Steroidogenic Effects of Salinity Change on the Hypothalamus–Pituitary–Gonad (HPG) Axis of Male Chinese Sea Bass (Lateolabrax maculatus)
Abstract
1. Introduction
2. Results and Discussion
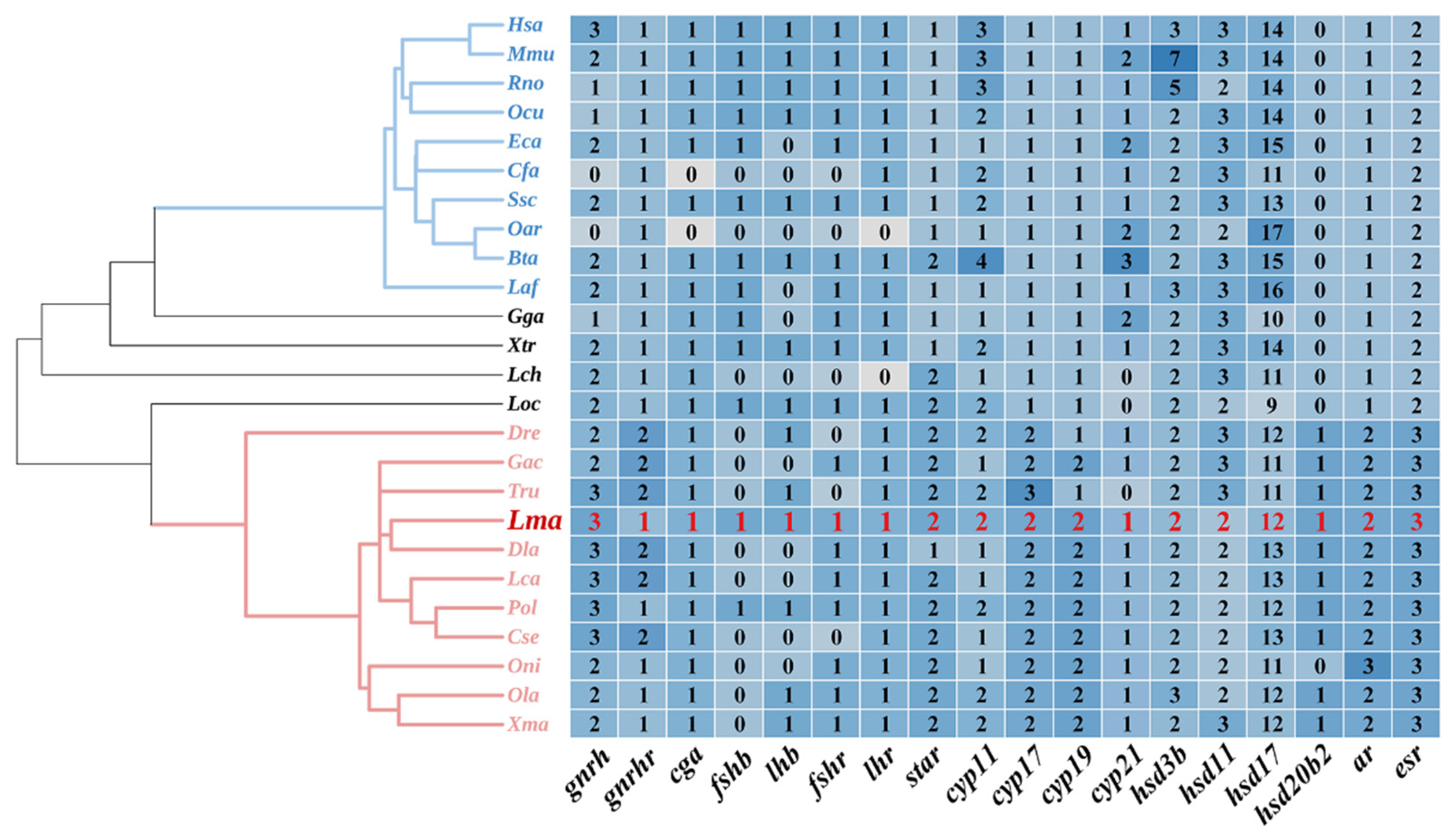
2.1. Genomic Landscape of HPG Axis Genes in L. maculatus
2.2. Phylogeny of HPG Axis Genes among Selected Vertebrates
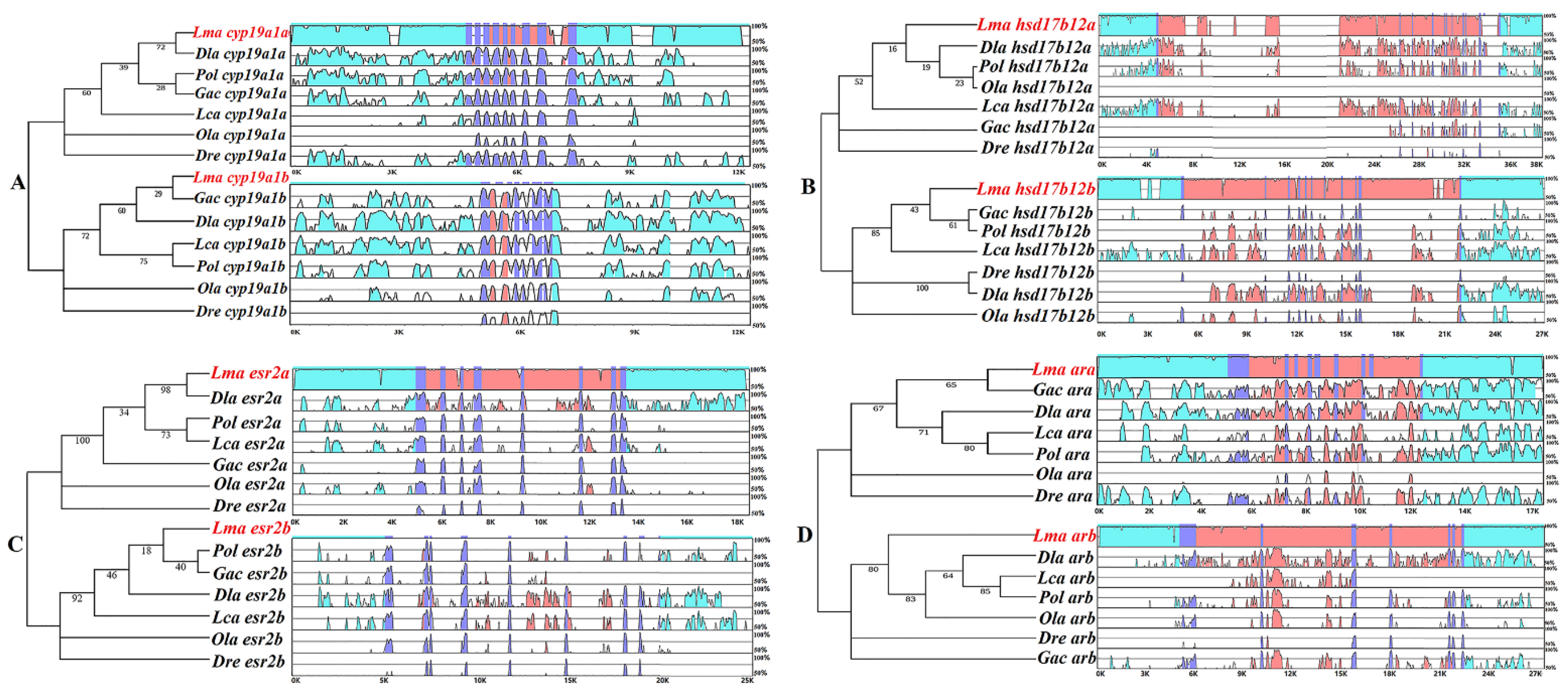
2.3. Conserved Sequence Structures of HPG Axis Genes among Teleost Species
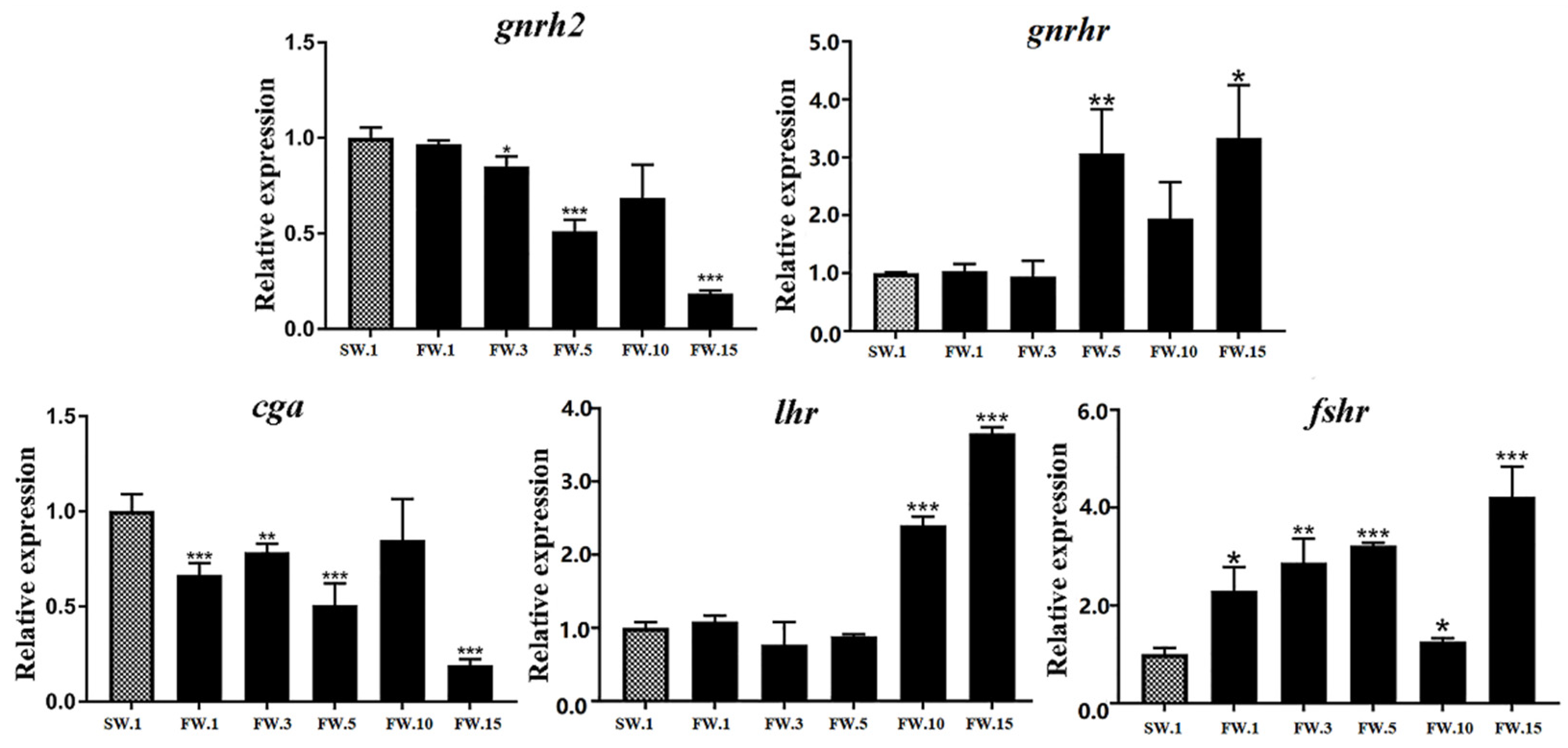
2.4. Expression of HPG Axis Genes in L. maculatus
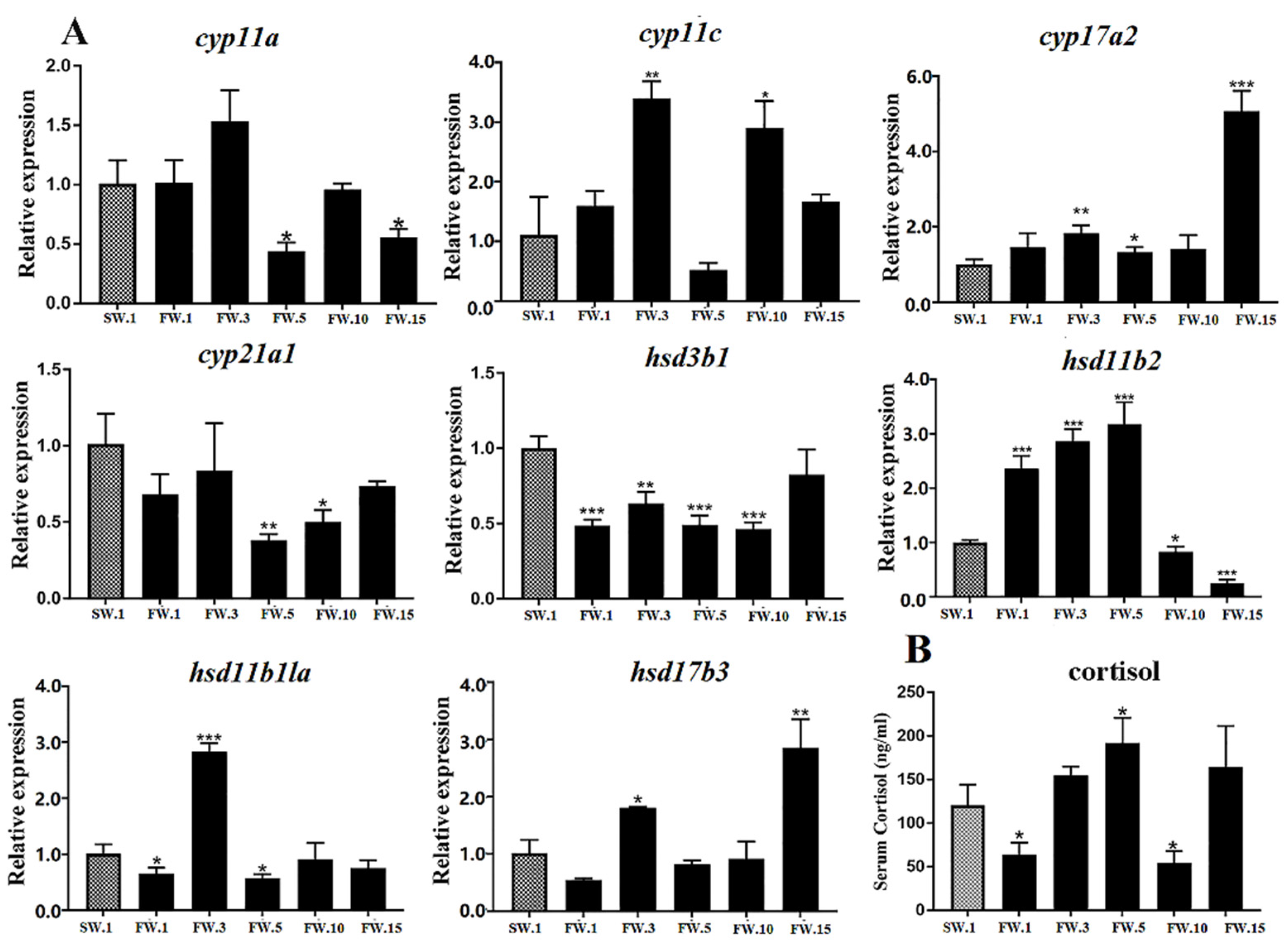
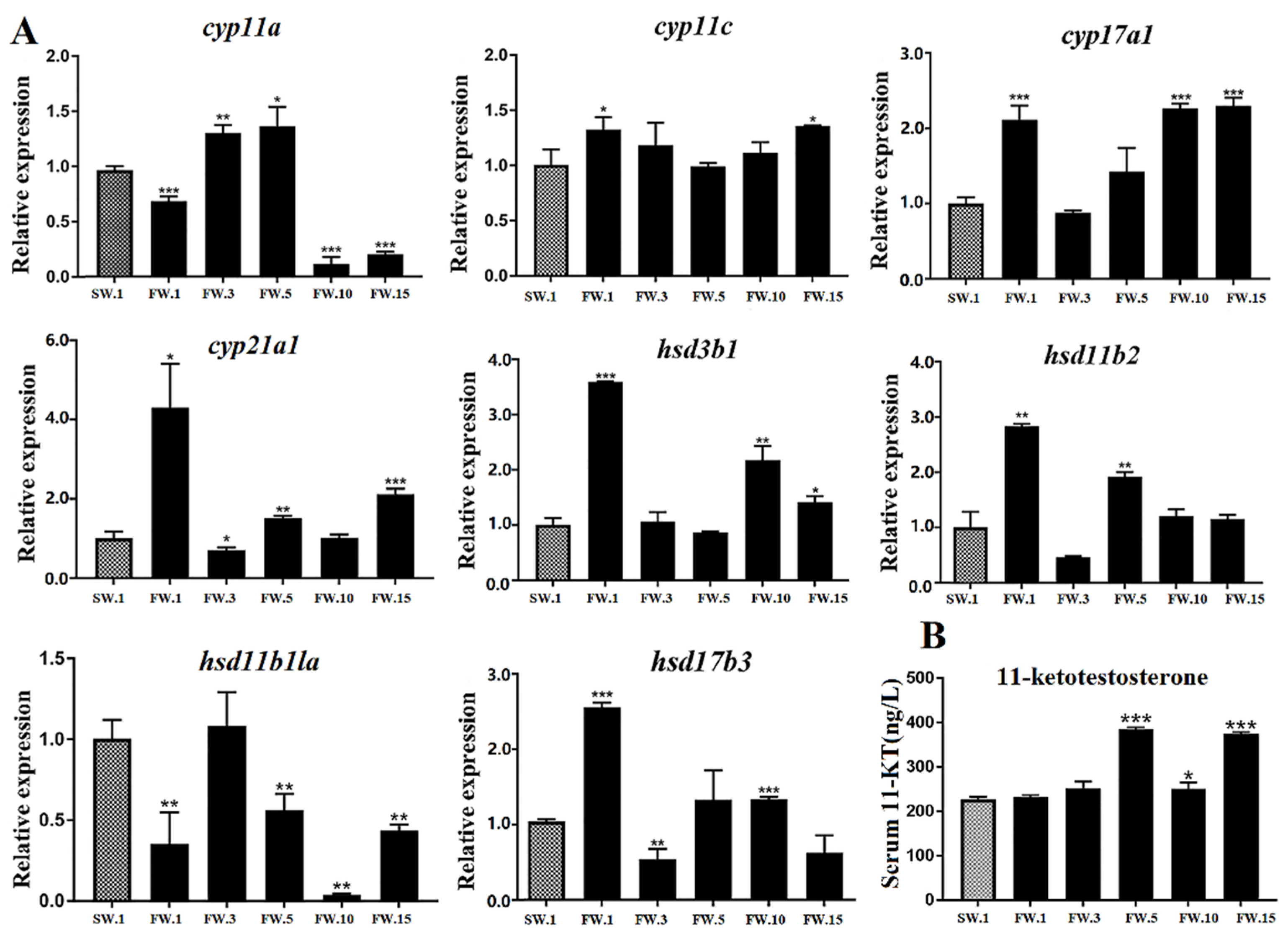
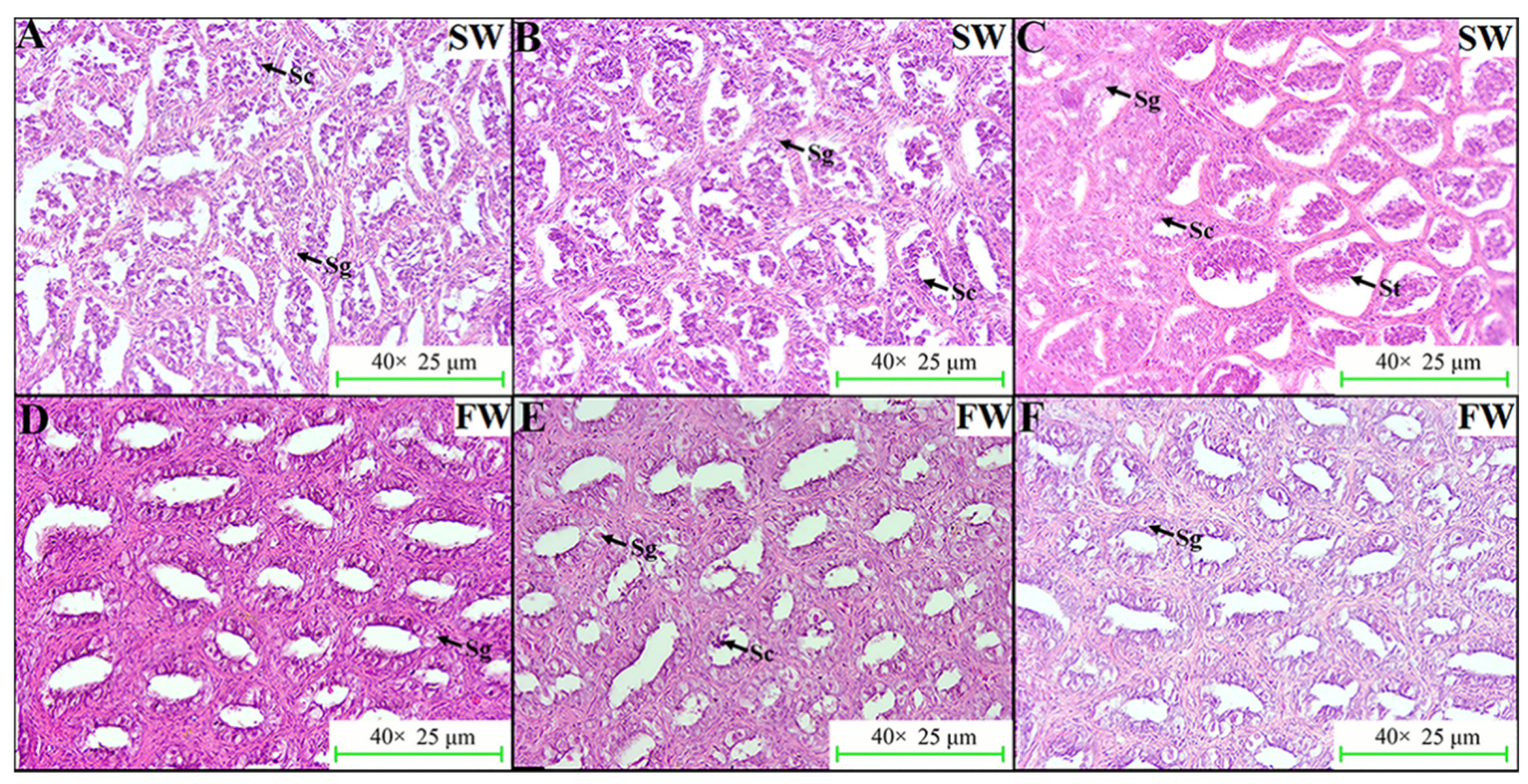
2.5. Effects of Salinity Change on HPG Axis Genes and the Sexual Development of Male L. maculatus
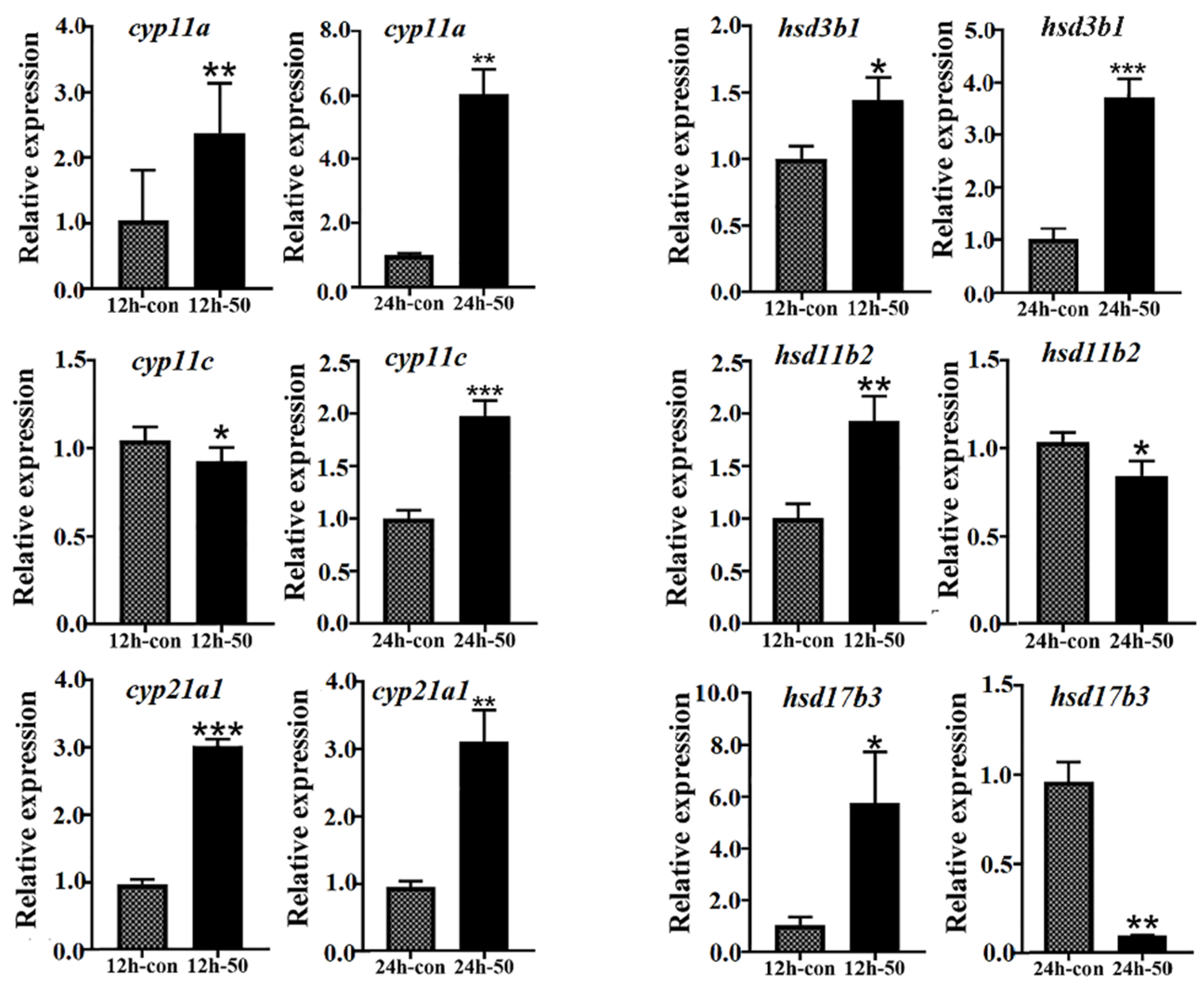
2.6. Putative Interaction between the HPG and HPI Axes in Male L. maculatus under Salinity Change
3. Materials and Methods
3.1. Identification and Annotation of HPG Axis Genes in L. maculatus
3.2. Phylogenetic and Conserved Motif Analysis
3.3. Conserved Sequence Analysis by mVista
3.4. Expression of HPG Axis Genes in L. maculatus Tissues
3.5. Salinity-Change Experiment on Maturing Male L. maculatus
3.6. RNA Extraction and qRT-PCR of HPG Axis Genes in L. maculatus
3.7. Quantification of Serum Steroid Hormones in L. maculatus
3.8. Histological Examination of L. maculatus Testes
3.9. Correlation of HPG Axis Genes in L. maculatus with Serum Hormones
