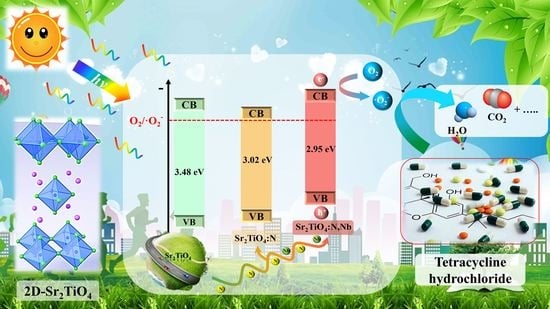
Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
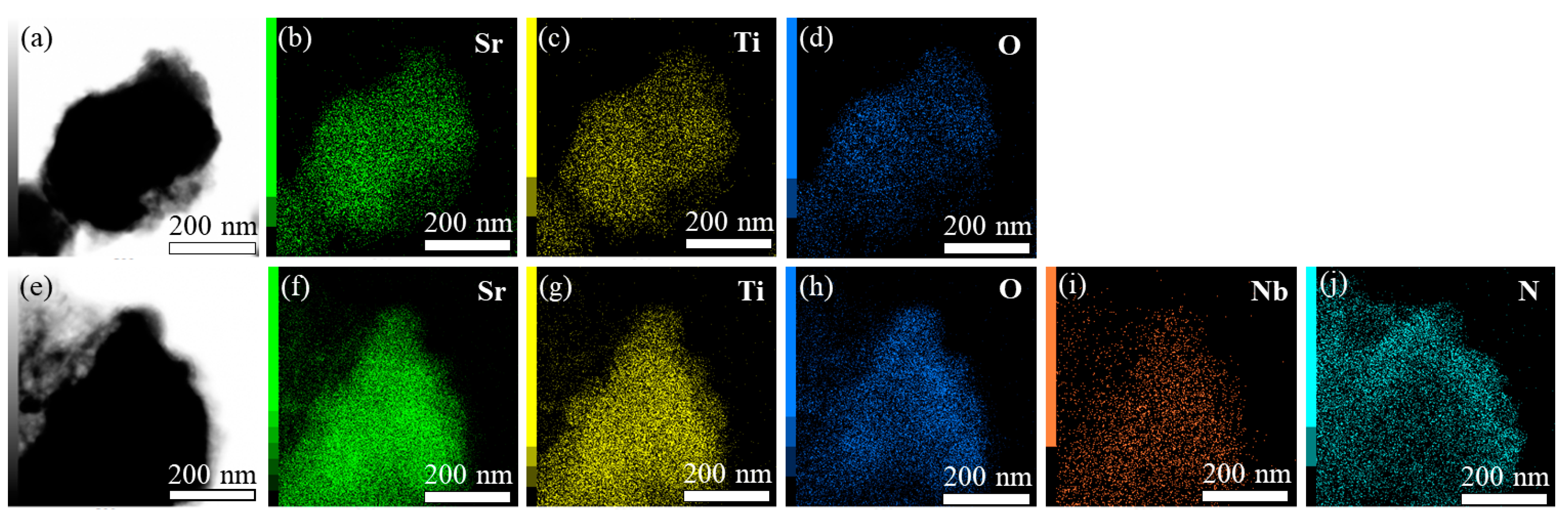
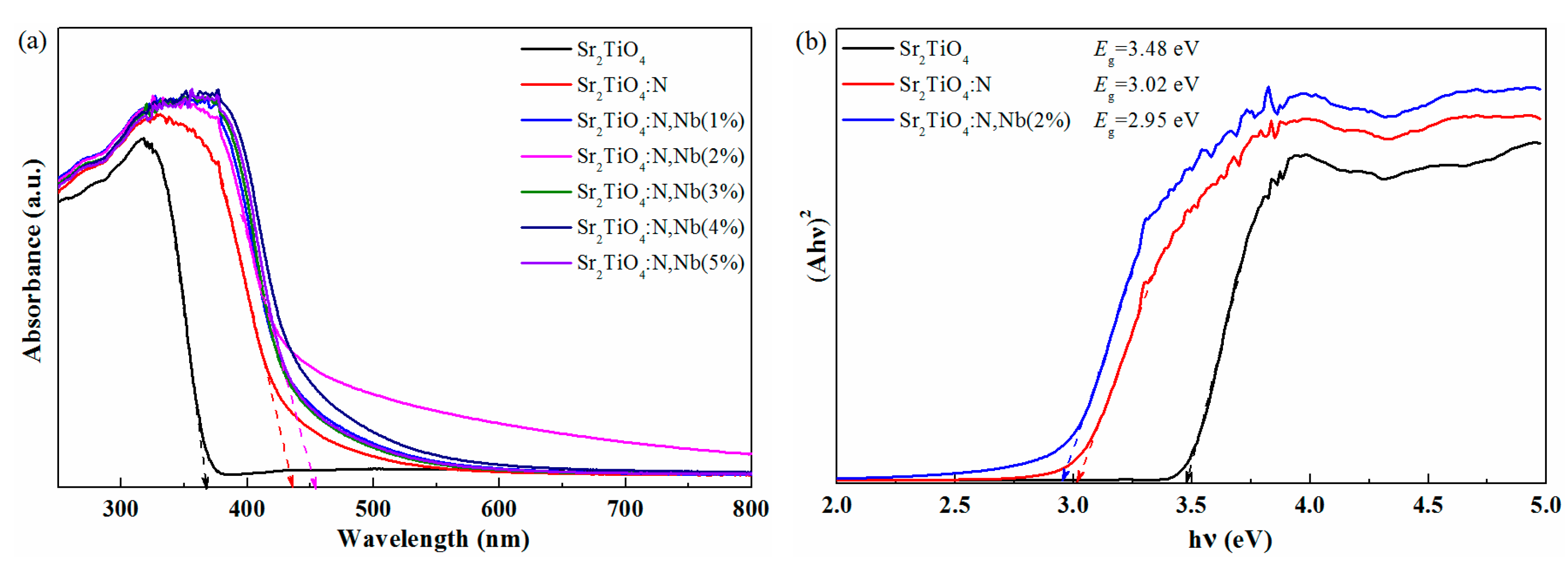
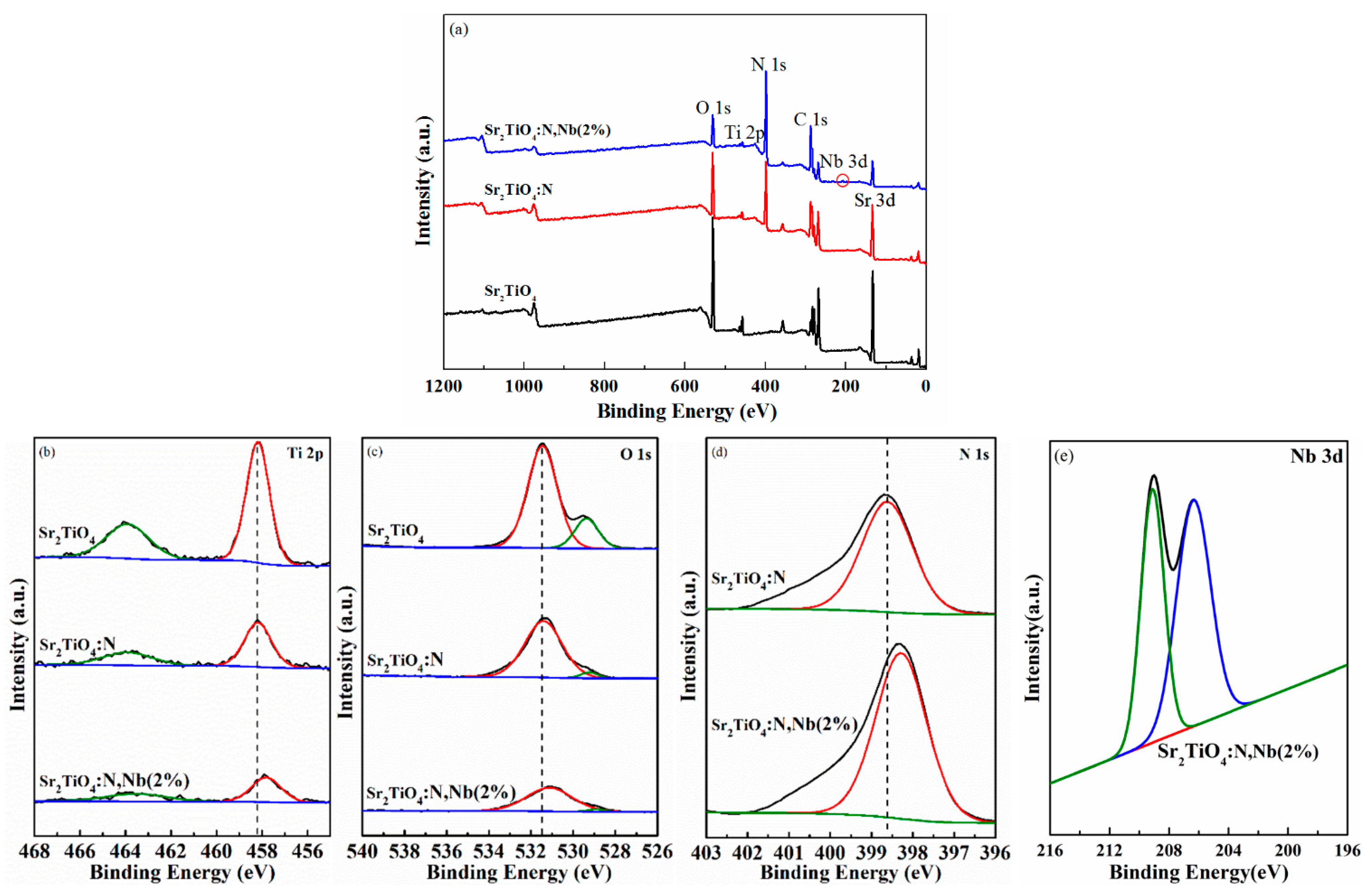
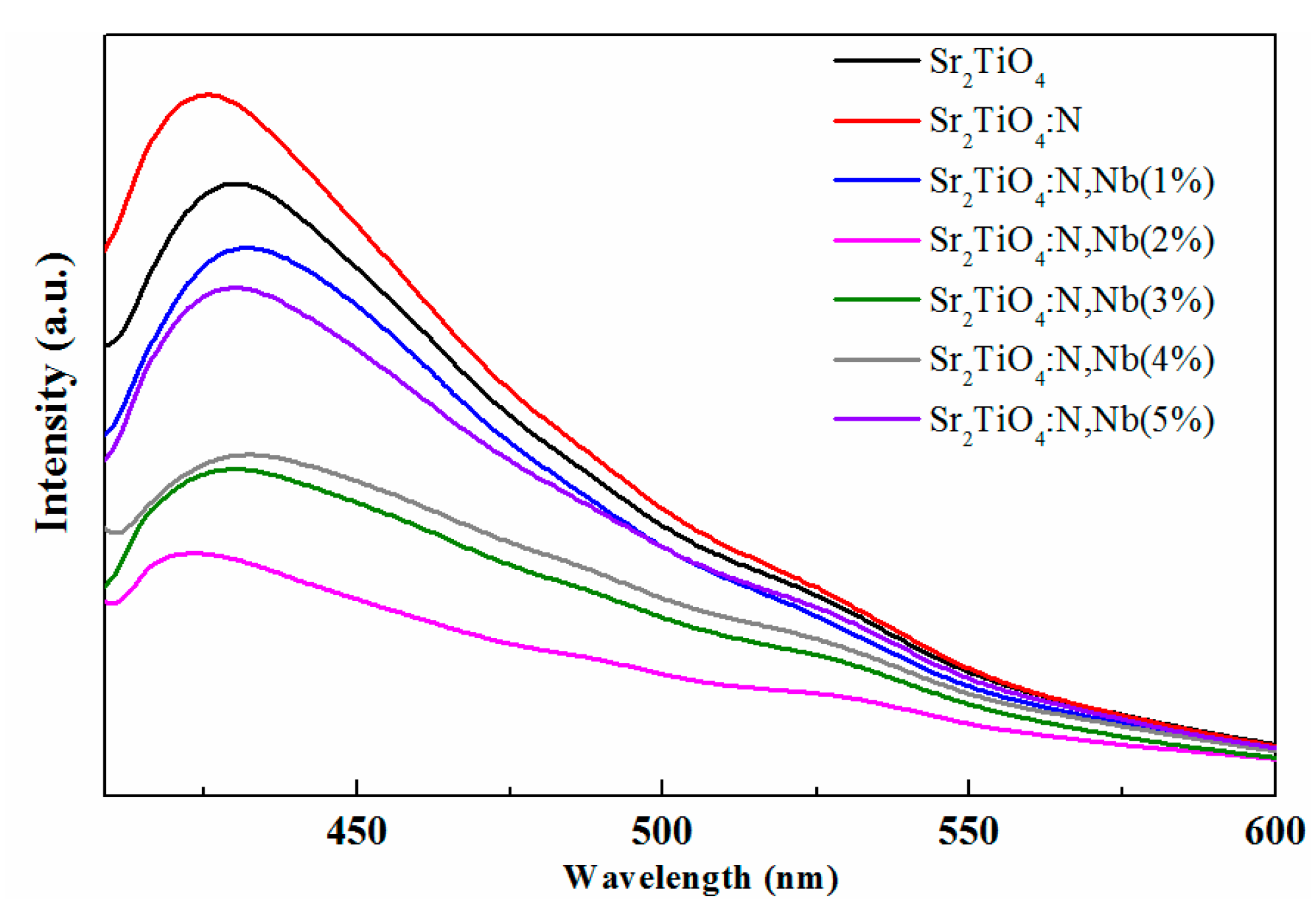
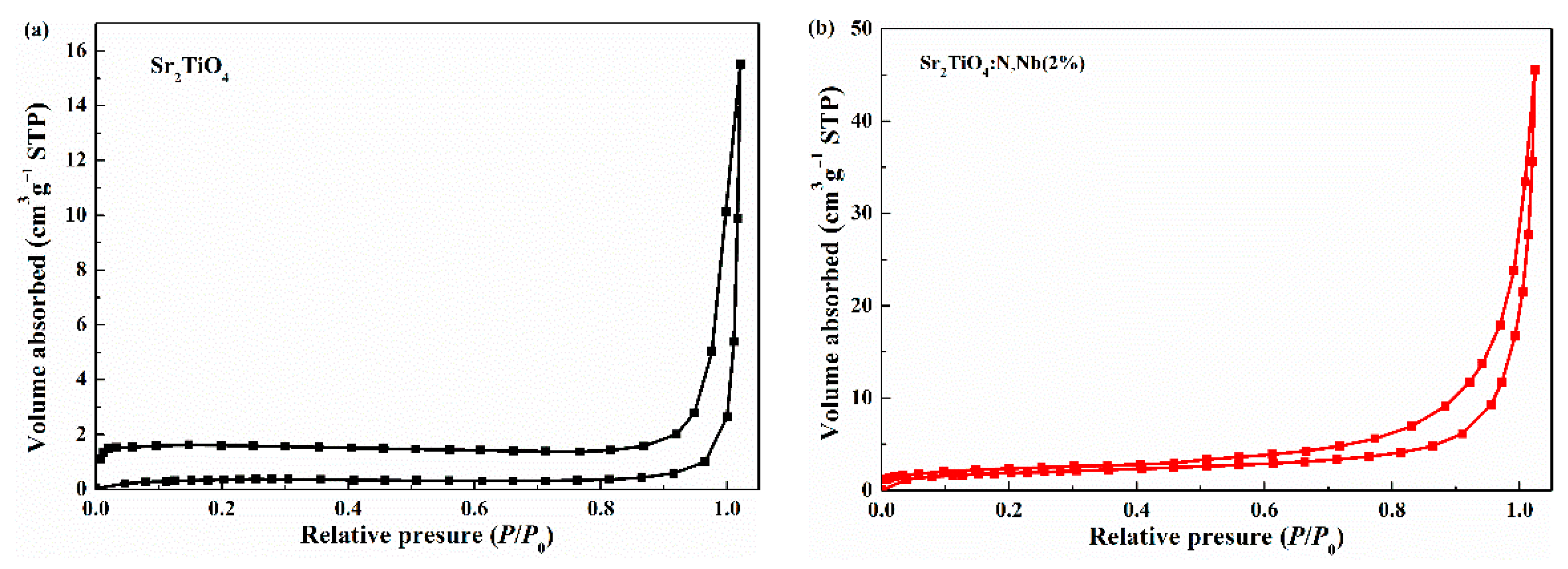
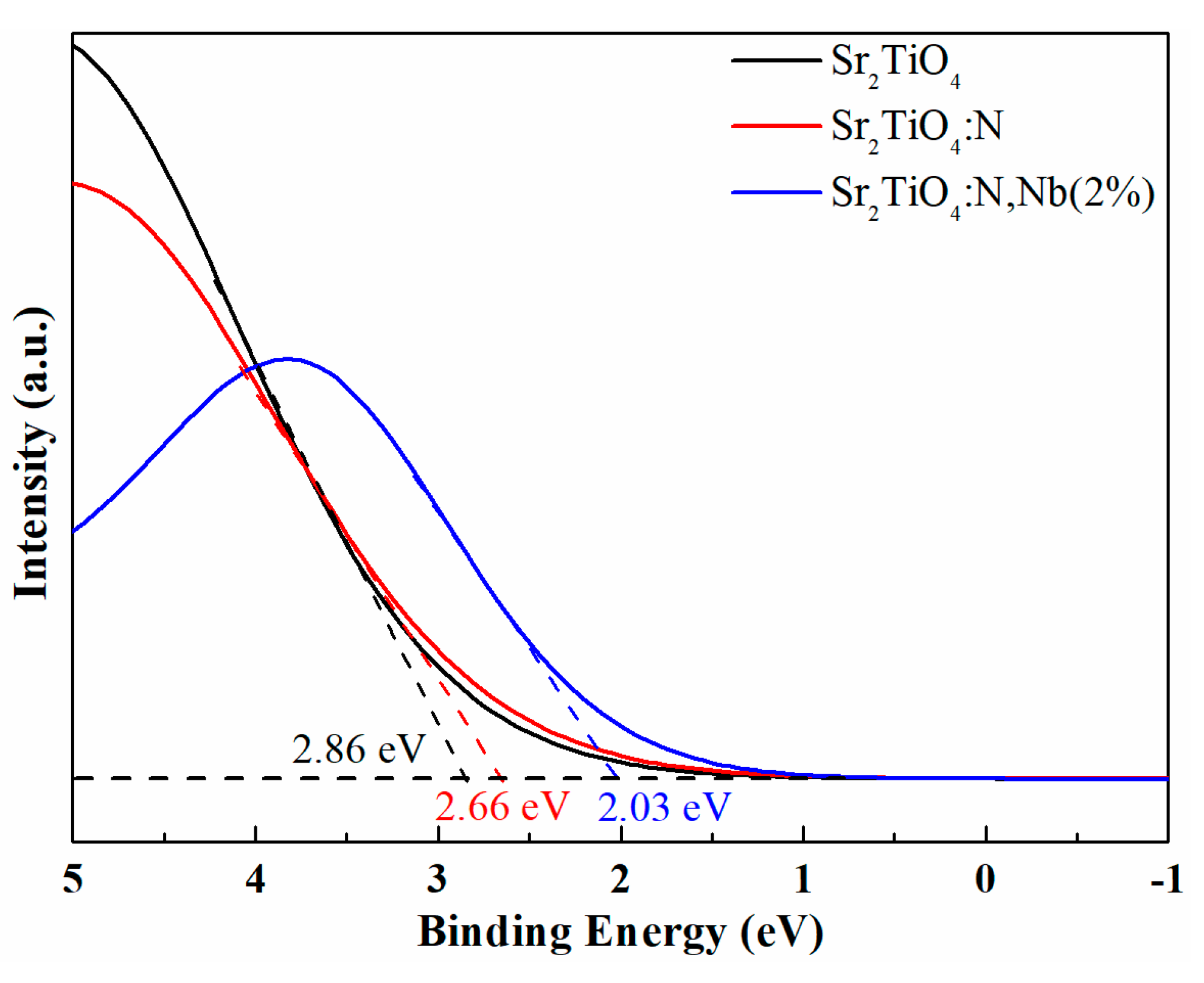
2.1. Materials Characterization
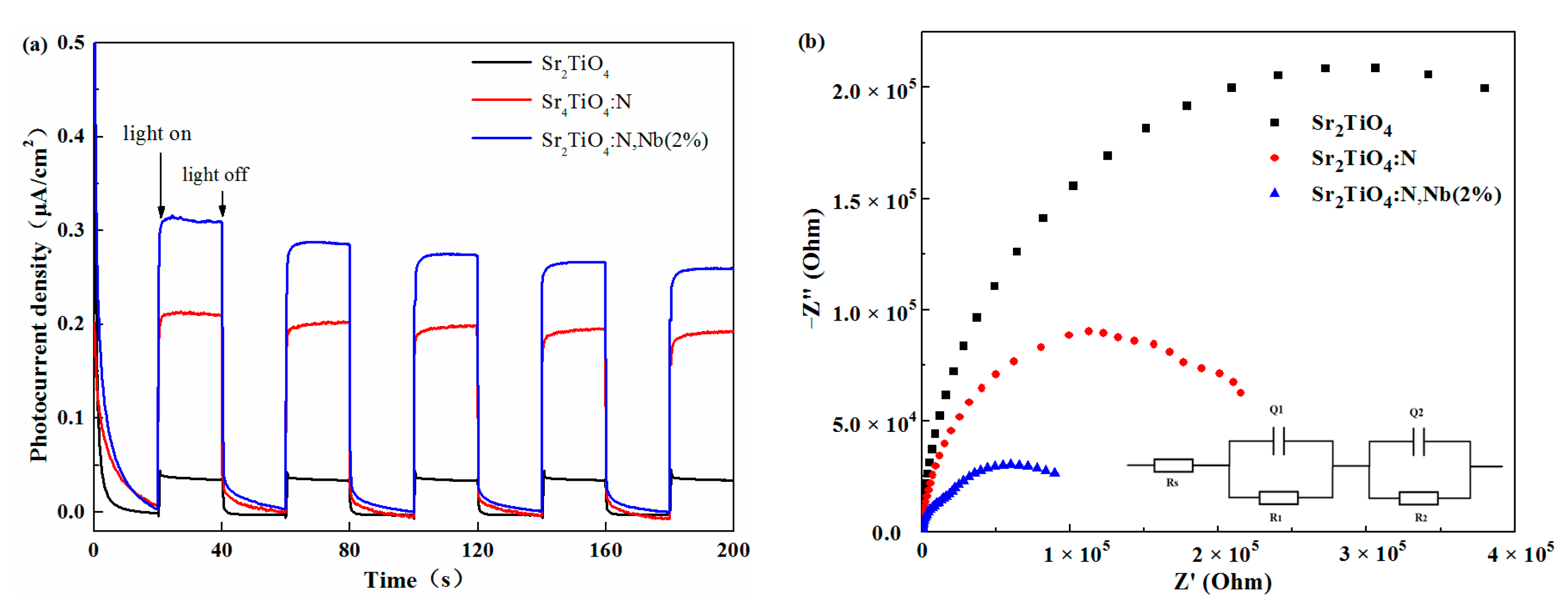
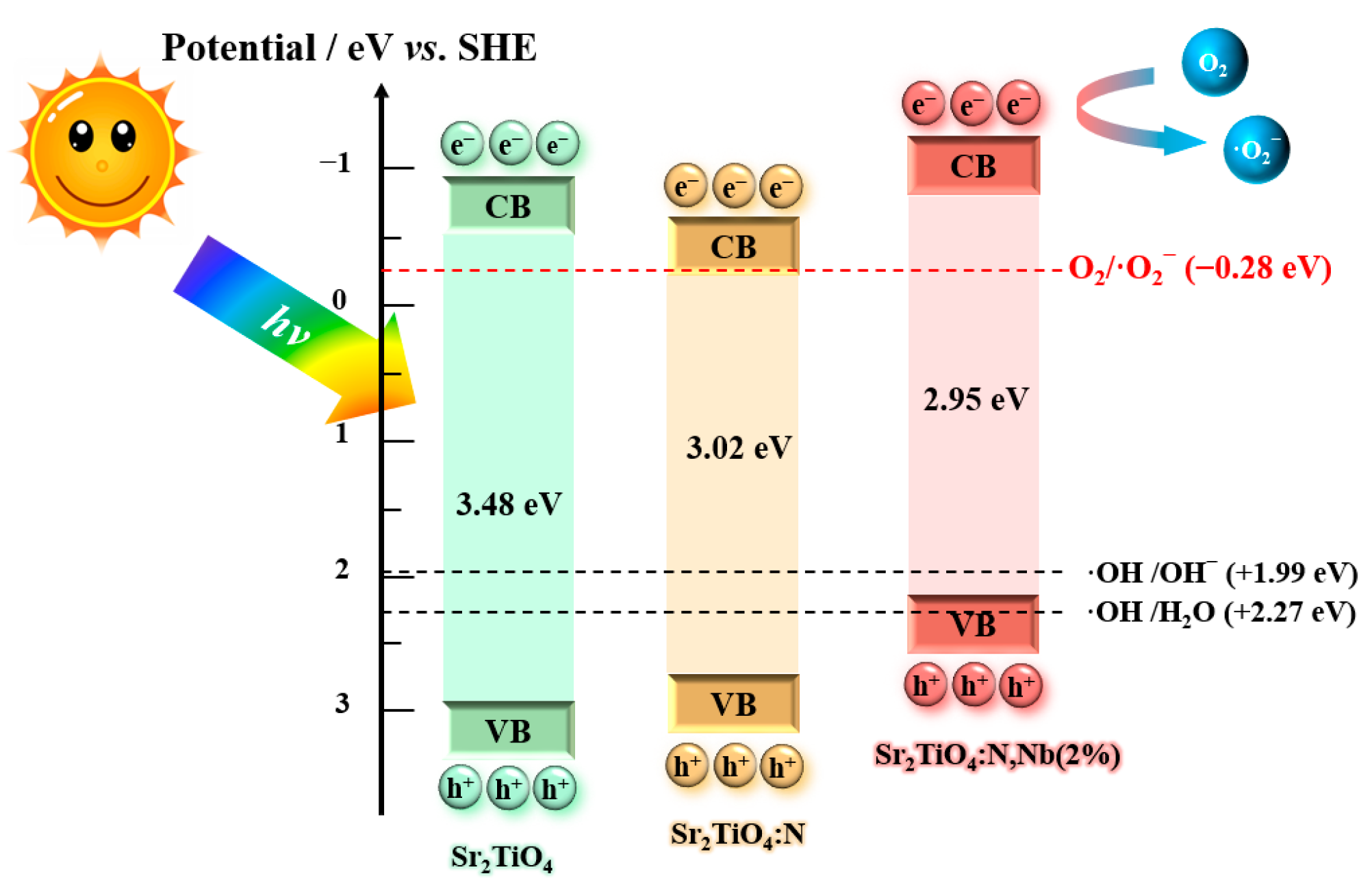
2.2. Photoelectrochemical Properties of Sr2TiO4:N,Nb
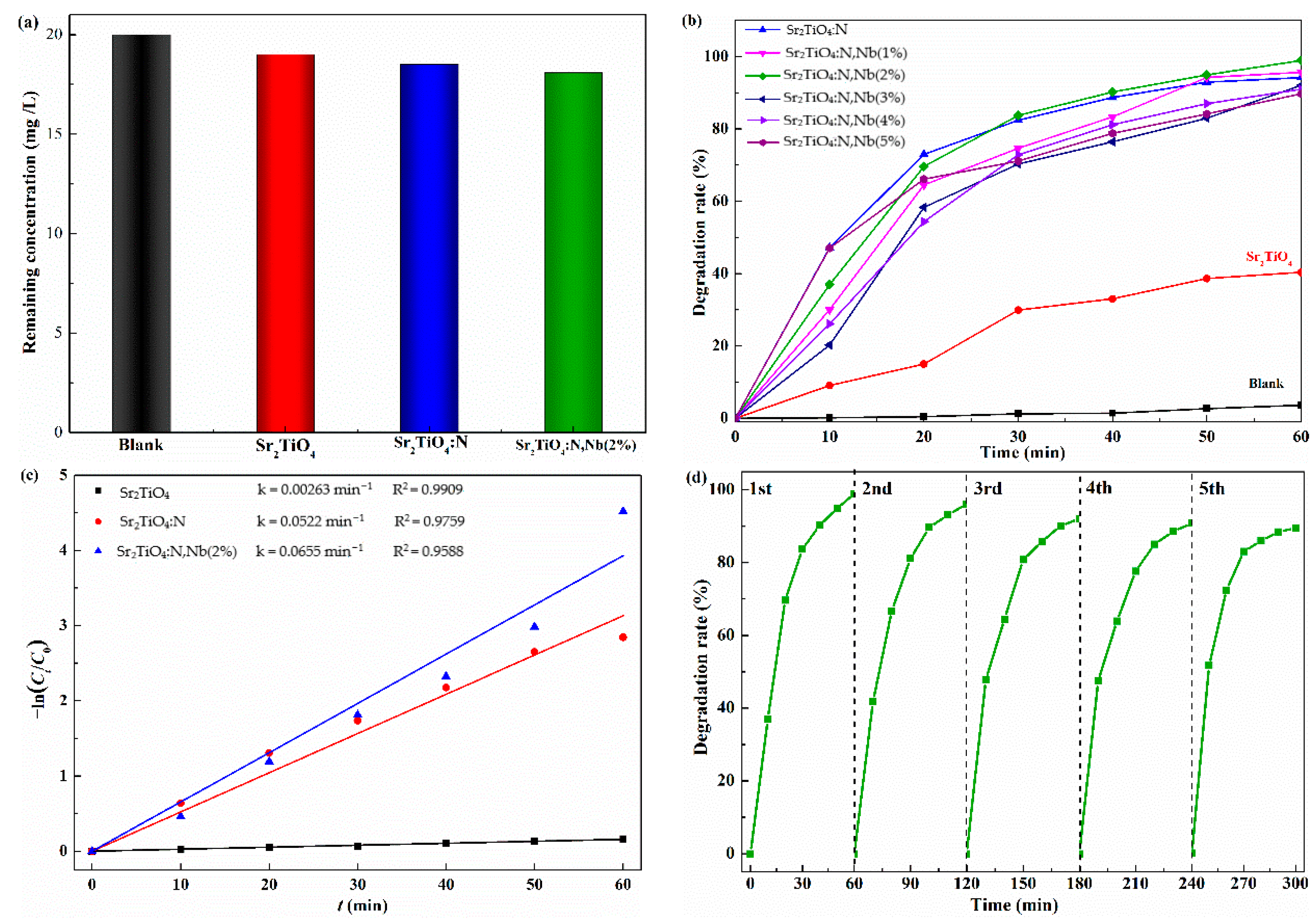
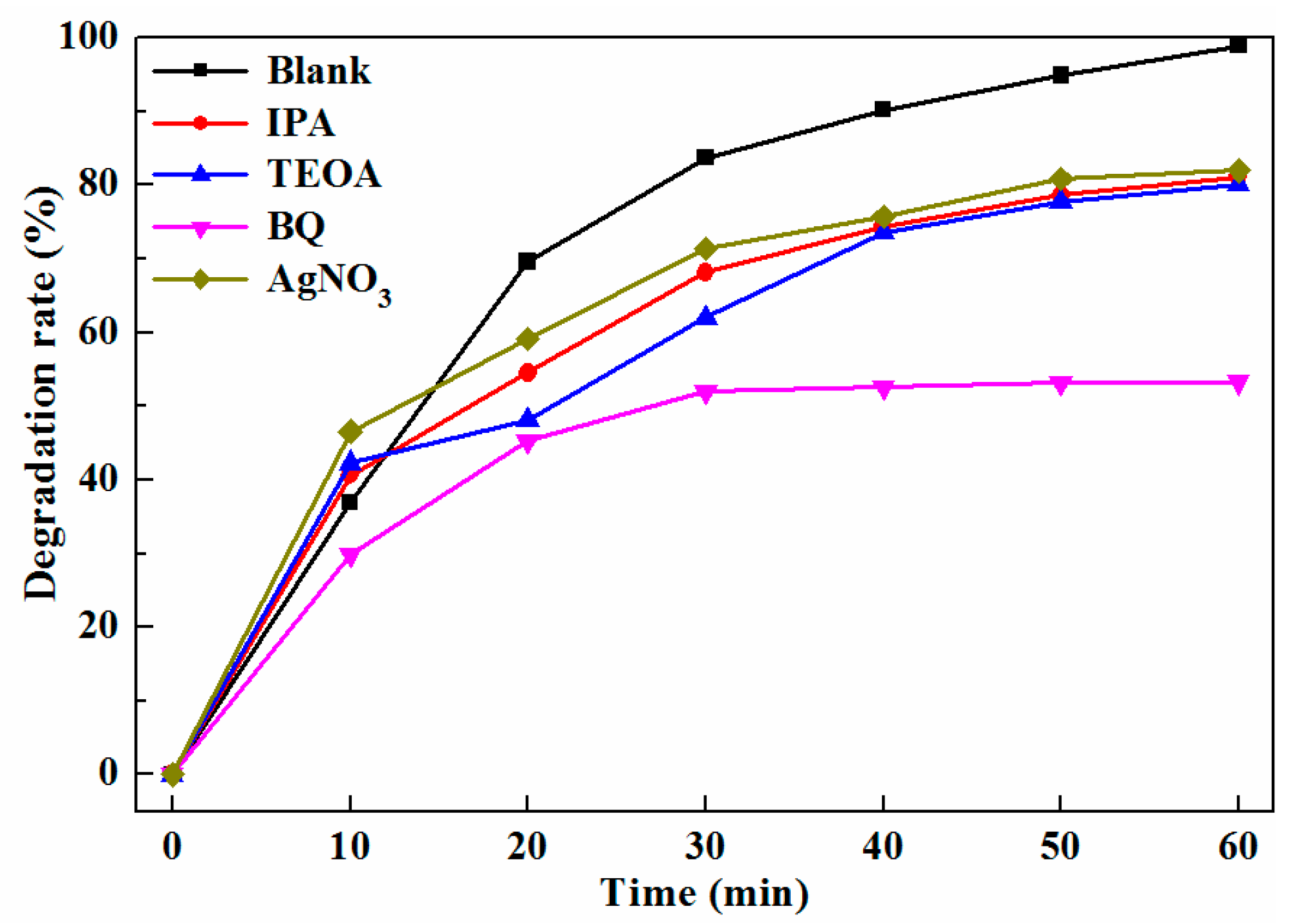
2.3. Photocatalytic Degradation of TC
2.4. Improvement Mechanism of Photocatalytic Performance
3. Materials and Methods
3.1. Chemicals
3.2. Materials Synthesis
3.2.1. Synthesis of Sr2TiO4
3.2.2. Synthesis of N-Doped Sr2TiO4
3.2.3. Synthesis of Nb/N Co-Doped Sr2TiO4
3.3. Characterization
3.4. Photoelectrochemical Measurements
3.5. Photocatalytic Degradation of TC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammad, A.; Khan, M.E.; Cho, M.H.; Yoon, T. Adsorption promoted visible-light-induced photocatalytic degradation of antibiotic tetracycline by tin oxide/cerium oxide nanocomposite. Appl. Surf. Sci. 2021, 565, 150337. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Bao, D.Y.; Li, J.H.; Jiang, X.Q.; Sun, X.S. Construction of Au modified direct Z-scheme g-C3N4/defective ZnO heterostructure with stable high-performance for tetracycline degradation. Appl. Surf. Sci. 2021, 555, 149696. [Google Scholar] [CrossRef]
