carcinoma and adenocarcinoma [1,2,6,7]. The histological subtype of EC with the highest incidence is esophageal squamous cell carcinoma (ESCC). The histology of human ESCC follows a stepwise pattern of dysplasia, hyperplasia, and SCC, and it originates from precancerous lesions [8].
Common risk factors for ESCC include smoking tobacco, excessive alcohol consumption, and chronic inflammation, which have been proven to have some synergistic impact on the development of EC [8,9]. Moreover, dietary variables, genetic factors, microbes, and other environmental factors may all have a role in the disease’s etiopathogenesis [8,9]. The geographical disparities show that genetic factors, ethnicity, and lifestyle play a significant influence in the development of ESCC, as demonstrated by the high incidence rates of ESCC in Southern Europe, Southern and Eastern Africa, and East Asia compared to North America and other regions of Europe [2]. Adenocarcinoma is the most predominant EC in Europe and North America [7]. Esophageal adenocarcinoma (EAC) and gastric cardiac adenocarcinoma (GCA) are two types of ACs that arise at the junction of the distal esophagus and the proximal stomach [10]. EAC is becoming a common source of mortality and morbidity, where patients who are suffering from this disease have a 17 percent survival rate, as this cancer is detected in later stages following the local invasion and/or metastasis [7]. Smoking, obesity, alcohol consumption, nutritional deficit, genetic factors, Helicobacter pylori infection, Barrett’s esophagus (BE), and chronic gastroesophageal reflux disease (GERD) are the main risk factors for EAC [1].
BE, a metaplastic alteration of the typical squamous mucosa of the esophagus into a columnar lining, is the solely recognized precursor for EAC [11]. Moreover, the presence of BE is linked to an elevated risk of EC by 30 to 40 folds [11]. Currently, only 5% of individuals with EAC have the precancer diagnosis of BE [2,12,13]. Although EC is asymptomatic in its initial stages; dysphagia, unintended weight loss, nausea, anorexia, abdominal pain, odynophagia, and bloating are the most common presenting symptoms at the early stage, which makes the diagnosis difficult [7,14,15]. Patients with EC have had a better general prognosis in recent decades because of surgical and medicinal advances, although overall survival remains dismal [16]. EC treatment is complicated and varies between nations and centers [17]. However, there is a need for a more effective biomarker for the treatment of EC because of its complications and aggressiveness.
The most utilized therapeutic modality before esophagectomy is radiochemotherapy [17,18]. However, the development of chemoresistance, significant adverse medication reactions, and high treatment costs present the main therapy hurdles for this disease at an advanced stage [19]. Therefore, the development of medications that are safe, effective, and economical continues to be a challenge in the field of EC research.
Many human diseases, such as metabolic disorders, autoimmune diseases, and cancer, are linked to dysregulated tissue metabolism and inflammation [20,21,22,23,24,25]. The transcriptional changes in metabolic and immunological cells in response to pathogenic stimuli received from the microenvironment are largely associated with transcription factor (TFs) abnormalities [20]. Nuclear receptors (NRs) are a type of TFs found in cell nuclei that bind with certain ligands, respond to hormones, and regulate several physiological processes in the cell [26,27]. They also act as a key regulator in reproduction, development, cellular differentiation, stress response, immunity and metabolism [28,29,30,31]. Moreover, these receptors have an impact on a wide range of genes and are involved in a complex network of signaling pathways [27,32,33]. NRs are essential for controlling several disease states, including diabetes, obesity, atherosclerosis, and cancer, in addition to normal homeostatic and metabolic processes [30]. Depending on the cell type, numerous coregulators and NR functions have been studied together in various contexts [34]. There are 48 TFs in the human NR family, including receptors for lipophilic vitamins, cholesterol metabolites such as retinoic acid and oxysterols, thyroid hormones, steroid hormones, and fatty acids, whose dysregulation frequently results in disease states [28,35,36,37]. Most NRs are notable therapeutic targets because of the ability of small compounds to specifically activate or inactivate them [28,36,38,39]. The classification of NRs’ superfamily was based on evolutionary sequence conservation observed among various receptors. These receptors are thought to have split into seven subfamilies thus far [27,28]. Adopted-Orphan-Receptors (Lipid-sensors and Enigmatic-Orphans), Endocrine-Receptors, and Orphan-Receptors are the three main groups of the 48 members of the superfamily of NRs [40]. The first category consists of receptors with a high affinity for their ligands, such as seco-steroidal receptors (VDR and RARs) and steroidal receptors (AR and ER). The FXRs, LXRs, and PPARs have modest binding affinities for a wider variety of lipophilic compounds and are included in the second category. NR4A1/NUR77 and ERRs are examples of the final group that have not yet been identified as ligands or do not have a ligand binding domain [40,41]. The structure is common by all members of the NR superfamily consists of a variable N-terminal region, a ligand-independent transactivation function (AF-1) domain, and a highly conserved DNA-binding domain (DBD), which binds to particular DNA sequences known as hormone response elements (HREs). These proteins’ C-terminal region contains the dimerization interface, ligand-dependent activation function (AF-2), and ligand binding domain (LBD) (Figure 1). The activation state of the NR is altered by ligand interaction, which either activates or inactivates its transcriptional output [42,43]. The majority of NR ligands are tiny, lipophilic molecules that may easily diffuse across the plasma membrane of cells and bind their associated receptors [28]. Most of the NRs’ activities can be regulated by endogenous and exogenous substances, such as metabolites, steroid hormones, and synthetic compounds [20].
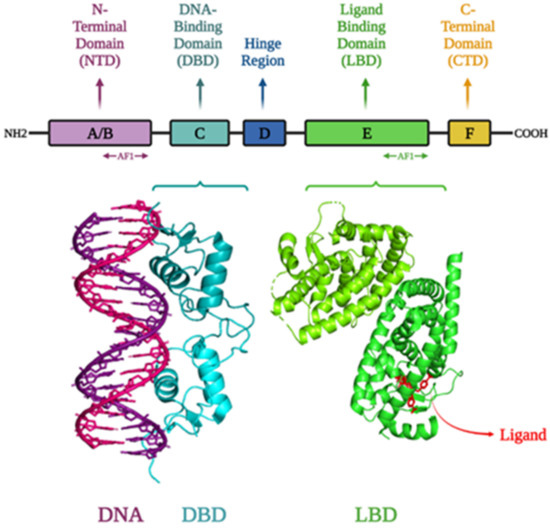
Figure 1.
Structure of different domains of NRs: N-terminal domain, DNA-binding domain, hinge, ligand/hormone binding domain, and C-terminal domain; Structure of the progesterone receptor-DNA complex at a resolution of 2.50 Å (PDB ID: 2C7A); Crystal structure of the complex between PPAR gamma ligand binding domain and the ligand AM-879 at a resolution of 2.69 Å (PDB ID: 6AN1). Visualization of the structures was performed using PyMOL and saved them as .jpg files [44,45,46,47,48].
Moreover, NRs have been intensively studied in cancer biology because they have shown tremendous promise as new therapeutic targets for various cancer types due to their high druggability and actionability qualities [26]. As a result, around 16 percent of FDA-approved medications now target NRs, emphasizing the relevance of NRs in human disease [49]. Moreover, NRs have become promising targets for anticancer drug development due to their impact on a variety of cancer-related processes (e.g., tumor initiation and therapeutic response) [49]. NRs are adaptable cellular ‘sensors’ because of their ability to respond fast and dynamically to numerous developmental and environmental signals by altering gene programs. As a result, NRs have long been used as biomarkers for the classification of a variety of solid tumors, including breast and prostate malignancies, as well as hormone therapy targets [50].
According to the growing number of data, NRs function as a modulator of signaling that connects the inflammatory response to the development and progression of cancer, and they control particular genes with tumor-suppressive or cancer-causing properties [34,51,52,53,54,55,56,57]. For example, the impact of steroid hormones on prostate cancer has been demonstrated by one of the earliest research projects on NRs. They demonstrated the activation of prostate cancer by androgen injections [58]. A surfeit number of investigations have demonstrated the significant role of NRs in tumor immunology and proved the roles of multiple NRs in modulating proliferation, differentiation, and apoptosis suggest that NRs and their ligands have direct antitumor effects on cancer cells and can be used as cancer immunotherapeutic targets [26,30]. It is well established that various NRs such as androgen receptors (ARs), estrogen receptors (ERs), farnesoid X receptors (FXRs), peroxisome proliferator-activated receptor γ (PPARγ), retinoic acid receptors (RARs), retinoid X receptors (RXRs), and vitamin D receptor (VDR), which shows association with cancer development [28,36,59,60]. Surprisingly, important roles of AR and ER in the etiological factors of breast cancer and prostate cancer have been discovered, respectively. AR expression in epithelial cells is thought to cause prostate cancer, and this cell type’s role in the development of the disease is significant. Moreover, prostate cancer is assumed to be caused by the excessive stimulation of the ER system because of increased ER levels [34]. Moreover, the oncogenic role of AR, ER, GR, PPAR and VDR in tumor-supporting cells is well characterized [31]. Further, estrogen and progesterone receptor expression remain therapeutically significant in predicting prognosis and deciding therapy options for breast cancer [36]. An in vitro study has demonstrated that NR4A1, which is an orphan receptor, is overexpressed in pancreatic cancer and regulates cancer cell survival and death [8]. According to a tissue-specific and FXR-null mice study, it has been shown that FXR has been linked to the development of gastrointestinal and liver malignancies and operates as a suppressor of hepatocellular cancer, primarily via maintaining BA homeostasis [61]. All other NRs, including RARs, RXRs, PPARs, GRs, and PRs, have also been extensively studied as cancer-therapeutic targets [62,63]. It was intriguing to discover that numerous NRs have been associated with EC and can be targeted as a new therapeutic target to prevent the disease’s progression. For instance, in a study, inhibition of FXR by FXR shRNA or guggulsterone decreased EC tumor development and growth in nude mice xenografts, as well as decreased tumor cell viability and incited apoptosis in vitro. Therefore, it is clear that EC can be effectively controlled by inhibiting FXR expression or activity and could be a therapeutic target [64].
There are a surplus number of studies conducted on NRs and their implications in EC, but there is still a lot to learn about how to target EC cells and stop them from spreading malignancy. The significance of NRs involved in EC using agonists and antagonists and their response to tumor growth is highlighted by this review. Synthetic medications have continued to exhibit severe side effects and the development of chemoresistance despite recent advancements in treatment approaches, restricting their applicability [65,66,67,68]. Because they have few adverse effects, phytochemicals are increasingly being used [69,70,71,72]. Furthermore, research over the previous four decades has illuminated the therapeutic and cancer-prevention potential of natural compounds as well as their underlying mechanisms of action [35,73,74,75,76,77,78,79]. Some natural substances have been discovered to be more effective in treating various types of cancer than the various modern chemotherapies [80,81,82,83,84,85,86,87,88]. Numerous studies have discovered the huge potential of a variety of non-toxic, multi-targeted natural compounds/agents in overcoming drug resistance in cancer cells and sensitizing them to chemotherapeutic medicines [89,90,91,92,93,94,95,96]. For example, a study on Daphne altaica Pall, a traditional Kazhak medicine, has demonstrated the anticancer effect of the medicine in EC through modulating PPARγ. The D. altaica extract (Da-Ea) has inhibited the cell proliferation of Eca-109 cells through upregulating PPARγ, which also induced apoptosis and S phase cell cycle arrest [97]. According to another study, inhibiting FXR with FXR shRNA or guggulsterone reduced tumor cell survival and metastasis and induced apoptosis in vitro, as well as decreased EC growth in nude mice xenografts [64]. Therefore, we have also concluded the role of natural products in modulating the expression of NRs in EC. Additionally, we emphasize NRs’ potential to serve as a novel therapeutic target and their capacity to treat and prevent EC.
2. Nuclear Receptor Signaling
NRs are a class of TFs, which are significant in both the development and progression of cancer [50]. The majority of NRs activate transcription as either homodimers or heterodimers with the RXR, even though a small fraction of NRs can bind and stimulate transcription as monomers [42]. When the ligand is bound, NRs undergo a conformational shift that allows the recruitment of coactivator proteins, which stimulate transcription, and co-ordinately dissociate the corepressor [42]. NR family members control transcription in several ways and can both activate and repress gene expression [98]. A subset of NRs that heterodimerize with RXR, including LXR, RAR, and TR, can actively silence target genes by binding to HREs in the absence of ligands [99,100]. Other NRs, such as LXR, PPAR, and GR, can inhibit the actions of other TFs, such as activator protein-1 (AP-1) and nuclear factor (NF)-κB, in a ligand-dependent manner [42].
NRs are divided into four categories based on their method of action: Type I-IV [101]. Type-I steroidal NRs, which include GR, PR, ER, and AR, are entrenched in the cytoplasmic membrane and connected to heat shock proteins (HSP90), and when the receptors bind to their ligand, the receptors release the chaperone, allowing homodimerization and are transported into the nucleus [101]. The type II category includes the non-steroidal NRs such as thyroid receptor, VDR, RAR, PPAR, and LXR, which are found in the nucleus and form an obligatory heterodimer with the RXR [102]. The heterodimeric complex is normally associated with corepressor proteins (e.g., NCoR and SMRT) in the absence of a ligand. However, when a ligand binds, corepressor proteins are released and coactivators are recruited, which modify chromatin structure and allow target genes to be activated [98,101,102] (Figure 2). The type III category contains orphan receptors which function as same as the Type I receptors but create a monomer that identifies an elusive DNA sequence, whereas Type IV, as monomers, attach to the half-site in HREs and function [38,101]. ER and AR signaling networks regulate reproduction, RAR and all-trans retinoic acid (ATRA) signaling pathways regulate mammalian embryonic development, VDR and metabolism regulate immune function and bone homeostasis, and GR and PPAR signaling pathways regulate inflammatory response [103,104,105,106,107,108,109,110]. Although ligands and coregulators are significant regulatory nodes in NR signaling pathways, different tissues and cell types have different ways of communicating the afferent physiologic signal through each channel [111].
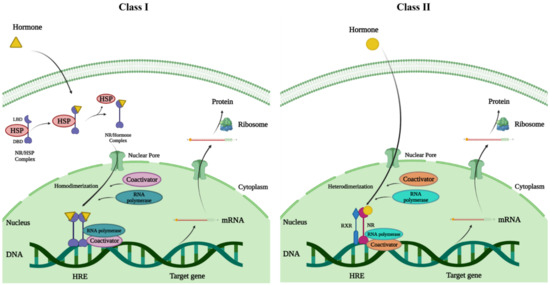
Figure 2.