3.10. In Vitro Stimulation of L. maculatus Testes with Cortisol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.A.; Dar, J.Y.; Ahmad, I.; Mir, I.N.; Bhat, H.; Bhat, R.A.H.; Ganie, P.A.; Sharma, R. Testicular development and spermatogenesis in fish: Insights into molecular aspects and regulation of gene expression by different exogenous factors. Rev. Aquac. 2021, 13, 2142–2168. [Google Scholar] [CrossRef]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Muñoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, J.; Möller, G.; Hrabě de Angelis, M.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Senthilkumaran, B. Steroidogenesis and its regulation in teleost—A review. Fish Physiol. Biochem. 2020, 46, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M. Hypothalamic- and pituitary-derived growth and reproductive hormones and the control of energy balance in fish. Gen. Comp. Endocrinol. 2020, 287, 113322. [Google Scholar] [CrossRef]
- Bliss, S.P.; Navratil, A.M.; Xie, J.; Roberson, M.S. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocrinol. 2010, 31, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, F.; Liu, S.; Zhao, H.; Lu, W.; Zhang, Q. Transcriptomic Analysis Reveals Functional Interaction of mRNA-lncRNA-miRNA in Steroidogenesis and Spermatogenesis of Gynogenetic Japanese Flounder (Paralichthys olivaceus). Biology 