- Li, Y.H.; Lai, Z.; Huang, Z.J.; Wang, H.Y.; Zhao, C.X.; Ruan, G.H.; Du, F.Y. Fabrication of BiOBr/MoS2/graphene oxide composites for efficient adsorption and photocatalytic removal of tetracycline antibiotics. Appl. Surf. Sci. 2021, 550, 149342. [Google Scholar] [CrossRef]
- Guo, Z.W.; Zheng, J.; Li, B.R.; Da, Z.L.; Meng, M.J. Fabrication of mixed matrix membranes blending with the TiO2/Bi3O4Cl 2D/2D heterojunction for photocatalytic degradation of tetracycline. Appl. Surf. Sci. 2022, 574, 151549. [Google Scholar] [CrossRef]
- Dong, X.; Xu, J.; Kong, C.; Zeng, X.; Wang, J.; Zhao, Y.; Zhang, W. Synthesis of β-FeOOH/TiO2/SiO2 by melting phase separation-hydrothermal method to improve photocatalytic performance. Ceram. Int. 2021, 47, 32303–32309. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.; Sun, C.; Cui, Y.; Liu, L.; Zhao, H.; Liang, B. Facile assembly of BiVO4/protonated g-C3N4/AgI with a novel dual Z-scheme mechanism for visible-light photocatalytic degradation of Rhodamine B. J. Mater. Sci. 2021, 56, 1328–1346. [Google Scholar] [CrossRef]
- Bae, H.S.; Manikandan, V.; Hwang, J.H.; Seo, Y.S.; Chung, H.S.; Ryu, H.I.; Chae, W.S.; Cho, M.; Ekambe, P.S.; Jang, J.S. Photocatalytic degradation of organic pollutants and inactivation of pathogens under visible light via CoOx surface-modified Rh/Sb-doped SrTiO3 nanocube. J. Mater. Sci. 2021, 56, 17235–17253. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, T.; Fan, W.; Xu, L. Recyclable visible-light photocatalytic composite materials based on tubular Au/TiO2/SiO2 ternary nanocomposites for removal of organic pollutants from water. Compos. Commun. 