The mode of action of NRs; NRs exert their transcriptional stimulation of target genes via two distinct mechanisms. Class I—In the class I type, the ligand is bound in the cytoplasm, which causes the chaperons that are bound to the receptors to dissociate, causing the receptor to move and dimerize. Class II—For the class II type, the receptor dimerizes with another receptor (heterodimerization) to bind to nuclear response elements; ligand binding then releases the co-repressor, activating the receptor’s transcriptional unit.
3. Nuclear Receptors in Esophageal Cancer
NRs are important not only in normal physiology but also in a variety of pathological disorders, the most prominent of which is cancer, where they regulate apoptosis, cellular growth, migration, and invasion [112]. NRs govern a range of biological activities that overlap with cancer cell characteristics; therefore, their functions in carcinogenesis and cancer progression have been extensively studied in recent decades [26,113]. Studies have shown that NRs expression and activation are highly expressed in cancer cells, and that leads to the survival of these cells. Therefore, inappropriate NR activation may contribute to the development and spread of cancer [114]. The significant role of numerous NRs such as ARs, ERs, FXRs, PPARs, RARs, RXRs, PXRs, and VDRs, has been identified in EC cells, and they likely contribute to the development and progression of this cancer by controlling several TFs and signaling pathways (Figure 3). In view of this, the main focus of this study is on relevant NRs associated with EC and the therapeutic value of utilizing small compounds such as agonists and antagonists. Table 1, Table 2 and Table 3 summarize the role of nuclear receptors in esophageal cancer in clinical, in vitro, and in vivo studies.
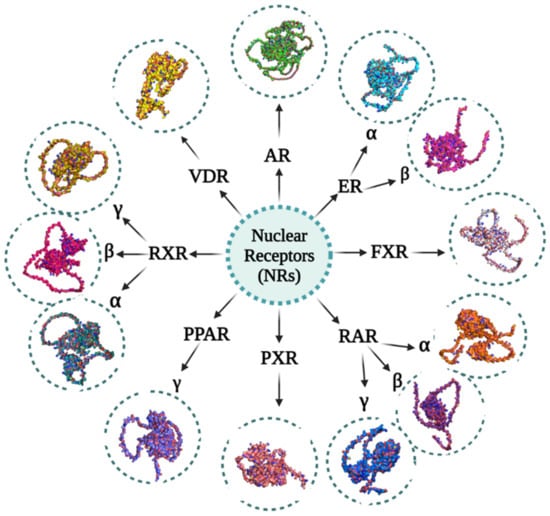
Figure 3.
Various NRs involved in esophageal cancer and their 3D structures: Androgen receptors (ARs) (UniProt ID: P10275), Estrogen receptor alpha (ERα) (UniProt ID: P03372), Estrogen receptor beta (ERβ) (UniProt ID: PQ92731), Farnesoid X receptors (FXRs) (UniProt ID: F1DAL1), Peroxisome proliferator-activated receptor gamma (PPARγ) (UniProt ID: P37231), Pregnane X receptors (PXRs) (UniProt ID: F1DAL3), Retinoic acid receptor alpha (RARα) (UniProt ID: P10276), Retinoic acid receptor beta (RARβ) (UniProt ID: P10826), Retinoic acid receptor gamma (RARγ) (UniProt ID: P13631), Retinoid X receptor alpha (RXRα) (UniProt ID: P19793), Retinoid X receptor beta (RXRβ) (UniProt ID: P28702), Retinoid X receptor gamma (RXRγ) (UniProt ID: P48443) and Vitamin D receptors (VDRs) (UniProt ID: P11473). These proteins’ primary structures were taken from the UniProt database. Using the AlphaFold protein structure database, the structures of these proteins were predicted. The image generation and visualization of the structures of these proteins were performed using PyMOL [47,48,115,116,117,118].

Table 1.
Nuclear receptor (NR) expression in esophageal cancer and various ESCC and EAC cell lines.

Table 2.
Mechanistic role of various nuclear receptors (NRs) in esophageal cancer in the presence of their agonists/antagonists.

Table 3.
Mechanistic role of various nuclear receptors (NRs) in esophageal cancer in clinical studies.
3.1. Androgen Receptors (ARs)
AR is a ligand-activated TFs in the steroid receptor family [180]. Growth factors, natural hormones, peptides, and synthetic compounds are all examples of ligands that can activate these receptors [180]. The AR is found in skeletal muscle, the prostate, the testes, the uterus, the breast, and other tissues [181]. The AR gene, which is 90 kb in size and situated on the X chromosome, is coded by eight exons [182]. Different domains in the AR include the N-terminal domain (NTD), DNA-binding domain (DBD), and ligand-binding domain (LBD). The least homologous section of the AR is its N-terminal region (amino acids 1–559), with less than 15–20% similarity among the class I members. AF-1, crucial for AR activity, is present in the NTD [183]. AR’s AF-1 contains all of AR’s phosphorylation sites except for three and is a target for several growth factors that phosphorylate the sites and activate the AR ligand on their own [184,185]. The DBD helps the AR to bind to the androgen Response Elements (ARE) in the regulatory regions of androgen-responsive genes. The DBD contains two zinc finger motifs necessary for DNA binding and dimerization and is highly conserved among receptors. The DBD and LBD’s lysine-rich hinge regions are essential for the nuclear localization of the receptor [186]. The AR’s LBD is responsible for ligand binding, is only minimally conserved among receptors, and contains AF-2, which is required for full receptor activation in the presence of ligand [180]. AF-2 refers to residues in the LBD that are implicated in transcription control. In a hormone-dependent way, this region of the AR recruits a set of coregulatory proteins known as p160 coactivators (e.g., steroid receptor coactivator-1 (SRC-1)) [187]. Interestingly, it was reported that AR is implicated in the development and progression of various cancer types, including prostate, breast, ovarian, etc. [188,189,190,191,192]. Therefore, AR’s expression and function are often investigated in cell lines and tumor specimens. However, the role of AR expression and function in the development and progression of EC is still poorly understood.
Increasing lines of evidence suggest that AR and AR responsive are highly overexpressed and activated in EC and controls the survival and prognosis of patients. For example, a study in 40 ESCC tumor tissues demonstrated high levels of AR expression in invasive ESCC tissues. In addition, this study also showed that the knockdown of the KYSE450 EC cell line with AR shRNA decreased the expression of AR, cell invasion, pAkt, and matrix metalloproteinase 2 (MMP2) [119]. Another clinical study showed high expression of AR in tissues from tobacco using ESCC patients compared with normal esophageal squamous tissues. Besides, higher expression of AR was also observed in the EC109, EC9706, HKESC-2, and TE-12 EC cell lines. Moreover, the inhibition of AR by shRNA reduced cell viability, cell growth, colony formation, anchorage-independent growth, and the S and G2/M phase. In addition, in mice with various androgen status, the overexpression of AR enhanced tumor growth. Further, AR promotes interleukin 6 (IL6), a common AR target gene in ESCC, transcription by binding directly to the IL6 promoter, and IL6 can then activate AR expression. Furthermore, prominent levels of AR and IL6 expression in human ESCC predict a worse clinical outcome in tobacco users [120]. Another clinical study demonstrated that AR gene expression was substantially higher in normal squamous epithelium than in esophageal adenocarcinomas [165]. According to another study, higher levels of dihydrotestosterone (DHT) inhibited the proliferation and cell division, induced cell cycle arrest and cell senescence and also altered androgen-responsive genes in OE33-AR, JH-AR, and OE19-AR EAC cell lines [148]. In addition, another study showed an increase of FK506-binding protein 5 (FKBP5), which is an androgen-responsive gene in AR-transduced OE33 cells (OE33-AR) [147]. Taken together, these findings demonstrated the significance of AR in the development and spread of EC, and additional research is required to identify the potential use AR as a therapeutic target in EAC and ESCC.
3.2. Estrogen Receptors (ERs)
Estrogen receptors (ERs) belong to the NR superfamily, which also comprises receptors that mediate the effects of thyroid hormones, steroid hormones, retinoids, and vitamin D [193]. ERs, similar to other steroid receptors, primarily serve as ligand-inducible TFs that bind chromatin at specific response regions as homodimers [193]. To interact with estrogen response elements (EREs) or other TFs, ERs dimerize and move to the nucleus, where they interact with them. This causes the recruitment of coregulatory proteins (coactivators or corepressors), an increase or decrease in mRNA levels and associated protein synthesis, as well as physiological responses [194,195,196]. The ligand-induced transcriptional activity of ER is mediated by two distinct activation functions, AF-1 and AF-2 [194,195,196]. ERs, similar to other members of the NR family, have structurally and functionally different domains. The DNA recognition and binding are carried out by the C or DNA-binding domain (DBD), which is the protein’s central and most conserved domain, while the COOH-terminal multifunctional D/E/F or ligand-binding domain (LBD) is responsible for ligand binding. The NH2-terminal or A/B domain is the least conserved and has the greatest variation in sequence and length [197,198]. Based on sequence homology with other receptors, the domains in the receptor have been split into six regions, A-F. Exon 1 codes for the N-terminal domain (regions A and B), exons 2 and 3 for the DNA-binding domain (region C), exon 4/hinge region (region D), and exons 5-8 for the hormone binding domain (regions E and F) [199]. ERs are divided into two subtypes: estrogen receptors α (ERα, also known as ER1 or ESR1) and estrogen receptors β (ERβ, also known as ER2 or ESR2), which are encoded by the estrogen receptor 1 (ESR1) and 2 (ESR2) genes, respectively. They are members who belong to the NR superfamily and carry out a range of biological processes [194,200,201].
In humans, ERα and ERβ play a critical role in the control of various intricate physiological processes. A multitude of disorders is linked to abnormal ER signaling, including cancer, metabolic and cardiovascular disease, neurodegeneration, inflammation, and osteoporosis [202,203,204]. For years, scientists have known that estrogen and its receptors play a critical role in cancer development [205]. Multiple investigations using esophageal tissues and various cell lines have demonstrated higher expression of both ERα and ERβ at variable levels, pointing to the significance of ER in the development of EC. For example, a recent study on EC has proved that apart from typical risk factors, the hormonal environment may play a crucial role in EC development [206]. Studies have demonstrated that positive ERα expression in combination with negative ERβ expression is an unfavorable independent prognostic predictor in ESCC [166,167]. In tumor tissues, the expression of ERβ is higher in AC and poorly differentiated SCC, and it increases with tumor stage and dedifferentiation. As a result, ERβ seems to be a sign of poor biological function, dedifferentiation, or a more advanced stage of disease [125]. Further studies on ESCC tissues showed that the levels of ERα and ERβ were inversely connected, and the downregulation of ERα and the overexpression of ERβ could indicate a poor prognosis [121]. Another study has demonstrated that the different isoforms of ERβ (ER-B1, ER-B2, ER-B3, and ER-B5) were shown to be overexpressed in EA tissues and suggests a possible role of antiestrogens in the treatment of EA [124]. Interestingly, it was noted that in EC cells, estrogen ligands such as 17β-estradiol and selective estrogen receptor modulators (SERM) inhibited cell proliferation. The amount of anti-growth effects caused by receptor agonists was proportional to the quantity of ER expression in the cell lines. Therefore, this research revealed that selective ER ligand treatment in EC and BE cells results in decreased cell growth and induced apoptosis [126,150]. In a distinct study, 1, 3, 5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), an ERα agonist, was shown to reduce the number of ECGI10 + ERα cells. Moreover, estradiol significantly increased the cell proliferation of ECGI10 + ERβ cells, and the addition of ICI 182780 dramatically reduced estradiol-mediated cell proliferation. In conclusion, this study’s findings unequivocally show that the presence of ERβ was strongly correlated with poor prognosis in ESCC, possibly by affecting the proliferation of carcinoma cells [123]. Another study showed that the ER system contributes to the spread of EC, and a highly selective ERα antagonist (MPP) and an ERβ-specific antagonist (PHTPP) elicited a concentration-dependent reduction in proliferation in EC cell lines. In addition, caspase 3/7 activity was significantly elevated in OE33 cell lines treated with MPP and PHTPP, and there was an increase in LDH activity in the presence of MPP-treated OE-33 cell lines [122]. However, a recent study showed that 17β-E2 inhibited the proliferation of human EC109 ESCC cells in a dose-dependent manner, which was inhibited by the ER antagonist ICI 182,780. Additionally, 17β-E2 significantly increased the release of intracellular Ca2+ and the entry of extracellular Ca2+ into ESCC cells, which was also inhibited by the ER antagonist IC1 82,780. When combined, this study shows that estrogen inhibits the proliferation of human ESCC cells, most likely via the ER-Ca2+ signaling pathway and it could a reason for the male predominance of ESCC [149]. In conclusion, it is evident that in a vast majority of cases of EC, ERs are markedly overexpressed and play a critical role in cell survival. Moreover, ES cancer cell invasion, migration, and proliferation have all been demonstrated to be inhibited by ER targeting, which also causes apoptosis. Additionally, the development of particular ER modulators would help in the prevention and treatment of ESCC patients.
3.3. Farnesoid X Receptors (FXRs)
The farnesoid X receptor (FXR) is a ligand-activated TF that belongs to the family of the NR, which is also classified as a nuclear bile acid (BA) receptor [207]. BAs operate as powerful endogenous ligands for FXR activation in the body [207]. FXR is a common receptor present in the intestine and liver that regulates bile acid, glucose, lipid metabolism, and energy balance to aid in maintaining systemic metabolic equilibrium [207,208]. FXR is encoded by the NR1H4 gene and controls the activities of several organs, including the brain, breast, cardiovascular system, gut, kidney, liver, and pancreas. As a result, FXR has become a popular therapeutic target for a wide range of disorders [207,208]. FXR detects physiologic and pathological metabolic changes and alters by regulating the transcription of genes related to cholesterol, fatty acid (FA), glucose, and amino acid balance. FXRα (NR1H4) and FXRβ (NR1H5) are two FXR genes that have been discovered, and the FXRα gene encodes four physiologically active versions (FXRα1, α2, α3, α4) as a result of several promoters and RNA splicing [209,210]. FXRα1/α2 and FXRα3/α4 are expressed at equal levels in the liver, whereas FXRα3/α4 isoforms are mostly expressed in the gut [211]. FXR binds to DNA (i.e., FXR response elements) as a monomer or as a heterodimer with the retinoid X receptor (RXR), another ligand-activated TF [210]. The N-terminal ligand-independent transcriptional activation AF-1 domain, DBD, a hinge region, and the C-terminal LBD comprising a transcriptional AF-2 comprises the structure of FXR, which is the same as the typical NR structure [212]. The hinge region sequence and the length of the AF-1 region differ between the four FXR isoforms. FXR agonists bind to the pocket produced by LBD, promoting its binding to FXR response regions in downstream target genes, which stimulates transcriptional activation [213].