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Y.; Lu, W.; Zong, W.; Zhu, Q.; Cheng, J. The comparative survey of coordinated regulation of steroidogenic pathway in Japanese flounder (Paralichthys olivaceus) and Chinese tongue sole (Cynoglossus Semilaevis). Int. J. Mol. Sci. 2022, 23, 5520. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. The role of the StAR protein in steroidogenesis: Challenges for the future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Tenugu, S.; Pranoty, A.; Mamta, S.K.; Senthilkumaran, B. Development and organization of gonadal steroidogenesis in bony fishes—A review. Aquac. Fish. 2021, 6, 223–246. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef]
- Liang, D.; Fan, Z.; Zou, Y.; Tan, X.; Wu, Z.; Jiao, S.; Li, J.; Zhang, P.; You, F. Characteristics of Cyp11a during Gonad Differentiation of the Olive Flounder Paralichthys olivaceus. Int. J. Mol. Sci. 2018, 19, 2641. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Senthilkumaran, B. Expression analysis of cyp11a1 during gonadal development, recrudescence and after hCG induction and sex steroid analog treatment in the catfish (Clarias batrachus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 176, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xiao, H.; Shi, H.; Wang, T.; Sun, L.; Tao, W.; Kocher, T.D.; Li, M.; Wang, D. Loss of Cyp11c1 causes delayed spermatogenesis due to the absence of 11-ketotestosterone. J. Endocrinol. 