2022, 32, 101154. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, Z.; Yi, X.; Chen, S.; Li, B.; Luo, W. Porous polyimide/carbon quantum dots/ZnS quantum dots material aerogel for efficient visible-light photocatalytic degradation over oxytetracycline. React. Funct. Polym. 2022, 178, 105330. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.; Zaman, M.; Aslam, M.Z.; Kousar, N.; Asim, M.; Rafique, M.; Siraj, K.; Tabish, A.N.; Usman, M.; et al. Photocatalysis and perovskite oxide-based materials: A remedy for a clean and sustainable future. RSC Adv. 2022, 12, 7009–7039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ni, S.; Mi, Y.; Xu, X. Ruddlesden-Popper compound Sr2TiO4 co-doped with La and Fe for efficient photocatalytic hydrogen production. J. Catal. 2018, 359, 112–121. [Google Scholar] [CrossRef]
- Sun, X.; Mi, Y.; Jiao, F.; Xu, X. Activating layered perovskite compound Sr2TiO4 via La/N codoping for visible light photocatalytic water splitting. ACS Catal. 2018, 8, 3209–3221. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Y.; Wu, F.; Chen, H.; Lv, M.; Ni, S.; Liu, G.; Xu, X. Photocatalytic hydrogen production over chromium doped layered perovskite Sr2TiO4. Inorg. Chem. 2015, 54, 7445–7453. [Google Scholar] [CrossRef]
- Xiao, H.B.; Liu, P.Y.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z.P. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy 2022, 23, 100899. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.Y.