According to recent research, FXR overexpression has been linked to the development and progression of breast, lung, pancreas, and esophageal malignancies. It has also been linked to tissue and cell-specific involvement in a variety of malignancies. It was also noted that FXR is strongly expressed in esophagitis, BE, and EAC [39]. For example, a study has demonstrated that FXR is overexpressed in BE, and guggulsterone, an FXR antagonist, significantly enhances apoptosis in a human BE-derived cell line which implies that FXR may play a role in apoptosis regulation [127]. According to another similar study, the suppression of FXR with FXR shRNA or guggulsterone reduced tumor cell survival and metastasis and induced apoptosis in vitro, as well as decreased EC growth in nude mice xenografts [64]. Another study demonstrated that, FXR was expressed in GERD tissues, and the level of expression has greatly increased in esophagitis [128]. In addition, the same study showed that FXR and basal TLR2 expression were linked, and TLR2 and FXR were significantly elevated during reflux esophagitis [128]. On the contrary, an in vitro and in vivo investigation has reported that the activation of FXR performs an antitumor role in the ESCC. FXR activation by its ligand GW4064 inhibited the ERK1/2 pathway and cell growth, increased apoptosis, and caused cell cycle arrest in ESCC cells. Further, the FXR ligand GW4064 reduced the growth of ESCC in a mouse xenograft model [151]. Altogether, it was identified that FXR could be a potential target for the management of ESCC.
3.4. Peroxisome Proliferator-Activated Receptors (PPARs)
Peroxisome proliferator-activated receptors (PPARs) are fatty acid-activated TFs, which belong to the nuclear hormone receptor superfamily, that control energy metabolism. PPARα (NR1C1), PPARγ (NR2C2), and PPARδ (NR3C3) (also known as PPARβ) are the three PPAR subtypes that have been discovered so far [214,215,216,217,218,219]. All PPARs, which have four functional domains termed A/B, C, D, and E/F, share the fundamental structural characteristics of the majority of NRs. The PPAR is phosphorylated by the ligand-independent AF-1 in the N-terminal (A/B) domain [220]. PPARs bind to the peroxisome proliferator response element (PPRE) in the promoter of PPAR target genes, and this interaction is mediated by the two-zinc fingered conserved core DBD, also referred to as the C domain. The cofactor docking site is the D domain, and the E domain is also known as the LBD. The E/F domain’s ligand-dependent AF-2 mediates the recruitment of PPAR cofactors involved in the transcription processes [220].
PPARα and PPARδ are also expressed in oxidative tissues and control gene expression involved in oxidative phosphorylation (OXPHOS), substrate delivery, and oxidation. PPARα stimulates energy dissipation and is found mostly in the brown adipose tissue (BAT), gut, heart, kidney, liver, and skeletal muscles [221,222]. PPARα influences esterification, fatty acid transport, and oxidation to mediate its actions. PPARβ/δ is widely expressed and plays a role in fatty acid oxidation as well as blood glucose control. White adipose tissue (WAT) has the highest levels of PPARγ expression, which is largely engaged in energy storage through promoting adipogenesis and lipid synthesis [220]. The PPARγ is expressed mainly in the gut, immune cells, liver, and skeletal muscles [221,223].
The binding of cognate lipid ligands, heterodimerization with another NR (RXR), the interaction of a few transcriptional coactivators, including PPAR coactivator-1 (PGC-1), as well as binding of the complex to PPAR response elements (PPREs) in the promoter of target genes are necessary for PPARs to function as NRs for transcription [223]. PPARs are triggered by several ligands. Eicosanoids and long-chain fatty acids (FAs) are examples of some common endogenous ligands for PPARα and PPARβ/δ, PPARγ on the other hand, is activated by arachidonic acid metabolites [224,225]. Pioglitazone, GW1929, and GW2090 are anti-diabetic thiazolidinedione (TZD) substances that specifically activate PPARγ, whereas GW501516 is a highly selective PPARβ/δ ligand [216,226].
The activation of PPAR by ligands has been linked with several malignancies. In vitro investigations on human cancer cells indicated growth-inhibitory effects such as cell-cycle arrest, differentiation, and death induced by PPAR ligands [227]. For example, a study has demonstrated the expression of PPARγ in T. Tn, and EC-GI-10 ESCC cell lines and revealed the marked growth inhibitory ability of PPARγ-ligands (Troglitazone, Pioglitazone, and 15d-PGJ2) to prevent the growth of human ESCC. Moreover, this effect was evident by the dose-dependent inhibition of deoxyribonucleic acid synthesis and G1 arrest and an increased level of cyclin-dependent kinase inhibitor p27 (Kip1), p21 (Cip1/Waf1), and p18(Ink4c). In addition, troglitazone treatment increased the expression of interleukin-1 alpha in EC-G1-10 cells [152]. Similarly, another study showed that troglitazone, a PPARγ-ligand, treatment in TE-13 cells inhibited the development of human ESCC through G1 cell cycle arrest by increasing p27 expression and induced apoptosis by increasing the expression of Bid, Bax, PARP, and caspase 3 and reducing the expression of cyclin E, MDM2, p16, cytochrome C, caspase 8, and Bcl-XL [153]. Interestingly, another study using 55 primary ESCC tissue samples has shown that the expression level of PPARγ mRNA was decreased in ESCC compared with normal esophageal mucosa, and this was correlated with poor prognosis [129]. Moreover, PPARγ and SIRT1 were substantially expressed in ESCC tissues, but high PPARγ expression was correlated with tumor grading but not with poor prognosis [168]. In this study, it was observed that increased tumor growth and poor prognosis were associated with the high expression of SIRT1, a protein that supports cell survival and angiogenesis in ESCC patients. However, SIRT1 expression was positively linked with EGFR but not with PPARγ or survivin [168]. In another study, it was observed that miR-10b was elevated while the expression of PPARγ was downregulated in EC tissues and ESCC cell lines EC109 and TE10, which established that PPARγ is a legitimate miR-10b target. Additionally, miR-10b suppression improved the chemosensitivity of EC cells to DDP in vitro and in vivo, and the overexpression of miR-10b decreased the PPARγ-mediated DDP sensitivity. The Akt/mTOR/p70S6K signaling pathway was also activated as a result of the overexpression of miR10b, and the deactivation of Akt/mTOR/p70S6K by Akt inhibitor (GSK690693) reduced miR-10b-induced DDP resistance in EC cells. Together, these findings show that PPARγ inhibition by miR-10b increased DDP resistance in EC by increasing Akt/mTOR/P70S6K signaling. Moreover, it was observed that after DDP treatment, the activation of PPARγ significantly aided DDP-induced apoptosis in EC109 and TE10 cells. In addition, elevated PPARγ consistently resulted in a rise in Bax levels and a decrease in Bcl2 levels after DDP treatment [133]. Interestingly, lycopene, a natural compound, was shown to suppress NF-κB and COX-2 expression and enhance the protein expression of PPARγ and cleaved caspase 3, which leads to an increase in apoptotic proteins and a decrease in inflammatory cytokines. These findings showed that an effective amount of lycopene could prevent the development of EC in NMBzA-injected F344 rats through potential anti-inflammatory and pro-apoptotic pathways [155]. Another study showed that Da Ea (ethyl acetate extract of D. altaica), which has anti-cancer effects, increased PPARγ expression levels, induced apoptosis and S phase cell cycle arrest, which prevented the proliferation of ECA 109 cells [97]. In addition, an in vitro and in vivo study in EC cells and ESO26 cells injected mice treated with T0070907 has demonstrated the transcriptional feedback loop between the PPARγ and the master regulator transcription factors (MRTF) that are particular to EC and fatty acid production. PPARγ overexpression was caused by MRTFs functioning together to promote PPARγ transcription by directly controlling its promoter and a distal EAC-specific enhancer. Moreover, this study also shows a decrease in cell proliferation and induced apoptosis in T0070907 treated OE33 AND ESO26 cell lines. In addition, in vivo study has demonstrated a decrease in the expression of FASN, ACC, ACLY, SCD and tumor growth in ESO26 cells injected mice [156]. Another study showed increased expressions of PPARγ, COX-2, HGF, gastrin, and NF-κB activity in BE tissues. Moreover, the increased NF-κB activity is probably linked to increased IL-8 and COX-2 expression [130]. Similarly, in EC tissues, upregulation of PPARγ was observed, and the treatment of EC cell lines with PPARγ antagonists (T0070907 and GW9662) decreased EC cell adhesion, expression of p-focal adhesion kinase (p-FAK) and pERK and induced apoptosis [131]. Another study has reported the reduced expression of PPARγ in esophageal tumor lesions and proved that ESCC cell proliferation could be inhibited by efatutazone, a PPARγ agonist, by inactivating the PI3K–Akt and MAPK pathways [154]. Interestingly, an in vitro and in vivo study demonstrated that the activation of PPARγ inhibits cancer cell growth in vitro by inducing apoptosis through increasing caspase 3 activity, but systemic PPARγ activation increased the growth of OE33-derived transplantable adenocarcinomas in vivo due to increased cell proliferation [132]. Collectively, these data suggest that PPARs play a critical role in the emergence of EC and might serve as a novel therapeutic target.
3.5. Retinoic Acid Receptors (RARs)
RARs are TFs that belong to the NR superfamily which can have non-genomic effects by triggering kinase signaling pathways that regulate the transcription of RA target genes [228,229]. RARs have a significant role in a variety of physiological processes, including embryonic development and organ homeostasis. RARs also help to regulate gene networks that control cell growth, differentiation, survival, and cell death at the cellular level [228,229]. RARs are divided into three different subtypes: RARα, RARβ, and RARγ and each subtype has different isoforms. RARβ is divided into four isoforms (β1, β2, β3, and β4), each with differing affinities for retinoids and biological roles [230]. The first nuclear RAR in humans, RARα (NR1B1), has a high affinity for ATRA and has preserved the NR modular organization structure. RARβ (NR1B2) and the RARγ (NR1B3) are the second and the third RAR gene respectively [228].
RAR’s modular structure, which includes many domains and functions, allows them to process both ligand binding and transcription [231]. The transactivation domain, AF-1, is found in the amino terminus (A/B region) and forms a recognition surface for co-activators and other TFs [231]. For DNA recognition, the DBD holds two zinc finger motifs, and the LBD of the family members are highly conserved. It has a ligand-induced activation factor called AF-2, which is important in transcriptional coregulator interactions [231]. RARs can bind to specific enhancer regions in DNA, known as retinoic acid response elements (RAREs) in target gene promoters, after dimerization with RXR, resulting in transcriptional activation of target genes in the presence of ligand [228,232].
Retinoids can induce cell differentiation and inhibit proliferation, which is one of the reasons why they are used to treat cancer [233]. Surfeit numbers of clinical evidence have demonstrated that RARβ2 expression is usually inversely linked with tumor grade and frequently lost or epigenetically silenced in human malignancies [230,234]. According to a clinical investigation, the state of squamous differentiation and the increase in RARβ-expression are early events connected to EC [169]. Several clinical, in vitro, and in vivo studies have reported the leading role of RARs in the development and growth of EC cells. For example, it was found that expression levels of RARα and RARβ increased significantly in the higher stages of Barrett’s adenocarcinoma while expression of RARγ was significantly reduced. Therefore, RARγ may have a tumor suppressor role in Barrett’s carcinogenesis [135]. In EC cases, RARβ2 mRNA expressions were markedly decreased, whereas RARβ4 mRNA expression was elevated. Additionally, when compared to normal tissues, tumors had higher expressions of cyclin D1 and EGFR, while lower expressions of RARβ1, COUP-TFI (COUP transcription factor 1), and COUP-TFII were observed. Therefore, in tumor samples, decreased RARβ2 expression was linked with increased RARβ4 expression and the inhibition of COUP-TFI and COUP-TFII [141]. Another study has proven that RARα was overexpressed in human EC tissues, and further, it was demonstrated that RARα knockdown by siRNA inhibited EC cell proliferation by downregulating proliferating cell nuclear antigen (PCNA), Ki67, MMP7, and MMP9 expression and increased the drug sensitivity to 5-fluorouracil and cisplatin [136]. Benzo-[a]pyrene diol epoxide (BPDE) is found to be an active metabolite of tobacco procarcinogens, and a study has proven that by suppressing RARβ2 transcription, BPDE reduced RARβ2 mRNA and protein levels. Moreover, retinoic acid was able to partially block BPDE’s inhibitory effect on RARβ2 expression while increasing the cell cycle G1 phase. Additionally, BPDE-induced COX-2 expression was linked to RARβ2 inhibition. The expression of EGFR, ERK1/2 phosphorylation, c-Jun, and COX-2 were decreased after the RARβ2-expression vector was transfected into EC cells. Additionally, there was little change in the expression of c-Jun and COX-2 after co-treatment of RARβ2 positive cells with BPDE. These studies have proved that BPDE may cause EC via inhibiting RARβ2 [158,161,163]. Another study showed that RARβ2′s tumor suppressor function may be linked to its ability to decrease COX-2 expression, which plays a role in carcinogenesis and metastasis, and 13 cis-RA mediated activation of RARβ2 suppressed COX-2 expression, implying that COX-2 inhibition is dependent on RARβ2 expression. BPDE significantly caused time-dependent methylation of the RARβ2 gene promoter in esophageal cancer cells, as well as suppression of EGFR, ERK1/2 phosphorylation, c-Jun, and COX-2 expression. RARβ2 expression is decreased by BPDE, and the restoration of RARβ2 expression lowers COX-2 protein in esophageal cancer cells, implying that RARβ2 plays a significant role in preventing esophageal carcinogenesis [159,162]. It was also observed that RARβ expression was gradually lost, starting with the mildly dysplastic stage of esophageal mucosae. Additionally, the expression of RARβ was reduced as a result of the differentiation of esophageal squamous. Further, P53 and Ki67 were accumulated in the later precancerous stage of EC. This study suggests that the expression of RARβ, P53, and Ki67 could be used as biomarkers for early EC diagnosis in high-risk populations [137,170,172]. In ESCC, DNA methylation frequently results in the inactivation of the genes RARβ, RARβ2, CRBP1, and TIG1, which are linked to retinoic acid signaling, and in contrast, another study revealed that RARβ2, p16, MGMT, CLDN3, CRBP, and MT1G were increased in ESCC tissues [171,173]. In mice tumors, 4- nitroquinoline 1-oxide (4-NQO), a carcinogen, inhibited RARβ2 but increased the expression of p-ERK1/2, c-FOS, and COX-2 proteins, as well as the methylation of the RARβ2 gene promoter. Moreover, it was shown that RARβ2 expression was decreased and p-ERK1/2, and COX-2 expression were increased by treatment with 4-NQO in human EC cells in vitro. Moreover, upregulated p-ERK1/2 and COX-2 expression were found in EC tissues, and p-ERK1/2 expressions were linked to a more advanced clinical tumor stage [164]. In addition, it was observed that overexpression of RARβ2 induced retinoid receptor-induced gene 1 (RRIG1) and inhibited Erk1/2 phosphorylation and COX-2 expression [142]. Another study showed that the knockdown of DNA (cytosine-5)-methyltransferase1 (DNMT1) in KYSE30 and TE-1 EC cells led to promoter demethylation and RARβ overexpression. This study showed that smoking status and low RARβ expression were associated with DNMT1 overexpression in esophageal SCC patients. Through the activation of DNMT1 in esophageal squamous epithelial cells, NNK, a tobacco-specific carcinogen, might cause RARβ promoter hypermethylation, which ultimately increased cell proliferation and inhibited apoptosis [138]. Another study showed that N-(4-hydroxyphenyl) retinamide (4HPR) but not RA suppressed the proliferation of the ESCC cell line EC109 in vitro. In addition, RARβ2 induction is correlated with growth inhibition in RA-responsive cells, whereas a failure in RARβ2 inducibility is correlated with RA resistance. These results suggest that 4HPR may operate as a growth inhibitor through direct or indirect interactions with RARβ2 [160]. Several clinical studies investigated the methylation status and the expression of the RARβ2 promoter area and revealed a significant relationship between RARβ2 methylation status and tumor grade. Further, only G2 stage (intermediate grade) tumors showed a link between methylation status and lower expression of RARβ2, and its restoration was accompanied by growth inhibition after 5-aza-dc treatment [139,140,174]. Therefore, RARs (α, β, γ) can be targeted and used as markers for the prevention and treatment of both EACs and ESCCs.