2020, 244, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Wang, D.S.; Shibata, Y.; Paul-Prasanth, B.; Suzuki, A.; Nagahama, Y. Characterization, expression and transcriptional regulation of P450c17-I and -II in the medaka (Oryzias latipes). Biochem. Biophys. Res. Commun. 2007, 362, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Wang, D.S.; Kobayashi, T.; Yano, A.; Paul-Prasanth, B.; Suzuki, A.; Sakai, F.; Nagahama, Y. A novel type of P450c17 lacking the lyase activity is responsible for C21-steroid biosynthesis in the fish ovary and head kidney. Endocrinology 2007, 148, 4282–4291. [Google Scholar] [CrossRef] [PubMed]
- Böhne, A.; Heule, C.; Boileau, N.; Salzburger, W. Expression and sequence evolution of aromatase cyp19a1 and other sexual development genes in East African cichlid fishes. Mol. Biol. Evol. 2013, 30, 2268–2285. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Guo, Y.; Wang, D.; Zhao, M.; Hou, X.; Li, S.; Lin, H.; Zhang, Y. Beta-Hydroxysteroid Dehydrogenase Genes in Orange-Spotted Grouper (Epinephelus coioides): Genome-Wide Identification and Expression Analysis during Sex Reversal. Front. Genet. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Ekstrand, B.; Zamaratskaia, G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ⁵-Δ⁴ isomerase: A review. Int. J. Mol. Sci. 2013, 14, 17926–17942. [Google Scholar] [CrossRef] [PubMed]