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z.P. Non-metal fluorine doping in Ruddlesden-Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Ziati, M.; Bekkioui, N.; Ez-Zahraouy, H. Ruddlesden-Popper compound Sr2TiO4 doped with chalcogens for optoelectronic applications: Insights from first-principle calculations. Chem. Phys. 2021, 548, 111221. [Google Scholar] [CrossRef]
- Yu, J.X.; Xu, X.X. Fluorination over Cr doped layered perovskite Sr2TiO4 for efficient photocatalytic hydrogen production under visible light illumination. J. Energy Chem. 2020, 51, 30–38. [Google Scholar] [CrossRef]
- Sun, X.; Xu, X. Efficient photocatalytic hydrogen production over La/Rh co-doped Ruddlesden-Popper compound Sr2TiO4. Appl. Catal. B-Environ. 2017, 210, 149–159. [Google Scholar] [CrossRef]
- Miyauchi, M.; Takashio, M.; Tobimatsu, H. Photocatalytic activity of SrTiO3 codoped with nitrogen and lanthanum under visible light illumination. Langmuir 2004, 20, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Rumaiz, A.K.; Woicik, J.C.; Cockayne, E.; Lin, H.Y.; Jaffari, G.H.; Shah, S.I. Oxygen vacancies in N doped anatase TiO2: Experiment and first-principles calculations. Appl. Phys. Lett. 2009, 95, 262111. [Google Scholar] [CrossRef]
- Corby, S.; Francàs, L.; Kafizas, A.; Durrant, J.R. Determining the role of oxygen vacancies in the photoelectrocatalytic performance of WO3 for water oxidation. Chem. Sci. 2020, 11, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Irshad, M.; Idrees, R.; Siraj, K.; Shakir, I.; Rafique, M.; Ain, Q.; Raza, R. Electrochemical evaluation of mixed ionic electronic perovskite cathode LaNi1-xCoxO3-δ for IT-SOFC synthesized by high temperature decomposition. Int. J. Hydrogen Energy 2021, 46, 10448–10456. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.; Siraj, K.; Raza, R.; Tabish, A.N.; Rafique, M.; Idrees, R.; Khan, F.; Majeed, S.; Ahsan, M. Evaluation of BaZr0.8X0.2 (X= Y, Gd, Sm) proton conducting electrolytes sintered at low temperature for IT-SOFC synthesized by cost effective combustion method. J. Alloys Compd. 2020, 815, 152389. [Google Scholar] [CrossRef]
- Cottineau, T.; Béalu, N.; Gross, P.A.; Pronkin, S.N.; Keller, N.; Savinova, E.R.; Keller, V. One step synthesis of niobium doped titania nanotube arrays to form (N,Nb) co-doped TiO2 with high visible light photoelectrochemical activity. J. Mater. Chem. A 2013, 1, 2151–2160. [Google Scholar] [CrossRef]
- Zou, F.; Jiang, Z.; Qin, X.; Zhao, Y.; Jiang, L.; Zhi, J.; Xiao, T.; Edwards, P.P. Template-free synthesis of mesoporous N-doped SrTiO3 perovskite with high visible-light-driven photocatalytic activity. Chem. Commun. 2012, 48, 8514–8516. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Sun, X.; Lv, M.; Ni, S. Layered perovskite compound NaLaTiO4 modified by nitrogen doping as a visible light active photocatalyst for water splitting. ACS Catal. 2020, 10, 9889–9898. [Google Scholar] [CrossRef]