3.6. Retinoid X Receptors (RXRs)
Retinoid X receptors (RXRs) are heterodimeric partners of other members of the NR superfamily [235]. There are three types of RXRs: RXRα, RXRβ, and RXRγ, all of which are nuclear transcriptional transactivator proteins that bind to DNA and are ligand-dependent [175]. The “permissive” subclass of heterodimers, such as PPAR, LXR, and FXR, is transcriptionally activated by RXR ligands (“rexinoids”) either independently or in conjunction with partner ligands in the “non-permissive” subclass, such as RAR, VDR, and TR [235]. The morphogenesis, development, growth, and differentiation of cells are all regulated by RXR, and its expression is found to be altered in several solid tumors [175]. RXR modulators have therapeutic potential for cancer and other disorders involving the acquisition and disposal of nutrients, such as metabolic diseases [236].
A surfeit number of studies have proven that RXR is essential for the development of EC. For example, in a study, it was demonstrated that the mRNA expression of the three different subtypes of RXR is significantly different in EC tissues and RXR mRNA expression levels may be useful biomarkers for BE and related adenocarcinoma since changes in the mRNA expression of all three RXR subtypes (RXRα, RXRβ, and RXRγ) are frequently observed in the development and progression of these diseases [175]. According to another study, EC tissues had higher levels of RXR mRNA and protein than normal esophageal tissues. The level of RXR overexpression was linked to tumor differentiation, TNM stage, and lymph node metastasis in EC patients. Further, EC patients with high RXR expression had considerably worse disease-free survival (DFS) and overall survival rates (OS). Moreover, multivariate analysis showed that the expression of RXR may be a predictor of DFS and OS in EC patients [176]. Another study showed that all six retinoid receptor subtypes, including RXR, were active in the tissues of EC patients. RXRβ was inversely correlated with patient lymph node metastatic status and was linked with a better clinical outcome across these receptor subtypes. According to these findings, retinoid receptors, particularly, RXRs play significant roles in ESCC and are associated with patient prognosis [172]. Furthermore, another study has revealed that both mRNA and protein of PPARγ and RXRα were expressed in ESCC cell lines from the KYSE series. Moreover, EC cell growth was decreased by the PPARγ ligand troglitazone (TRO), and RXRα ligand 9-cis retinoic acid (9CRA) administration had a synergistic impact. The combined treatment with TRO and 9CRA, which also markedly elevated the sub-G1 phase, showed that ligand administration was predominantly responsible for inducing apoptotic cell death in EC cells. Additionally, TRO + 9CRA treatment significantly inhibited the growth of tumors implanted in nude mice [157].
3.7. Vitamin D Receptor (VDR)
The vitamin D receptor (VDR) belongs to the NR superfamily and is involved in vitamin D’s biological activities [237]. The VDR ligand regulates the expression of many genes involved in calcium/phosphate balance, cellular proliferation and differentiation, and immunological response [237]. VDR is abundantly expressed in cardiomyocytes, vascular endothelial cells, and vascular smooth muscle cells [238]. One of three retinoid X receptors (RXRα, RXRβ, and RXRγ) forms dimers with VDR. The VDR homodimer or VDR-RXR heterodimer attaches to vitamin D response elements (VDREs), which are enhancer elements [239]. In combination with the RXR, ligand binding induces VDR nuclear localization and promotes VDR–DNA complexation. Particular VDREs have been discovered in the promoter sequences of genes that are activated or repressed by VDR. Interactions with coregulators are required for VDR-mediated gene regulation (coactivator and corepressor) [240]. The natural ligand of the VDR is 1,25-dihydroxy vitamin D (1,25(OH)2D3), a hormonal metabolite of vitamin D. VDR enters the nucleus after binding to 1,25(OH)2D3 and forms a heterodimer with retinoid X receptor (RXR), which regulates gene transcription by interacting with response elements in target gene promoters [241].
An N-terminal domain, a conserved DNA-binding domain, a flexible hinge region, and a conserved ligand-binding domain make up VDR’s structure [241,242]. The LBD has 12 helices and takes the form of a small, 3D structure when bound to a ligand. Deep inside the receptor, the ligand-binding pocket enables highly selective interactions with natural ligands such as 1,25(OH)2D3 [240].
In a study, it was demonstrated that through a bile acid ligand, VDR plays a role in the early development of EC. Interestingly, it has been shown that in both EAC and columnar cell metaplasia (CCM), VDR expression was considerably higher in male patients than in females. Moreover, VDR amplification was linked to a worse prognosis but not VDR protein expression [144]. However, another study has shown that both JNK1 and VDR were decreased in ESCC epithelial cells in comparison to the normal esophagus. JNK1 and VDR stromal expression also reduced the motility, migration, and proliferation of ESCC cells by blocking signaling pathways involved in proliferation and metastasis. Therefore, stromal JNK1 and VDR function as tumor suppressors in ESCC, and the degree of their stromal expression may affect the prognosis of ESCC [146]. Moreover, it was observed that EAC exhibits VDR expression, and as the tumor dedifferentiates, the expression level of VDR also decreases [143,177]. In contrast, another clinical study has demonstrated that the mRNA expression of VDR was higher in BE tissues compared to the normal squamous epithelium tissues [145]. In addition, it was shown that variable polymorphisms in genes involved in vitamin D metabolism are connected to the probability of reflux-BE-EAC development. In addition, low expression of VDR and CYP27B1 and high expression of CYP24A1 were observed in EAC tumor tissues compared to normal esophageal tissues [179]. Another study showed that claudin-2 was found to be strongly expressed in EAC and ESCC tissues, and its expression was linked to the expression of the bile acid receptors VDR and TGR5 [178]. These studies showed that dysregulation of VDR plays a critical role in the development of EC.
3.8. Other Nuclear Receptors
Several other NRs have also been thoroughly investigated and examined for their crucial function in esophageal carcinogenesis. One such receptor is the pregnane X receptor (PXR, NR1I2), also known as PAR (the receptor activated by pregnane) and SXR (steroid and xenobiotic receptor), which is the NR super family’s archetypal member [243]. Both endobiotics and xenobiotics can activate PXR. PXR’s biological function as a major xenobiotic receptor is primarily mediated by its ligand-dependent binding to regulatory gene sequences [244]. The 50 kDa PXR protein is composed of the DBD, the relatively short hinge region, and LBD with AF-1 and AF-2 regions [245]. PXR signaling has also been linked to cancer-related processes such as cell survival, proliferation, angiogenesis, and oxidative stress [246]. It was noted that PXR is associated with the development of EC. For example, a study has reported that PXR is highly overexpressed in BE and EAC patients and revealed their nuclear localization in adenocarcinoma tissues. Furthermore, PXR translocates to the nuclei of adenocarcinoma cells after being stimulated with lithocholic acid. This result, together with the discovery of a link between a PXR polymorphism and BE, suggests that PXR may have a role in esophageal illness prognosis and treatment [134]. Hence, insights into NRs and how they interact with TFs can lead to the discovery of novel drug targets that can be used to treat esophageal carcinogenesis.
4. NRs as Biomarkers in Esophageal Cancer
Biomarkers are any objective testing and evaluation characteristics that serve as indications for normal biological processes, case processes, or pharmaceutical responses [247]. Biomarkers are widely used for human illness investigation and play an essential role in early diagnosis, disease prevention, and the discovery of targeted drugs and drug reactions [247]. Patients with poorly differentiated esophageal carcinoma often have a bad prognosis, and various agonists and antagonists of NRs have been widely used for the treatment of EC. Agonists and antagonists with the structure-based design that can either induce or impede NR activity will give practical treatment methods for this disease [248]. The role of NRs as a potential biomarker has been suggested by the research undertaken for the early detection, prevention, and treatment of esophageal carcinogenesis. Moreover, some studies noted that AR is implicated in tumor growth, so it could be a good target for molecularly targeted ESCC therapies [119]. For example, a study on 77 EAC patients reported that 94.7% (75/77) of them were seen with high AR expression, and it also implies that AR influences overall survival. Therefore, this study suggests new treatment options for EAC, such as drugs that target AR signaling or androgen-responsive genes [147]. In tumor tissues, the ERβ is expressed more in AC and in poorly differentiated SCC. ER seems to be a marker of poor biological behaviour, such as dedifferentiation or an advanced stage of disease ER expression rises with tumor stage and dedifferentiation in both AC and SCC [125]. In EC tissues, the low expression of PPARγ was observed compared with normal esophageal epithelium; therefore, PPARγ mRNA expression level can act as a prognostic marker in post-operative EC patients [129]. According to another study, Barrett’s tissues have significantly different levels of RAR mRNA expression than normal esophageal tissues, while Barrett’s dysplasia and adenocarcinoma tissues have dramatically different RAR mRNA levels. Therefore, these findings suggest that RAR mRNA levels may be useful biomarkers for this disease [135]. Even though NRs have the potential to serve as biomarkers and have been the subject of numerous studies, further clinical research is required to demonstrate their ability to serve as diagnostic and prognostic biomarkers for the treatment of EC.
5. Epigenetic Alterations in NRs in Esophageal Cancer
The majority of epigenetic regulation of gene expression is dependent on DNA methylation and histone modifications because there are no inherent changes in the DNA sequence [249,250,251]. In human tumors, abnormal epigenetic alterations are more common than gene mutations, and in the preliminary stages of cancer, epigenetic dysregulation is a common event [252,253,254]. Therefore, the disruption of the “epigenetic machinery” is significant in the genesis of cancer [249,255]. It is well-established that epigenetic changes play a crucial role in the development and progression of EC [256]. Further, epigenetic changes, especially in the form of DNA hypermethylation of tumor suppressor genes, have been seen in ESCC and EAC, as well as the EAC precursor lesion BE. A subset of these abnormal methylation of tumor suppressor genes is thought to be involved in the etiology of esophageal malignancies [257,258]. In a study, 125 ESCC tissues were analyzed and found that 98/125 patients (78.4%) had RARβ2 hypermethylation and concluded that hypermethylation of the tumor suppressor gene RARβ2 has been linked to the onset and severity of ESCC [174]. Another similar study reported that RARβ2 gene methylation is frequent in the esophageal mucosa of ESCC patients, and it tends to grow in prevalence in mucosal foci as the disease’s histological severity worsens [173]. In another investigation of 28 ESCC tissues, diminished RARβ expression was found in 42.9% (12/28) of the cases, with half of these instances showing RARβ DNA methylation. Although RARβ DNA methylation was found in non-neoplastic samples (10.0%), the incidence of methylation in ESCC (25.0%) was higher, suggesting that methylation of the RARβ gene may play a pivotal role in esophageal carcinogenesis [171].
6. Conclusions
ECs are one of the most aggressive and poorly understood deadliest diseases worldwide and better treatment options are urgently needed. Despite the recent advances in oncological and surgical treatment, new methods for predicting disease outbreaks and curing EC must be investigated to enhance results. For developing a better treatment system, understanding the vital roles played by the proteins and genes that are being altered due to epigenetics and mutations is necessary. NRs play a key role in the initiation, development, and progression of various cancers. Some of the NRs involved in EC development are ARs, ERs, FXRs, PPARs, RARs, RXRs, and VDRs, and their subtypes. A surfeit number of studies have reported the role of these receptors in esophageal carcinogenesis, and are expressed differently depending on the receptor’s function. Interestingly, a few studies have shown that NRs exhibit both tumor-promoting and tumor-inhibiting properties. In most cases, the majority of the NRs (ARs, ERs, PPARs, and RARs) are overexpressed in the ESCC and EAC tissues and cell lines. Moreover, NRs are also involved in the development, growth, and progression of ESCC and EA. This implies that NRs can be a suitable target for the diagnosis and prognosis of EC. Further, NRs also has the potential to be a biomarker for the early detection, prevention, and treatment of esophageal carcinogenesis. Multiple studies have reported the importance of agonists and antagonists that play a vital role in the function and expression of NRs in EC. Agonists and antagonists have the potential to inhibit tumor growth, migration, and invasion and cause apoptosis by modifying the expression of NRs and by controlling a wide range of genes involved in cell differentiation, proliferation, and apoptosis. Furthermore, epigenetic changes also play a significant role in the development and progression of EC. Several studies have proved that hypermethylation alters the expression patterns of RARβ and RARβ2 in EC [171,173,174]. It is also noted that there are very few major clinical trials conducted on NRs’ influence on EC. Therefore, more clinical studies on the role of NRs in the development of EC will aid in the discovery of novel therapeutic targets for better management of this disease.