- Rasheeda, M.K.; Kagawa, H.; Kirubagaran, R.; Dutta-Gupta, A.; Senthilkumaran, B. Cloning, expression and enzyme activity analysis of testicular 11beta-hydroxysteroid dehydrogenase during seasonal cycle and after hCG induction in air-breathing catfish (Clarias gariepinus). J. Steroid Biochem. Mol. Biol. 2010, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adamski, J.; Jakob, F.J. A guide to 17β-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2001, 171, 1–4. [Google Scholar] [CrossRef]
- Mindnich, R.; Möller, G.; Adamski, J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Wang, D.S.; Senthilkumaran, B.; Yoshikuni, M.; Shibata, Y.; Kobayashi, T.; Sudhakumari, C.C.; Nagahama, Y. Cloning, expression and characterization of three types of 17beta-hydroxysteroid dehydrogenases from the Nile tilapia, Oreochromis niloticus. J. Mol. Endocrinol. 2005, 35, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef]
- Vieira, A.B.C.; Weber, A.A.; Ribeiro, Y.M.; Luz, R.K.; Bazzoli, N.; Rizzo, E. Influence of salinity on spermatogenesis in adult Nile tilapia (Oreochromis niloticus) testis. Theriogenology 2019, 131, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Wang, Y.; Lu, W.; Zhang, Q.; Cheng, J. Genomic and transcriptomic landscape and evolutionary dynamics of heat shock proteins in spotted sea bass (Lateolabrax maculatus) under salinity change and alkalinity stress. Biology 2022, 11, 353. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Wang, H.; Ren, Y.; Zhao, J.; Li, Y. RNA-Seq analysis of salinity stress-responsive transcriptome in the liver of spotted sea bass (Lateolabrax maculatus). PLoS ONE 2017, 12, e0173238. [Google Scholar] [CrossRef]