- Atkinson, I.; Parvulescu, V.; Pandele Cusu, J.; Anghel, E.M.; Voicescu, M.; Culita, D.; Somacescu, S.; Munteanu, C.; Šćepanović, M.; Popovic, Z.V.; et al. Influence of preparation method and nitrogen (N) doping on properties and photo-catalytic activity of mesoporous SrTiO3. J. Photoch. Photobiol. A 2019, 368, 41–51. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Shen, C.; Xu, X. Efficient photocatalytic hydrogen production over Rh-doped inverse spinel Zn2TiO4. ChemCatChem 2016, 8, 2289–2295. [Google Scholar] [CrossRef]
- Nachaithong, T.; Moontragoon, P.; Chanlek, N.; Thongbai, P. Fe3+/Nb5+co-doped rutile–TiO2 nanocrystalline powders prepared by a combustion process: Preparation and characterization and their giant dielectric response. RSC Adv. 2020, 10, 24784–24794. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Iyo, A.; Sekita, Y.; Ishibashi, S.; Ihara, H. In-situ characterization of doping-effect on electronic structure of epitaxial films of infinite layer SrCuO2. Czech. J. Phys. 1996, 46, 2683–2684. [Google Scholar] [CrossRef]
- Salma, A.; Thoroeboveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Liu, D.; Tian, J.; Wang, W.; Ni, J.; Wang, X. Visible-light-excited humic acid for peroxymonosulfate activation to degrade bisphenol A. Chem. Eng. J. 2020, 400, 125853. [Google Scholar] [CrossRef]
- Sorkh-Kaman-Zadeh, A.; Dashtbozorg, A. Facile chemical synthesis of nanosize structure of Sr2TiO4 for degradation of toxic dyes from aqueous solution. J. Mol. Liq. 2016, 223, 921–926. [Google Scholar] [CrossRef]
- Hu, C.; Chen, T.S.; Huang, H.X. Heterojunction of n-type Sr2TiO4 with p-type Bi5O7I with enhanced photocatalytic activity under irradiation of simulated sunlight. Appl. Surf. Sci. 2017, 426, 536–544. [Google Scholar] [CrossRef]