Author Contributions
S.J.: Writing original draft preparation, Investigation, Visualization, Figure and Table preparation; M.H.: Writing review and editing; S.G.: Writing review and editing; M.S.A.: Writing review and editing; M.A.: Writing review and editing; E.H.C.L.: Writing review and editing; K.C.-H.Y.: Writing review and editing; G.S.: Writing, review and editing; A.P.K. and A.B.K.: Conceptualization, funding, supervision, review development and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by RMBH/PNH/SB/12/2015-1438 grant awarded to Ajaikumar B. Kunnumakkara by Department of Biotechnology (DBT), Government of India. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University (KKU) for funding this work through the Research Group Program under the Grant Number: (R.G.P.1/256/43). E. Hui Clarissa Lee was supported by a Ph.D. scholarship from Yong Loo Lin School of Medicine, National University of Singapore. Kenneth Chun-Hong Yap was supported by the President’s Ph.D. Scholarship, National University of Singapore. Singapore Ministry of Education (MOE-T2EP30120-0016) awarded to Alan Prem Kumar supported this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were created in BioRender.com. Mangala Hegde acknowledges Science and Engineering Board (SERB)-National Post-Doctoral Fellowship (NPDF) (PDF/2021/004053).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5-aza-dc-5-aza-20 | deoxycytidine |
| 4HPR | N-(4-hydroxyphenyl) retinamide |
| AC | Adenocarcinoma |
| AF | Activation function |
| AR | Androgen receptors |
| ARE | Androgen Responsive Elements |
| ATRA | All-trans retinoic acid |
| BA | Bile acid |
| BE | Barrett’s esophagus |
| BPDE | Benzo-[a]pyrene diol epoxide |
| CCM | Columnar cell metaplasia |
| CCK2 | Cholecystokinin 2 |
| CDDP | Cisplatin, cisplatinum, or cis-diamminedichloroplatinum (II) |
| COUP TF | Chicken ovalbumin upstream promoter transcription factor |
| COX-2 | Cyclooxygenase-2 |
| CRBP-1 | Cellular retinol-binding protein-1 |
| CTD | C-terminal domain |
| Da-Ea | Ethyl acetate extract of D. altaica |
| DBD | DNA-binding domain |
| DDP | Diamminedichloroplatinum |
| DFS | Disease-free survival |
| DHT | Dihydrotestosterone |
| DNMT1 | DNA (cytosine-5)-methyltransferase1 |
| DNMT3A | DNA (cytosine-5)-methyltransferase 3A |
| DPN | Diarylpropionitrile |
| E2 | 17β-estradiol |
| EAC | Esophageal adenocarcinoma |
| EC | Esophageal cancer |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| EREs | Estrogen response elements |
| ERK | Extracellular-signal regulated kinase |
| ERRs | Estrogen related receptors |
| ESCC | Esophageal squamous cell cancer |
| ESR | Estrogen receptor |
| FAK | Focal adhesion kinase |
| FBXO32 | F-Box Protein 32 |
| FDA | Food and drug administration |
| FGFR | Fibroblast growth factor receptors |
| FKBP5 | FK506-binding protein 5 |
| FU | Fluorouracil |
| FXR | Farnesoid X receptor |
| GCA | Gastric cardia adenocarcinoma |
| GERD | Gastroesophageal reflux disease |
| GRs | Glucocorticoid receptors |
| HGF | Hepatocyte growth factor |
| HMOX1 | Heme Oxygenase 1 |
| HREs | Hormone response elements |
| HSP90 | Heat shock protein 90 |
| IL-6 | Interleukin-6 |
| JNK | c-Jun N-terminal kinases |
| KLK3 | Kallikrein Related Peptidase 3 |
| LBD | Ligand binding domain |
| LCA | Lithocholic acid |
| LDH | Lactate dehydrogenase |
| LINE-1 | Long Interspersed Element-1 |
| LXRs | Liver X receptor |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| MPP | Methyl-piperidinopyrazole |
| MRTF | Master Regulator Transcription Factors |
| NCoR | Nuclear receptor corepressor |
| NF-κB | Nuclear factor kappa B |
| NNK | 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone |
| NQO | Nitroquinoline 1-oxide |
| NRs | Nuclear receptors |
| NR1H4 | Nuclear Receptor Subfamily 1 Group H Member 4 |
| NR4A1 | Nuclear receptor 4A1 |
| NTD | N-terminal domain |
| OS | Overall survival |
| PAR | Pregnane-activated receptor |
| PCNA | Proliferating cell nuclear antigen |
| PG | Prostaglandin |
| PGC-1 | PPAR coactivator-1 |
| PHTPP | 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol |
| PLIN2 | Perilipin 2 |
| PPAR | Peroxisome Proliferator Activated Receptor |
| PPRE | Peroxisome proliferator response element |
| PPT | 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole |
| PRs | Progesterone receptors |
| PXR | Pregnane X receptor |
| RA | Retinoic acid |
| RAR | Retinoic acid receptor |
| RAREs | Retinoic acid receptor elements |
| RRIG1 | Retinoid receptor-induced gene-1 |
| RXR | Retinoid X receptor |
| SERMs | Selective estrogen receptor modulators |
| SCC | Squamous cell carcinoma |
| SIRT1 | Sirtulin 1 |
| SMRT | Silencing mediator of retinoic acid and thyroid hormone receptor |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SXR | Steroid and xenobiotic receptor |
| TFs | Transcription factors |
| TGR5 | Takeda G protein-coupled receptor 5 |
| TIG1 | Tazarotene-induced gene-1 |
| TLRs | Toll-like receptors |
| TNM | Tumor nodes metastases |
| TR | Thyroid hormone receptor |
| TRO | Troglitazone |
| TZD | Thiazolidinedione |
| VDR | Vitamin D receptor |
| VDR | Vitamin D receptor elements |
| VEGFA | Vascular endothelial growth factor A |
| WNT5A | Wnt Family Member 5A |
References
- Arnal, M.J.D.; Arenas, Á.F.; Arbeloa, Á.L. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-L.; Yu, S.-J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J. Emerging multimodality approaches to treat localized esophageal cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nakajima, M. Treatments for esophageal cancer: A review. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 330–335. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hirano, H.; Kato, K. Systemic treatment of advanced esophageal squamous cell carcinoma: Chemotherapy, molecular-targeting therapy and immunotherapy. Jpn. J. Clin. Oncol. 2019, 49, 412–420. [Google Scholar] [CrossRef]
- Short, M.W.; Burgers, K.; Fry, V. Esophageal cancer. Am. Fam. Physician 2017, 95, 22–28. [Google Scholar]
- Li, Y.; Li, Y.; Chen, X. NOTCH and esophageal squamous cell carcinoma. Adv. Exp. Med. Biol. 2021, 1287, 59–68. [Google Scholar] [CrossRef]
- Lam, A.K. Introduction: Esophageal squamous cell carcinoma—current status and future advances. In Esophageal Squamous Cell Carcinoma; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2020; Volume 2129, pp. 1–6. [Google Scholar] [CrossRef]
- Siersema, P.D. Esophageal cancer. Gastroenterol. Clin. N. Am. 2008, 37, 943–964. [Google Scholar] [CrossRef]
- Snider, E.J.; Freedberg, D.E.; Abrams, J.A. Potential role of the microbiome in Barrett’s esophagus and esophageal adenocarcinoma. Dig. Dis. Sci. 2016, 61, 2217–2225. [Google Scholar] [CrossRef]
- Layke, J.C.; Lopez, P.P. Esophageal cancer: A review and update. Am. Fam. Physician 2006, 73, 2187–2194. [Google Scholar]
- Wang, D.H.; Souza, R.F. Biology of Barrett’s esophagus and esophageal adenocarcinoma. Gastrointest. Endosc. Clin. 2011, 21, 25–38. [Google Scholar] [CrossRef]
- Ghavamzadeh, A.; Moussavi, A.; Jahani, M.; Rastegarpanah, M.; Iravani, M. Esophageal cancer in Iran. Semin. Oncol 2001, 28, 153–157. [Google Scholar] [CrossRef]
- Hou, X.; Wen, J.; Ren, Z.; Zhang, G. Non-coding RNAs: New biomarkers and therapeutic targets for esophageal cancer. Oncotarget 2017, 8, 43571. [Google Scholar] [CrossRef]
- Vendrely, V.; Launay, V.; Najah, H.; Smith, D.; Collet, D.; Gronnier, C. Prognostic factors in esophageal cancer treated with curative intent. Dig. Liver Dis. 2018, 50, 991–996. [Google Scholar] [CrossRef]
- Borggreve, A.S.; Kingma, B.F.; Domrachev, S.A.; Koshkin, M.A.; Ruurda, J.P.; van Hillegersberg, R.; Takeda, F.R.; Goense, L. Surgical treatment of esophageal cancer in the era of multimodality management. Ann. N. Y. Acad. Sci. 2018, 1434, 192–209. [Google Scholar] [CrossRef]
- Dupuis, O.; Ganem, G.; Béra, G.; Pointreau, Y.; Pradier, O.; Martin, P.; Mirabel, X.; Denis, F. Esophageal cancer. Cancer Radiother. 2010, 14, S74–S83. [Google Scholar] [CrossRef]
- Parama, D.; Boruah, M.; Yachna, K.; Rana, V.; Banik, K.; Harsha, C.; Thakur, K.K.; Dutta, U.; Arya, A.; Mao, X.; et al. Diosgenin, a steroidal saponin, and its analogs: Effective therapies against different chronic diseases. Life Sci. 2020, 260, 118182. [Google Scholar] [CrossRef]
- Fan, R.; Pineda-Torra, I.; Venteclef, N. Editorial: Nuclear receptors and coregulators in metabolism and immunity. Front. Endocrinol. 2021, 12, 828635. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Mostafavi, E.; Aref, A.R.; Sethi, G.; Wang, L.; Tergaonkar, V. Non-coding RNA-based regulation of inflammation. Semin. Immunol. 2022, 101606, in press. [Google Scholar] [CrossRef]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer 2007, 120, 2545–2556. [Google Scholar] [CrossRef]
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2022, 80, 379–390. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Yang, Z.; Gimple, R.C.; Zhou, N.; Zhao, L.; Gustafsson, J.-Å.; Zhou, S. Targeting nuclear receptors for cancer therapy: Premises, promises, and challenges. Trends Cancer 2021, 7, 541–556. [Google Scholar] [CrossRef]
- Mazaira, G.I.; Zgajnar, N.R.; Lotufo, C.M.; Daneri-Becerra, C.; Sivils, J.C.; Soto, O.B.; Cox, M.B.; Galigniana, M.D. Nuclear receptors: A historical perspective. In Nuclear Receptors; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1966, pp. 1–5. [Google Scholar] [CrossRef]
- Frigo, D.E.; Bondesson, M.; Williams, C. Nuclear receptors: From molecular mechanisms to therapeutics. Essays Biochem. 2021, 65, 847–856. [Google Scholar] [CrossRef]
- Zuo, H.; Wan, Y. Nuclear receptors in skeletal homeostasis. Curr. Top. Dev. Biol. 2017, 125, 71–107. [Google Scholar] [CrossRef]
- Sherman, M.H.; Downes, M.; Evans, R.M. Nuclear receptors as modulators of the tumor microenvironment. Cancer Prev. Res. 2012, 5, 3–10. [Google Scholar] [CrossRef]
- Cheng, H.S.; Lee, J.X.T.; Wahli, W.; Tan, N.S. Exploiting vulnerabilities of cancer by targeting nuclear receptors of stromal cells in tumor microenvironment. Mol. Cancer 2019, 18, 51. [Google Scholar] [CrossRef]
- Cai, W.; Xiong Chen, Z.; Rane, G.; Satendra Singh, S.; Choo, Z.; Wang, C.; Yuan, Y.; Zea Tan, T.; Arfuso, F.; Yap, C.T.; et al. Wanted DEAD/H or alive: Helicases winding up in cancers. JNCI J. Natl. Cancer Inst. 2017, 109, djw278. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, K.I.; Baek, S.H. Nuclear receptors and coregulators in inflammation and cancer. Cancer Lett. 2008, 267, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the therapeutic potential of apigenin against cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.B.; Graham, J.D.; Clarke, C.L. Emerging functional roles of nuclear receptors in breast cancer. J. Mol. Endocrinol. 2017, 58, R169–R190. [Google Scholar] [CrossRef]
- Gangwar, S.K.; Kumar, A.; Yap, K.C.; Jose, S.; Parama, D.; Sethi, G.; Kumar, A.P.; Kunnumakkara, A.B. Targeting nuclear receptors in lung cancer-novel therapeutic prospects. Pharmaceuticals 2022, 15, 624. [Google Scholar] [CrossRef]
- Gangwar, S.K.; Kumar, A.; Jose, S.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. Nuclear receptors in oral cancer-Emerging players in tumorigenesis. Cancer Lett. 2022, 536, 215666. [Google Scholar] [CrossRef]
- Girisa, S.; Henamayee, S.; Parama, D.; Rana, V.; Dutta, U.; Kunnumakkara, A.B. Targeting Farnesoid X receptor (FXR) for developing novel therapeutics against cancer. Mol. Biomed. 2021, 2, 21. [Google Scholar] [CrossRef]
- Thorne, J.L.; Campbell, M.J. Nuclear receptors and the Warburg effect in cancer. Int. J. Cancer 2015, 137, 1519–1527. [Google Scholar] [CrossRef]
- Garattini, E.; Bolis, M.; Paroni, G.; Fratelli, M.; Terao, M. Lipid-sensors, enigmatic-orphan and orphan nuclear receptors as therapeutic targets in breast-cancer. Oncotarget 2016, 7, 42661. [Google Scholar] [CrossRef][Green Version]
- Valledor, A.F.; Ricote, M. Nuclear receptor signaling in macrophages. Biochem. Pharmacol. 2004, 67, 201–212. [Google Scholar] [CrossRef]
- Flanagan, J.J.; Neklesa, T.K. Targeting nuclear receptors with PROTAC degraders. Mol. Cell. Endocrinol. 2019, 493, 110452. [Google Scholar] [CrossRef]
- Ribeiro Filho, H.V.; Bernardi Videira, N.; Bridi, A.V.; Tittanegro, T.H.; Helena Batista, F.A.; de Carvalho Pereira, J.G.; de Oliveira, P.S.L.; Bajgelman, M.C.; Le Maire, A.; Figueira, A.C.M. Screening for PPAR non-agonist ligands followed by characterization of a hit, AM-879, with additional no-adipogenic and cdk5-mediated phosphorylation inhibition properties. Front. Endocrinol. 2018, 9, 11. [Google Scholar] [CrossRef]