- Chi, M.; Ni, M.; Jia, Y.; Gu, Z.; Wen, H. Blood physiological responses and steroidogenetic effects of decreasing salinity on maturing male spotted sea bass (Lateolabrax maculatus). Aquac. Res. 2018, 49, 3517–3528. [Google Scholar] [CrossRef]
- Lynch, M.; Force, A. The probability of duplicate gene preservation by subfunctionalization. Genetics 2000, 154, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.F.; Yan, Y.L.; Guiguen, Y.; Postlethwait, J.; Chung, B.C. Two Cyp19 (P450 aromatase) genes on duplicated zebrafish chromosomes are expressed in ovary or brain. Mol. Biol. Evol. 2001, 18, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Moulik, S.R.; Pal, P.; Majumder, S.; Das, S.; Guha, P.; Juin, S.K.; Panigrahi, A.K.; Mukherjee, D. Estrogen-regulated expression of cyp19a1a and cyp19a1b genes in swim-up fry of Labeo rohita. Gen. Comp. Endocrinol. 2017, 251, 85–93. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Zhao, C.; Miao, Y.; Cai, J.; Song, L.; Wei, J.; Chakraborty, T.; Wu, L.; Wang, D. The role of StAR2 gene in testicular differentiation and spermatogenesis in Nile tilapia (Oreochromis niloticus). J. Steroid Biochem. Mol. Biol. 2021, 214, 105974. [Google Scholar] [CrossRef]