- Zhang, W.J.; Sun, X.; Yang, B. Sol-gel preparation of La doped strontium titanate. Asian J. Chem. 2013, 25, 3263–3266. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef]
- Kwak, B.S.; Do, J.Y.; Park, N.K.; Kang, M. Surface modification of layered perovskite Sr2TiO4 for improved CO2 photoreduction with H2O to CH4. Sci. Rep. 2017, 7, 16370. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Bi, Y.; Jin, J.; Ehsan, M.F.; Fu, M.; He, T. Preparation of 2D hydroxyl-rich carbon nitride nanosheets for photocatalytic reduction of CO2. RSC Adv. 2015, 5, 33254–33261. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, S.; Yang, Y.; Qin, H.; Ni, Z.; Yang, S.; Li, X. Facile synthesis of Bi/Bi2WO6 nanocomposite with enhanced photocatalytic activity under visible light. Appl. Catal. B-Environ. 2016, 196, 89–99. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]


| Doping Elements | A-Site/B-Site or O-Site Doping | Doping Content | Eg (eV) | References |
|---|---|---|---|---|
| Cr | B | 5 at% | ~1.5 | [15] |
| Ag | A | 2.5 at% | 3.05 | [16] |
| F | O | 3 at% | 3.20 | [17] |
| Chalcogens (S, Se, Te) | O | -- | 0~2.99 | [18] |
| La/N | A/O | 10 at%/- | 2.2 | [13] |
| Cr/F | B/O | 5 at%/40 at % | ~2.5 | [19] |
| La/Rh | A/B | 1.5 at%/3 at % | 2.43 | [20] |
| La/Fe | A/B | 1.5 at%/3 at % | ~2.75 | [12] |
| Sr2TiO4 | Sr2TiO4:N | Sr2TiO4:N,Nb(1%) | Sr2TiO4:N,Nb(2%) | Sr2TiO4:N,Nb(3%) | Sr2TiO4:N,Nb(4%) | Sr2TiO4:N,Nb(5%) | |
|---|---|---|---|---|---|---|---|
| Average crystallite size | 43 nm | 65 nm | 64 nm | 55 nm | 68 nm | 71 nm | 61 nm |