- Roemer, S.C.; Donham, D.C.; Sherman, L.; Pon, V.H.; Edwards, D.P.; Churchill, M.E. Structure of the progesterone receptor-deoxyribonucleic acid complex: Novel interactions required for binding to half-site response elements. Mol. Endocrinol. 2006, 20, 3042–3052. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Schrödinger LLC. The PyMOL Molecular Graphics System; Schrödinger LLC.: New York, NY, USA, 2020. [Google Scholar]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Parris, T.Z. Pan-cancer analyses of human nuclear receptors reveal transcriptome diversity and prognostic value across cancer types. Sci. Rep. 2020, 10, 1873. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Liu, X.; Liu, Y.; Gu, Z.; Cao, H.; Dickerson, K.E.; Chen, M.; Chen, W.; Shao, Z. Noncoding variants connect enhancer dysregulation with nuclear receptor signaling in hematopoietic malignanciesnoncoding variants in hematopoietic malignancies. Cancer Discov. 2020, 10, 724–745. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Seyed Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into biological role of LncRNAs in epithelial-mesenchymal transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious toggling of mTOR activity to combat insulin resistance and cancer: Current evidence and perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Bordoloi, D.; Banik, K.; Shabnam, B.; Padmavathi, G.; Monisha, J.; Arfuso, F.; Dharmarajan, A.; Mao, X.; Lim, L.H.K.; Wang, L.; et al. TIPE Family of Proteins and Its Implications in Different Chronic Diseases. Int. J. Mol. Sci. 2018, 19, 2974. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Z.W.; Duan, W.; Qian, C.Y.; Wang, S.N.; Deng, M.S.; Zi, D.; Wang, J.M.; Mao, C.Y.; Song, G.; et al. Inhibiting the redox function of APE1 suppresses cervical cancer metastasis via disengagement of ZEB1 from E-cadherin in EMT. J. Exp. Clin. Cancer Res. 2021, 40, 220. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, W.; Zeng, W.; Zhang, L.; Zhang, C.; Qiu, Y.; Wang, L.; Yin, X.; Zhang, C.; Liang, W. Naringenin prevents TGF-β1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation. Breast Cancer Res. 2016, 18, 38. [Google Scholar] [CrossRef]
- Yin, H.; Que, R.; Liu, C.; Ji, W.; Sun, B.; Lin, X.; Zhang, Q.; Zhao, X.; Peng, Z.; Zhang, X.; et al. Survivin-targeted drug screening platform identifies a matrine derivative WM-127 as a potential therapeutics against hepatocellular carcinoma. Cancer Lett. 2018, 425, 54–64. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Leach, D.A.; Powell, S.M.; Bevan, C.L. Women in cancer thematic review: New roles for nuclear receptors in prostate cancer. Endocr.-Relat. Cancer 2016, 23, T85–T108. [Google Scholar] [CrossRef]
- Shishodia, S.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007, 74, 118–130. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef]
- Conzen, S.D. Minireview: Nuclear receptors and breast cancer. Mol. Endocrinol. 2008, 22, 2215–2228. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, J.; Hu, Z.; Xu, M.; Zhang, Y.; Long, M.; Fan, Y.; Montone, K.T.; Tanyi, J.L.; Tavana, O. Systematic pan-cancer characterization of nuclear receptors identifies potential cancer biomarkers and therapeutic targetsnuclear receptors in human cancers. Cancer Res. 2022, 82, 46–59. [Google Scholar] [CrossRef]
- Guan, B.; Li, H.; Yang, Z.; Hoque, A.; Xu, X. Inhibition of farnesoid X receptor controls esophageal cancer cell growth in vitro and in nude mouse xenografts. Cancer 2013, 119, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sandur, S.K.; Lin, X.; Chaturvedi, M.M.; Aggarwal, B.B. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J. Biol. Chem. 2006, 281, 23425–23435. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone inhibits bone metastasis of breast cancer cells through abrogation of the CXCR4 signaling axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ho, P.C.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand through modulation of NF-kappaB pathway. Int. J. Cancer 2008, 123, 1733–1740. [Google Scholar] [CrossRef]
- Awasthee, N.; Rai, V.; Chava, S.; Nallasamy, P.; Kunnumakkara, A.B.; Bishayee, A.; Chauhan, S.C.; Challagundla, K.B.; Gupta, S.C. Targeting IkappaappaB kinases for cancer therapy. Semin. Cancer Biol. 2019, 56, 12–24. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Harsha, C.; Bordoloi, D.; Lalduhsaki Sailo, B.; Sethi, G.; Leong, H.C.; Arfuso, F.; Mishra, S.; Wang, L.; Kumar, A.P.; et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Simvastatin potentiates TNF-α-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: Role of IkappaBα kinase and TGF-β-activated kinase-1. J. Immunol. 2007, 178, 2507–2516. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Choudhury, B.; Kandimalla, R.; Bharali, R.; Monisha, J.; Kunnumakara, A.B.; Kalita, K.; Kotoky, J. Anticancer activity of garcinia morella on T-Cell murine lymphoma via apoptotic induction. Front. Pharmacol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations: An open-label parallel-arm study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Nair, A.S.; Ahn, K.S.; Pandey, M.K.; Yi, Z.; Liu, M.; Aggarwal, B.B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor β-activated kinase-1-mediated NF-κB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 2007, 109, 5112–5121. [Google Scholar] [CrossRef]
- Thomas, D.; Govindhan, S.; Baiju, E.C.; Padmavathi, G.; Kunnumakkara, A.B.; Padikkala, J. Cyperus rotundus L. prevents non-steroidal anti-inflammatory drug-induced gastric mucosal damage by inhibiting oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 485–490. [Google Scholar] [CrossRef]
- Muralimanoharan, S.B.; Kunnumakkara, A.B.; Shylesh, B.; Kulkarni, K.H.; Haiyan, X.; Ming, H.; Aggarwal, B.B.; Rita, G.; Kumar, A.P. Butanol fraction containing berberine or related compound from nexrutine inhibits NFkappaB signaling and induces apoptosis in prostate cancer cells. Prostate 2009, 69, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kunnumakkara, A.B. Molecular Targets and Therapeutic Uses of Spices: Modern Uses for Ancient Medicine; World Scientific: Singapore, 2009. [Google Scholar]
- Kunnumakkara, A.B.; Koca, C.; Dey, S.; Gehlot, P.; Yodkeeree, S.; Danda, D.; Sung, B.; Aggarwal, B.B. Traditional uses of spices: An overview. In Molecular Targets and Therapeutic Uses of Spices; World Scientific Publishing: Singapore, 2009; pp. 1–24. [Google Scholar] [CrossRef]
- Nair, A.S.; Shishodia, S.; Ahn, K.S.; Kunnumakkara, A.B.; Sethi, G.; Aggarwal, B.B. Deguelin, an Akt inhibitor, suppresses IkappaBα kinase activation leading to suppression of NF-κB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J. Immunol. 2006, 177, 5612–5622. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Kim, S.H.; Sethi, G.; Ahn, K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015, 360, 280–293. [Google Scholar] [CrossRef]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- Babu, B.H.; Jayram, H.N.; Nair, M.G.; Ajaikumar, K.B.; Padikkala, J. Free radical scavenging, antitumor and anticarcinogenic activity of gossypin. J. Exp. Clin. Cancer Res. 2003, 22, 581–589. [Google Scholar]
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-kappaB signaling by Calebin A, a compound of turmeric, in multicellular tumor microenvironment: Potential role of apoptosis induction in crc cells. Biomedicines 2020, 8, 236. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Damayenti, Y.D.; Deka, A.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Kunnumakkara, A.B. Acorus calamus: A bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 107–122. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.; Dey, S.; Koca, C.; Tong, Z.; Gelovani, J.G.; Guha, S.; et al. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int. J. Cancer 2012, 131, E292–E303. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Rajendran, P.; Ong, T.H.; Chen, L.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin. Cancer Res. 2011, 17, 1425–1439. [Google Scholar] [CrossRef]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma: A systematic, comprehensive, and critical review. Anticancer Drugs 2015, 26, 475–486. [Google Scholar] [CrossRef]
- Kizaibek, M.; Wubuli, A.; Gu, Z.; Bahetjan, D.; Tursinbai, L.; Nurhamit, K.; Chen, B.; Wang, J.; Tahan, O.; Cao, P. Effects of an ethyl acetate extract of Daphne altaica stem bark on the cell cycle, apoptosis and expression of PPARγ in Eca109 human esophageal carcinoma cells. Mol. Med. Rep. 2020, 22, 1400–1408. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar] [CrossRef]
- Wagner, B.L.; Valledor, A.F.; Shao, G.; Daige, C.L.; Bischoff, E.D.; Petrowski, M.; Jepsen, K.; Baek, S.H.; Heyman, R.A.; Rosenfeld, M.G. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 2003, 23, 5780–5789. [Google Scholar] [CrossRef]
- Hörlein, A.J.; Näär, A.M.; Heinzel, T.; Torchia, J.; Gloss, B.; Kurokawa, R.; Ryan, A.; Kamei, Y.; Söderström, M.; Glass, C.K. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 1995, 377, 397–404. [Google Scholar] [CrossRef]
- Jonathan, M.-C.; Adrián, S.-H.; Gonzalo, A. Type II nuclear receptors with potential role in Alzheimer disease. Mol. Asp. Med. 2021, 78, 100940. [Google Scholar] [CrossRef]
- Bain, D.L.; Heneghan, A.F.; Connaghan-Jones, K.D.; Miura, M.T. Nuclear receptor structure: Implications for function. Annu. Rev. Physiol. 2007, 69, 201–220. [Google Scholar] [CrossRef]
- Carpenter, K.D.; Korach, K.S. Potential biological functions emerging from the different estrogen receptors. Ann. N. Y. Acad. Sci. 2006, 1092, 361–373. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor β: An overview and update. Nucl. Recept. Signal. 2008, 6, e003. [Google Scholar] [CrossRef]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARα: Energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 2010, 8, e002. [Google Scholar] [CrossRef] [PubMed]
- Granner, D.K.; Wang, J.-C.; Yamamoto, K.R. Regulatory actions of glucocorticoid hormones: From organisms to mechanisms. Adv. Exp. Med. Biol. 2015, 872, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor-negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- McKenna, N.J. Research resources for nuclear receptor signaling pathways. Mol. Pharmacol. 2016, 90, 153–159. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Meyer, C.; Geistlinger, T.; Lupien, M.; Wang, Q.; Liu, T.; Zhang, Y.; Brown, M.; Liu, X.S. A comprehensive view of nuclear receptor cancer cistromesa comprehensive view of nuclear receptor cancer cistromes. Cancer Res. 2011, 71, 6940–6947. [Google Scholar] [CrossRef]
- Bharathkumar, H.; Mohan, C.D.; Ananda, H.; Fuchs, J.E.; Li, F.; Rangappa, S.; Surender, M.; Bulusu, K.C.; Girish, K.S.; Sethi, G.; et al. Microwave-assisted synthesis, characterization and cytotoxic studies of novel estrogen receptor α ligands towards human breast cancer cells. Bioorg. Med. Chem. Lett. 2015, 25, 1804–1807. [Google Scholar] [CrossRef]
- Nacusi, L.P.; Debes, J.D. Primers on molecular pathways: Nuclear receptors in pancreatic cancer: The ligand-independent way. Pancreatology 2008, 8, 422–424. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- UniProt, Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Huang, H.; Huang, W.; Chen, Y.; McGarvey, P.B.; Wu, C.H.; Arighi, C.N.; UniProt, C. A crowdsourcing open platform for literature curation in UniProt. PLoS Biol. 2021, 19, e3001464. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. Alphafold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, T.; Zhong, X.; Cheng, C. Androgen receptor promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 2. Tumor Biol. 2015, 36, 5859–5864. [Google Scholar] [CrossRef]
- Dong, H.; Xu, J.; Li, W.; Gan, J.; Lin, W.; Ke, J.; Jiang, J.; Du, L.; Chen, Y.; Zhong, X. Reciprocal androgen receptor/interleukin-6 crosstalk drives oesophageal carcinoma progression and contributes to patient prognosis. J. Pathol. 2017, 241, 448–462. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, S.-W.; Niu, Y.; Chen, L.; Liu, S.; Ma, T.; Chen, X.; Xu, L.; Su, Z.; Chen, H. Expression of estrogen receptor α and β in esophageal squamous cell carcinoma. Oncol. Rep. 2013, 30, 2771–2776. [Google Scholar] [CrossRef][Green Version]
- Al-Khyatt, W.; Tufarelli, C.; Khan, R.; Iftikhar, S.Y. Selective oestrogen receptor antagonists inhibit oesophageal cancer cell proliferation in vitro. BMC Cancer 2018, 18, 121. [Google Scholar] [CrossRef]
- Zuguchi, M.; Miki, Y.; Onodera, Y.; Fujishima, F.; Takeyama, D.; Okamoto, H.; Miyata, G.; Sato, A.; Satomi, S.; Sasano, H. Estrogen receptor α and β in esophageal squamous cell carcinoma. Cancer Sci. 2012, 103, 1348–1355. [Google Scholar] [CrossRef]
- Liu, L.; Chirala, M.; Younes, M. Expression of estrogen receptor-β isoforms in Barrett’s metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res. 2004, 24, 2919–2924. [Google Scholar]
- Kalayarasan, R.; Ananthakrishnan, N.; Kate, V.; Basu, D. Estrogen and progesterone receptors in esophageal carcinoma. Dis. Esophagus 2008, 21, 298–303. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Qi, Y.-J.; Jiang, Q.; Ma, Y.-F.; Wang, L.-D. Relevance of serum estradiol and estrogen receptor β expression from a high-incidence area for esophageal squamous cell carcinoma in China. Med. Oncol. 2011, 28, 188–193. [Google Scholar] [CrossRef]
- De Gottardi, A.; Dumonceau, J.M.; Bruttin, F.; Vonlaufen, A.; Morard, I.; Spahr, L.; Rubbia-Brandt, L.; Frossard, J.L.; Dinjens, W.N.; Rabinovitch, P.S.; et al. Expression of the bile acid receptor FXR in Barrett’s esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol. Cancer 2006, 5, 48. [Google Scholar] [CrossRef]
- Nortunen, M.; Vakiparta, N.; Porvari, K.; Saarnio, J.; Karttunen, T.J.; Huhta, H. Pathophysiology of reflux oesophagitis: Role of Toll-like receptors 2 and 4 and Farnesoid X receptor. Virchows Arch. 2021, 479, 285–293. [Google Scholar] [CrossRef]
- Terashita, Y.; Sasaki, H.; Haruki, N.; Nishiwaki, T.; Ishiguro, H.; Shibata, Y.; Kudo, J.; Konishi, S.; Kato, J.; Koyama, H.; et al. Decreased peroxisome proliferator-activated receptor gamma gene expression is correlated with poor prognosis in patients with esophageal cancer. Jpn. J. Clin. Oncol. 2002, 32, 238–243. [Google Scholar] [CrossRef][Green Version]