| Samples | Doping Elements | Organic Pollutants | Degradation Rates | Irradiation System | References |
|---|---|---|---|---|---|
| Sr2TiO4 | —— | methylene orange | 32% (120 min) | UV lamp (400 W Osram lamps) | [36] |
| Sr2TiO4 | —— | methylene orange | 3.2% (180 min) | Xenon lamp (300 W without filter) | [37] |
| Sr2TiO4 | —— | methylene orange | 78% (120 min) | UV lamp (λ = 253.7 nm, 2200 mW·cm−2) | [38] |
| Sr2TiO4 | La | methylene orange | 90.5% (120 min) | UV lamp (λ = 253.7 nm, 2200 mW·cm−2) | [38] |
| Sr2TiO4 | —— | tetracycline | 40% (60 min) | LED lamp (λ > 420 nm) | This work |
| Sr2TiO4 | Nb/N | tetracycline | 99% (60 min) | LED lamp (λ > 420 nm) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, P.; Zhao, Y.; Zeng, X. Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light. Int. J. Mol. Sci. 2022, 23, 10927. https://doi.org/10.3390/ijms231810927
Wang J, Li P, Zhao Y, Zeng X. Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light. International Journal of Molecular Sciences. 2022; 23(18):10927. https://doi.org/10.3390/ijms231810927
Chicago/Turabian StyleWang, Jiansheng, Pengwei Li, Yingna Zhao, and Xiongfeng Zeng. 2022. "Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light" International Journal of Molecular Sciences 23, no. 18: 10927. https://doi.org/10.3390/ijms231810927
APA StyleWang, J., Li, P., Zhao, Y., & Zeng, X. (2022). Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light. International Journal of Molecular Sciences, 23(18), 10927. https://doi.org/10.3390/ijms231810927