- Konturek, P.C.; Nikiforuk, A.; Kania, J.; Raithel, M.; Hahn, E.G.; Muhldorfer, S. Activation of NFκB represents the central event in the neoplastic progression associated with Barrett’s esophagus: A possible link to the inflammation and overexpression of COX-2, PPARγ and growth factors. Dig. Dis. Sci. 2004, 49, 1075–1083. [Google Scholar] [CrossRef]
- Takahashi, H.; Fujita, K.; Fujisawa, T.; Yonemitsu, K.; Tomimoto, A.; Ikeda, I.; Yoneda, M.; Masuda, T.; Schaefer, K.; Saubermann, L.J.; et al. Inhibition of peroxisome proliferator-activated receptor gamma activity in esophageal carcinoma cells results in a drastic decrease of invasive properties. Cancer Sci. 2006, 97, 854–860. [Google Scholar] [CrossRef]
- Al-Taie, O.H.; Graf, T.; Illert, B.; Katzenberger, T.; Mörk, H.; Kraus, M.R.; Barthelmes, H.U.; Scheurlen, M.; Seufert, J. Differential effects of PPARγ activation by the oral antidiabetic agent pioglitazone in Barrett’s carcinoma in vitro and in vivo. J. Gastroenterol. 2009, 44, 919–929. [Google Scholar] [CrossRef]
- Wu, K.; Hu, Y.; Yan, K.; Qi, Y.; Zhang, C.; Zhu, D.; Liu, D.; Zhao, S. microRNA-10b confers cisplatin resistance by activating AKT/mTOR/P70S6K signaling via targeting PPARγ in esophageal cancer. J. Cell. Physiol. 2020, 235, 1247–1258. [Google Scholar] [CrossRef]
- van de Winkel, A.; Menke, V.; Capello, A.; Moons, L.M.; Pot, R.G.; van Dekken, H.; Siersema, P.D.; Kusters, J.G.; van der Laan, L.J.; Kuipers, E.J. Expression, localization and polymorphisms of the nuclear receptor PXR in Barrett’s esophagus and esophageal adenocarcinoma. BMC Gastroenterol. 2011, 11, 108. [Google Scholar] [CrossRef]
- Lord, R.V.; Tsai, P.I.; Danenberg, K.D.; Peters, J.H.; Demeester, T.R.; Tsao-Wei, D.D.; Groshen, S.; Salonga, D.; Park, J.M.; Crookes, P.F.; et al. Retinoic acid receptor-α messenger RNA expression is increased and retinoic acid receptor-γ expression is decreased in Barrett’s intestinal metaplasia, dysplasia, adenocarcinoma sequence. Surgery 2001, 129, 267–276. [Google Scholar] [CrossRef]
- Mao, X.M.; Li, H.; Zhang, X.Y.; Zhou, P.; Fu, Q.R.; Chen, Q.E.; Shen, J.X.; Liu, Y.; Chen, Q.X.; Shen, D.Y. Retinoic acid receptor α knockdown suppresses the tumorigenicity of esophageal carcinoma via Wnt/β-catenin pathway. Dig. Dis. Sci. 2018, 63, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Y.L.; Fu, J.; Huang, H.; Chen, S.Z.; Qu, P.; Tian, H.M.; Liu, Z.Y.; Zhang, W. The abnormal expression of retinoic acid receptor-β, p 53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J. Gastroenterol. 2002, 8, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, S.L.; Li, Y.; Meng, M.; Qin, C.Y. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone induces retinoic acid receptor β hypermethylation through DNA methyltransferase 1 accumulation in esophageal squamous epithelial cells. Asian Pac. J. Cancer Prev. 2012, 13, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Ding, F.; Li, J.; Guo, M.; Li, W.; Wang, Y.; Yu, Z.; Zhan, Q.; Wu, M.; et al. 5-Aza-2′-deoxycytidine induces retinoic acid receptor-β2 demethylation and growth inhibition in esophageal squamous carcinoma cells. Cancer Lett. 2005, 230, 271–283. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ding, F.; Guo, M.Z.; Zhang, L.Y.; Wu, M.; Liu, Z.H. Downregulation of retinoic acid receptor-β2 expression is linked to aberrant methylation in esophageal squamous cell carcinoma cell lines. World J. Gastroenterol. 2004, 10, 771–775. [Google Scholar] [CrossRef]
- Xu, X.C.; Lee, J.J.; Wu, T.T.; Hoque, A.; Ajani, J.A.; Lippman, S.M. Increased retinoic acid receptor-β4 correlates in vivo with reduced retinoic acid receptor-β2 in esophageal squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 826–829. [Google Scholar] [CrossRef]
- Liang, Z.D.; Lippman, S.M.; Wu, T.T.; Lotan, R.; Xu, X.C. RRIG1 mediates effects of retinoic acid receptor β2 on tumor cell growth and gene expression through binding to and inhibition of RhoA. Cancer Res. 2006, 66, 7111–7118. [Google Scholar] [CrossRef]
- Trowbridge, R.; Sharma, P.; Hunter, W.J.; Agrawal, D.K. Vitamin D receptor expression and neoadjuvant therapy in esophageal adenocarcinoma. Exp. Mol. Pathol. 2012, 93, 147–153. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.; Xia, Y.; Bandla, S.; Zakharov, V.; Wu, S.; Peters, J.; Godfrey, T.E.; Sun, J. Vitamin D receptor is highly expressed in precancerous lesions and esophageal adenocarcinoma with significant sex difference. Hum. Pathol. 2014, 45, 1744–1751. [Google Scholar] [CrossRef]
- Janmaat, V.T.; Van De Winkel, A.; Peppelenbosch, M.P.; Spaander, M.C.; Uitterlinden, A.G.; Pourfarzad, F.; Tilanus, H.W.; Rygiel, A.M.; Moons, L.M.; Arp, P.P.; et al. Vitamin D receptor polymorphisms are associated with reduced esophageal vitamin D receptor expression and reduced esophageal adenocarcinoma risk. Mol. Med. 2015, 21, 346–354. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, S.; Guo, Y.; Wei, X.; Zhang, Y.; Yang, Y.; Zhang, H.; Ma, M.; Yang, W. Stromal expression of JNK1 and VDR is associated with the prognosis of esophageal squamous cell carcinoma. Clin. Transl. Oncol. 2018, 20, 1185–1195. [Google Scholar] [CrossRef]
- Smith, E.; Palethorpe, H.M.; Ruszkiewicz, A.R.; Edwards, S.; Leach, D.A.; Underwood, T.J.; Need, E.F.; Drew, P.A. Androgen receptor and androgen-responsive gene FKBP5 are independent prognostic indicators for esophageal adenocarcinoma. Dig. Dis. Sci. 2016, 61, 433–443. [Google Scholar] [CrossRef]
- Palethorpe, H.M.; Drew, P.A.; Smith, E. Androgen signaling in esophageal adenocarcinoma cell lines in vitro. Dig. Dis. Sci. 2017, 62, 3402–3414. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Q.; Fu, S.; Zheng, Z. Estrogen receptors in regulating cell proliferation of esophageal squamous cell carcinoma: Involvement of intracellular Ca2+ signaling. Pathol. Oncol. Res. 2017, 23, 329–334. [Google Scholar] [CrossRef]
- Sukocheva, O.; Wee, C.; Ansar, A.; Hussey, D.; Watson, D. Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells. Dis. Esophagus 2013, 26, 628–635. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, H.; Yao, D.; Zhang, X.; Chen, W.D.; Wang, Y.D. Activation of FXR suppresses esophageal squamous cell carcinoma through antagonizing ERK1/2 signaling pathway. Cancer Manag. Res. 2021, 13, 5907–5918. [Google Scholar] [CrossRef]
- Rumi, M.A.; Sato, H.; Ishihara, S.; Ortega, C.; Kadowaki, Y.; Kinoshita, Y. Growth inhibition of esophageal squamous carcinoma cells by peroxisome proliferator-activated receptor-γ ligands. J. Lab. Clin. Med. 2002, 140, 17–26. [Google Scholar] [CrossRef]
- Fujii, D.; Yoshida, K.; Tanabe, K.; Hihara, J.; Toge, T. The ligands of peroxisome proliferator-activated receptor (PPAR) gamma inhibit growth of human esophageal carcinoma cells through induction of apoptosis and cell cycle arrest. Anticancer Res. 2004, 24, 1409–1416. [Google Scholar]
- Sawayama, H.; Ishimoto, T.; Watanabe, M.; Yoshida, N.; Sugihara, H.; Kurashige, J.; Hirashima, K.; Iwatsuki, M.; Baba, Y.; Oki, E. Small molecule agonists of PPAR-γ exert therapeutic effects in esophageal cancer. Cancer Res. 2014, 74, 575–585. [Google Scholar] [CrossRef]
- Cui, L.; Xu, F.; Wu, K.; Li, L.; Qiao, T.; Li, Z.; Chen, T.; Sun, C. Anticancer effects and possible mechanisms of lycopene intervention on N-methylbenzylnitrosamine induced esophageal cancer in F344 rats based on PPARγ. Eur. J. Pharmacol. 2020, 881, 173230. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, B.; Yang, Q.; Pan, Y.; Yang, W.; Freedland, S.J.; Ding, L.W.; Freeman, M.R.; Breunig, J.J.; Bhowmick, N.A.; et al. A transcriptional regulatory loop of master regulator transcription factors, PPARG, and fatty acid synthesis promotes esophageal adenocarcinoma. Cancer Res. 2021, 81, 1216–1229. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Shimada, Y.; Itami, A.; Ito, T.; Kawamura, J.; Kawabe, A.; Kaganoi, J.; Maeda, M.; Watanabe, G.; Imamura, M. Growth inhibition through activation of peroxisome proliferator-activated receptor γ in human oesophageal squamous cell carcinoma. Eur. J. Cancer 2003, 39, 2239–2246. [Google Scholar] [CrossRef]
- Song, S.; Xu, X.C. Effect of benzo[a]pyrene diol epoxide on expression of retinoic acid receptor-β in immortalized esophageal epithelial cells and esophageal cancer cells. Biochem. Biophys. Res. Commun. 2001, 281, 872–877. [Google Scholar] [CrossRef]
- Li, M.; Song, S.; Lippman, S.M.; Zhang, X.K.; Liu, X.; Lotan, R.; Xu, X.C. Induction of retinoic acid receptor-β suppresses cyclooxygenase-2 expression in esophageal cancer cells. Oncogene 2002, 21, 411–418. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Wu, M.; Levi, G.; Ferrari, N. Inhibition of cancer cell growth by all-trans retinoic acid and its analog N-(4-hydroxyphenyl) retinamide: A possible mechanism of action via regulation of retinoid receptors expression. Int. J. Cancer 1998, 78, 248–254. [Google Scholar] [CrossRef]
- Song, S.; Lippman, S.M.; Zou, Y.; Ye, X.; Ajani, J.A.; Xu, X.C. Induction of cyclooxygenase-2 by benzo[a]pyrene diol epoxide through inhibition of retinoic acid receptor-β 2 expression. Oncogene 2005, 24, 8268–8276. [Google Scholar] [CrossRef][Green Version]
- Song, S.; Guan, B.; Men, T.; Hoque, A.; Lotan, R.; Xu, X.C. Antitumor effect of retinoic acid receptor-β2 associated with suppression of cyclooxygenase-2. Cancer Prev. Res. 2009, 2, 274–280. [Google Scholar] [CrossRef]
- Ye, F.; Xu, X.C. Benzo[a]pyrene diol epoxide suppresses retinoic acid receptor-β2 expression by recruiting DNA (cytosine-5-)-methyltransferase 3A. Mol. Cancer 2010, 9, 93. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, B.; Men, T.; Fujimoto, J.; Xu, X. Comparable molecular alterations in 4-nitroquinoline 1-oxide-induced oral and esophageal cancer in mice and in human esophageal cancer, associated with poor prognosis of patients. In Vivo 2013, 27, 473–484. [Google Scholar]
- Awan, A.K.; Iftikhar, S.; Morris, T.; Clarke, P.; Grabowska, A.; Waraich, N.; Watson, S. Androgen receptors may act in a paracrine manner to regulate oesophageal adenocarcinoma growth. Eur. J. Surg. Oncol. 2007, 33, 561–568. [Google Scholar] [CrossRef]
- Nozoe, T.; Oyama, T.; Takenoyama, M.; Hanagiri, T.; Sugio, K.; Yasumoto, K. Significance of immunohistochemical expression of estrogen receptors α and β in squamous cell carcinoma of the esophagus. Clin. Cancer Res. 2007, 13, 4046–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ku, J.; Liu, R.; Wu, H.; Liang, G.; Wei, Y.; Chen, P.; Zhao, X.; Liu, S.; Li, Y. Characterization of serum estradiol level and tissue estrogen receptor immunostaining with clinical response and reproductive factor changes in Chinese female patients with esophageal squamous cell carcinoma. Biomed. Pharmacother. 2017, 93, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, S.; Liang, J.; Qiu, W. Clinicopathological and predictive significance of SIRT1 and peroxisome proliferator-activated receptor gamma in esophageal squamous cell carcinoma: The correlation with EGFR and Survivin. Pathol. Res. Pract. 2018, 214, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhang, W.; El-Naggar, A.K.; Lippman, S.M.; Lin, P.; Lotan, R.; Xu, X.C. Loss of retinoic acid receptor-β expression is an early event during esophageal carcinogenesis. Am. J. Pathol. 1999, 155, 1519–1523. [Google Scholar] [CrossRef]
- Zhang, W.; Rashid, A.; Wu, H.; Xu, X.C. Differential expression of retinoic acid receptors and p53 protein in normal, premalignant, and malignant esophageal tissues. J. Cancer Res. Clin. Oncol. 2001, 127, 237–242. [Google Scholar] [CrossRef]
- Mizuiri, H.; Yoshida, K.; Toge, T.; Oue, N.; Aung, P.P.; Noguchi, T.; Yasui, W. DNA methylation of genes linked to retinoid signaling in squamous cell carcinoma of the esophagus: DNA methylation of CRBP1 and TIG1 is associated with tumor stage. Cancer Sci. 2005, 96, 571–577. [Google Scholar] [CrossRef]
- Fujishima, F.; Suzuki, T.; Nakamura, Y.; Taniyama, Y.; Ono, K.; Sugawara, A.; Miyazaki, S.; Moriya, T.; Sato, A.; Satomi, S.; et al. Retinoid receptors in human esophageal squamous cell carcinoma: Retinoid X receptor as a potent prognostic factor. Pathol. Int. 2011, 61, 401–408. [Google Scholar] [CrossRef]
- Roth, M.J.; Abnet, C.C.; Hu, N.; Wang, Q.H.; Wei, W.Q.; Green, L.; D’Alelio, M.; Qiao, Y.L.; Dawsey, S.M.; Taylor, P.R.; et al. p16, MGMT, RARβ2, CLDN3, CRBP and MT1G gene methylation in esophageal squamous cell carcinoma and its precursor lesions. Oncol. Rep. 2006, 15, 1591–1597. [Google Scholar] [CrossRef]
- Li, R.N.; Yu, F.J.; Wu, C.C.; Chen, Y.K.; Yu, C.C.; Chou, S.H.; Lee, J.Y.; Cheng, Y.J.; Wu, M.T.; Wu, I.C. Methylation status of retinoic acid receptor β2 promoter and global DNA in esophageal squamous cell carcinoma. J. Surg. Oncol. 2014, 109, 623–627. [Google Scholar] [CrossRef]
- Brabender, J.; Lord, R.V.; Metzger, R.; Park, J.; Salonga, D.; Danenberg, K.D.; Holscher, A.H.; Danenberg, P.V.; Schneider, P.M. Role of retinoid X receptor mRNA expression in Barrett’s esophagus. J. Gastrointest. Surg. 2004, 8, 413–422. [Google Scholar] [CrossRef]
- Ren, H.Y.; Guo, Y.H.; Yang, L.M.; Dai, T.J.; Lin, M.Z.; Yu, L.; Zhu, Q.G.; Meng, J.R. Expression and clinical significance of retinoid X receptor α in esophageal carcinoma. Ann. Diagn. Pathol. 2018, 34, 110–115. [Google Scholar] [CrossRef]
- Trowbridge, R.; Mittal, S.K.; Sharma, P.; Hunter, W.J.; Agrawal, D.K. Vitamin D receptor expression in the mucosal tissue at the gastroesophageal junction. Exp. Mol. Pathol. 2012, 93, 246–249. [Google Scholar] [CrossRef]
- Abu-Farsakh, S.; Wu, T.; Lalonde, A.; Sun, J.; Zhou, Z. High expression of Claudin-2 in esophageal carcinoma and precancerous lesions is significantly associated with the bile salt receptors VDR and TGR5. BMC Gastroenterol. 2017, 17, 33. [Google Scholar] [CrossRef]
- Singhal, S.; Kapoor, H.; Subramanian, S.; Agrawal, D.K.; Mittal, S.K. Polymorphisms of genes related to function and metabolism of vitamin D in esophageal adenocarcinoma. J. Gastrointest. Cancer 2019, 50, 867–878. [Google Scholar] [CrossRef]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of selective androgen receptor modulators (SARMs). Mol. Cell. Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef]
- Tan, J.-a.; Joseph, D.R.; Quarmby, V.E.; Lubahn, D.B.; Sar, M.; French, F.S.; Wilson, E.M. The rat androgen receptor: Primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Mol. Endocrinol. 1988, 2, 1276–1285. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Joseph, D.R.; Sar, M.; Tan, J.-a.; Higgs, H.N.; Larson, R.E.; French, F.S.; Wilson, E.M. The human androgen receptor: Complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol. Endocrinol. 1988, 2, 1265–1275. [Google Scholar] [CrossRef]
- Jenster, G.; van der Korput, H.A.; Trapman, J.; Brinkmann, A.O. Identification of two transcription activation units in the n-terminal domain of the human androgen receptor. J. Biol. Chem. 1995, 270, 7341–7346. [Google Scholar] [CrossRef]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef]
- Ward, R.D.; Weigel, N.L. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. Biofactors 2009, 35, 528–536. [Google Scholar] [CrossRef]
- Ylikomi, T.; Bocquel, M.; Berry, M.; Gronemeyer, H.; Chambon, P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 1992, 11, 3681–3694. [Google Scholar] [CrossRef]
- Culig, Z.; Klocker, H.; Bartsch, G.; Hobisch, A. Androgen receptors in prostate cancer. Endocr.-Relat. Cancer 2002, 9, 155–170. [Google Scholar] [CrossRef]
- Di Leone, A.; Fragomeni, S.M.; Scardina, L.; Ionta, L.; Mule, A.; Magno, S.; Terribile, D.; Masetti, R.; Franceschini, G. Androgen receptor expression and outcome of neoadjuvant chemotherapy in triple-negative breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1910–1915. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Wang, S.; Zeng, K.; Wei, S.; Sun, N.; Sun, G.; Wang, M.; Zou, R.; Liu, W.; et al. PRPF6 promotes androgen receptor/androgen receptor-variant 7 actions in castration-resistant prostate cancer cells. Int. J. Biol. Sci. 2021, 17, 188–203. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, X.; Zheng, L.; Hu, X.; Sun, L.; Zhu, X. The role of the androgen receptor in ovarian cancer carcinogenesis and its clinical implications. Oncotarget 2017, 8, 29395–29405. [Google Scholar] [CrossRef]
- Anestis, A.; Zoi, I.; Papavassiliou, A.G.; Karamouzis, M.V. Androgen receptor in breast cancer-clinical and preclinical research insights. Molecules 2020, 25, 358. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Pakdel, F. Molecular pathways of estrogen receptor action. Int. J. Mol. Sci. 2018, 19, 2591. [Google Scholar] [CrossRef]
- Arnal, J.-F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B. Membrane and nuclear estrogen receptor alpha actions: From tissue specificity to medical implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef]
- Ascenzi, P.; Bocedi, A.; Marino, M. Structure–function relationship of estrogen receptor α and β: Impact on human health. Mol. Asp. Med. 2006, 27, 299–402. [Google Scholar] [CrossRef]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Makela, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.r.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.-Å. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Parker, M. Structure and function of estrogen receptors. Vitam. Horm. 1995, 51, 267–287. [Google Scholar] [CrossRef]
- Eyster, K.M. The estrogen receptors: An overview from different perspectives. In Estrogen Receptors; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1366, pp. 1–10. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C. Estrogen receptors and endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Loo, S.Y.; Syn, N.L.; Koh, A.P.; Teng, J.C.; Deivasigamani, A.; Tan, T.Z.; Thike, A.A.; Vali, S.; Kapoor, S.; Wang, X.; et al. Epigenetic derepression converts PPARγ into a druggable target in triple-negative and endocrine-resistant breast cancers. Cell Death Discov. 2021, 7, 265. [Google Scholar] [CrossRef]
- Kirtonia, A.; Gala, K.; Fernandes, S.G.; Pandya, G.; Pandey, A.K.; Sethi, G.; Khattar, E.; Garg, M. Repurposing of drugs: An attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 2021, 68, 258–278. [Google Scholar] [CrossRef]
- Chen, G.G.; Zeng, Q.; Tse, G.M. Estrogen and its receptors in cancer. Med. Res. Rev. 2008, 28, 954–974. [Google Scholar] [CrossRef]
- Kalita, D.; Bannoth, S.; Purkayastha, J.; Talukdar, A.; Das, G.; Singh, P. A study of hormonal receptors in esophageal carcinoma: Northeast indian tertiary cancer center study. South Asian J. Cancer 2020, 9, 222–226. [Google Scholar] [CrossRef]
- Hou, Y.; Fan, W.; Yang, W.; Samdani, A.Q.; Jackson, A.O.; Qu, S. Farnesoid X receptor: An important factor in blood glucose regulation. Clin. Chim. Acta 2019, 495, 29–34. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Feng, Q.; Chen, W.-D.; Wang, Y.-D. Farnesoid X receptor: A potential therapeutic target in multiple organs. Histol. Histopathol. 2021, 35, 1403–1414. [Google Scholar] [CrossRef]
- Massafra, V.; van Mil, S. FXR: A “homeostat” for hepatic nutrient metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1864, 45–59. [Google Scholar] [CrossRef]
- Han, C.Y. Update on FXR biology: Promising therapeutic target? Int. J. Mol. Sci. 2018, 19, 2069. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Q.; Wang, G.; Xu, X.; Hao, H. FXR modulators for enterohepatic and metabolic diseases. Expert Opin. Ther. Pat. 2018, 28, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Gadaleta, R.M.; Moschetta, A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal. 2010, 8, e005. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Goode, E.; Chen, J.; Oro, A.E.; Bradley, D.J.; Perlmann, T.; Noonan, D.J.; Burka, L.T.; McMorris, T.; Lamph, W.W.; et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995, 81, 687–693. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Keaton, M.; Baranova, A.; Liu, S.; Hu, X.; Li, Q.; Cheng, L.; Zhou, P.; Cao, H.; et al. Variants and expression changes in PPAR-encoding genes display no significant association with schizophrenia. Biosci. Rep. 2020, 40, BSR20201083. [Google Scholar] [CrossRef]
- Gee, V.M.; Wong, F.S.; Ramachandran, L.; Sethi, G.; Kumar, A.P.; Yap, C.W. Identification of novel peroxisome proliferator-activated receptor-gamma (PPARγ) agonists using molecular modeling method. J. Comput.-Aided Mol. Des. 2014, 28, 1143–1151. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338. [Google Scholar] [CrossRef]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef]
- Sikka, S.; Chen, L.; Sethi, G.; Kumar, A.P. Targeting PPARγ signaling cascade for the prevention and treatment of prostate cancer. PPAR Res. 2012, 2012, 968040. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Yu, K.; Bayona, W.; Kallen, C.B.; Harding, H.P.; Ravera, C.P.; McMahon, G.; Brown, M.; Lazar, M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995, 270, 23975–23983. [Google Scholar] [CrossRef]
- Altmann, R.; Hausmann, M.; Spottl, T.; Gruber, M.; Bull, A.W.; Menzel, K.; Vogl, D.; Herfarth, H.; Scholmerich, J.; Falk, W.; et al. 13-Oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochem. Pharmacol. 2007, 74, 612–622. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Kar, S.; Kanchi, M.M.; Arora, S.; Kim, J.E.; Koh, P.F.; Yousef, E.; Samy, R.P.; Shanmugam, M.K.; et al. PPARγ ligand-induced annexin A1 expression determines chemotherapy response via deubiquitination of death domain kinase RIP in triple-negative breast cancers. Mol. Cancer Ther. 2017, 16, 2528–2542. [Google Scholar] [CrossRef]
- di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015, 41, 1–115. [Google Scholar] [CrossRef]
- Sun, S.Y.; Lotan, R. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol./Hematol. 2002, 41, 41–55. [Google Scholar] [CrossRef]
- Xu, X.C. Tumor-suppressive activity of retinoic acid receptor-β in cancer. Cancer Lett. 2007, 253, 14–24. [Google Scholar] [CrossRef]
- Alvarez, S.; Bourguet, W.; Gronemeyer, H.; de Lera, A.R. Retinoic acid receptor modulators: A perspective on recent advances and promises. Expert Opin. Ther. Pat. 2011, 21, 55–63. [Google Scholar] [CrossRef]
- Breems-de Ridder, M.C.; Lowenberg, B.; Jansen, J.H. Retinoic acid receptor fusion proteins: Friend or foe. Mol. Cell. Endocrinol. 2000, 165, 1–6. [Google Scholar] [CrossRef]
- Mongan, N.P.; Gudas, L.J. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation 2007, 75, 853–870. [Google Scholar] [CrossRef]
- Altucci, L.; Leibowitz, M.D.; Ogilvie, K.M.; de Lera, A.R.; Gronemeyer, H. RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 2007, 6, 793–810. [Google Scholar] [CrossRef]
- Dominguez, M.; Alvarez, S.; de Lera, A.R. Natural and Structure-based RXR Ligand Scaffolds and Their Functions. Curr. Top. Med. Chem. 2017, 17, 631–662. [Google Scholar] [CrossRef]
- Garcia, P.; Lorenzo, P.; de Lera, A.R. Natural ligands of RXR receptors. Methods Enzymol. 2020, 637, 209–234. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and vitamin D receptor: New insights in the treatment of hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. The function of vitamin D receptor in vitamin D action. J. Biochem. 2000, 127, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Mutchie, T.R.; Yu, O.B.; Di Milo, E.S.; Arnold, L.A. Alternative binding sites at the vitamin D receptor and their ligands. Mol. Cell. Endocrinol. 2019, 485, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W. Vitamin D3 receptors: Structure and function in transcription. Annu. Rev. Nutr. 1991, 11, 189–216. [Google Scholar] [CrossRef]
- Yang, S.; Li, A.; Wang, J.; Liu, J.; Han, Y.; Zhang, W.; Li, Y.C.; Zhang, H. Vitamin D receptor: A novel therapeutic target for kidney diseases. Curr. Med. Chem. 2018, 25, 3256–3271. [Google Scholar] [CrossRef]
- Oladimeji, P.O.; Chen, T. PXR: More than just a master xenobiotic receptor. Mol. Pharmacol. 2018, 93, 119–127. [Google Scholar] [CrossRef]
- Pondugula, S.R.; Mani, S. Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response. Cancer Lett. 2013, 328, 1–9. [Google Scholar] [CrossRef]
- Xing, Y.; Yan, J.; Niu, Y. PXR: A center of transcriptional regulation in cancer. Acta Pharm. Sin. B 2020, 10, 197–206. [Google Scholar] [CrossRef]
- Noreault, T.L.; Kostrubsky, V.E.; Wood, S.G.; Nichols, R.C.; Strom, S.C.; Trask, H.W.; Wrighton, S.A.; Evans, R.M.; Jacobs, J.M.; Sinclair, P.R. Arsenite decreases CYP3A4 and RXRα in primary human hepatocytes. Drug Metab. Dispos. 2005, 33, 993–1003. [Google Scholar] [CrossRef]
- Ding, D.; Han, S.; Zhang, H.; He, Y.; Li, Y. Predictive biomarkers of colorectal cancer. Comput. Biol. Chem. 2019, 83, 107106. [Google Scholar] [CrossRef]
- Jin, L.; Li, Y. Structural and functional insights into nuclear receptor signaling. Adv. Drug Deliv. Rev. 2010, 62, 1218–1226. [Google Scholar] [CrossRef]
- Guo, M.; Peng, Y.; Gao, A.; Du, C.; Herman, J.G. Epigenetic heterogeneity in cancer. Biomark. Res. 2019, 7, 23. [Google Scholar] [CrossRef]
- Kaypee, S.; Sudarshan, D.; Shanmugam, M.K.; Mukherjee, D.; Sethi, G.; Kundu, T.K. Aberrant lysine acetylation in tumorigenesis: Implications in the development of therapeutics. Pharmacol. Ther. 2016, 162, 98–119. [Google Scholar] [CrossRef]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Sethi, G. Role of epigenetics in inflammation-associated diseases. In Epigenetics: Development and Disease; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2013; Volume 61, pp. 627–657. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Arfuso, F.; Arumugam, S.; Chinnathambi, A.; Jinsong, B.; Warrier, S.; Wang, L.Z.; Kumar, A.P.; Ahn, K.S.; Sethi, G.; et al. Role of novel histone modifications in cancer. Oncotarget 2018, 9, 11414–11426. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.Y.; Xin, H.W.; Wang, L.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Wang, H.; Tang, F.R.; Warrier, S.; et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 108, 17–20. [Google Scholar] [CrossRef]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef]
- Kit, O.I.; Vodolazhskiy, D.I.; Kolesnikov, E.N.; Timoshkina, N.N. [Epigenetic markers of esophageal cancer: DNA methylation]. Biomed. Khim. 2016, 62, 520–526. [Google Scholar] [CrossRef]
- Kaz, A.M.; Grady, W.M. Epigenetic biomarkers in esophageal cancer. Cancer Lett. 2014, 342, 193–199. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Guo, M. DNA N6-methyladenine increased in human esophageal squamous cell carcinoma. Discov. Med. 2020, 29, 85–90. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).