- Yu, X.; Wu, L.; Xie, L.; Yang, S.; Charkraborty, T.; Shi, H.; Wang, D.; Zhou, L. Characterization of two paralogous StAR genes in a teleost, Nile tilapia (Oreochromis niloticus). Mol. Cell. Endocrinol. 2014, 392, 152–162. [Google Scholar] [CrossRef]
- Tokarz, J.; Lintelmann, J.; Möller, G.; Adamski, J. Substrate multispecificity among 20β-hydroxysteroid dehydrogenase type 2 members. Mol. Cell. Endocrinol. 2020, 510, 110822. [Google Scholar] [CrossRef]
- Castaneda-Cortes, D.C.; Fernandino, J.I. Stress and sex determination in fish: From brain to gonads. Int. J. Dev. Biol. 2021, 65, 207–214. [Google Scholar] [CrossRef]
- Goikoetxea, A.; Todd, E.V.; Gemmell, N.J. Stress and sex: Does cortisol mediate sex change in fish? Reproduction 2017, 154, R149–R160. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Sharma, P.; Purohit, S.; Kothiyal, S.; Negi, S.; Bhattacharya, I. Sex Specific Transcriptional Regulation of Gonadal Steroidogenesis in Teleost Fishes. Front. Endocrinol. 2022, 13, 820241. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Kobayashi, T.; Todo, T.; Mark Lokman, P.; Nagahama, Y.; Young, G. Molecular cloning and expression during spermatogenesis of a cDNA encoding testicular 11β-hydroxylase (P45011beta) in rainbow trout (Oncorhynchus mykiss). Mol. Reprod. Dev. 2002, 62, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Higuchi, M.; Miura, C.; Yamaguchi, S.; Tozawa, Y.; Miura, T. Roles of 11beta-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology 2006, 147, 5139–5146. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Wang, H.; Wen, H.; Li, Y.; Zhang, K.; Liu, Y. Evaluation of potential reference genes for quantitative RT-PCR analysis in spotted sea bass (Lateolabrax maculatus) under normal and salinity stress conditions. PeerJ 2018, 6, e5631. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Li, X.; Wang, Y.; Lu, W.; Hou, J.; Cheng, J. Steroidogenic Effects of Salinity Change on the Hypothalamus–Pituitary–Gonad (HPG) Axis of Male Chinese Sea Bass (Lateolabrax maculatus). Int. J. Mol. Sci. 2022, 23, 10905. https://doi.org/10.3390/ijms231810905
Fang Z, Li X, Wang Y, Lu W, Hou J, Cheng J. Steroidogenic Effects of Salinity Change on the Hypothalamus–Pituitary–Gonad (HPG) Axis of Male Chinese Sea Bass (Lateolabrax maculatus). International Journal of Molecular Sciences. 2022; 23(18):10905. https://doi.org/10.3390/ijms231810905
Chicago/Turabian StyleFang, Zhenru, Xujian Li, Yapeng Wang, Wei Lu, Juncheng Hou, and Jie Cheng. 2022. "Steroidogenic Effects of Salinity Change on the Hypothalamus–Pituitary–Gonad (HPG) Axis of Male Chinese Sea Bass (Lateolabrax maculatus)" International Journal of Molecular Sciences 23, no. 18: 10905. https://doi.org/10.3390/ijms231810905
APA StyleFang, Z., Li, X., Wang, Y., Lu, W., Hou, J., & Cheng, J. (2022). Steroidogenic Effects of Salinity Change on the Hypothalamus–Pituitary–Gonad (HPG) Axis of Male Chinese Sea Bass (Lateolabrax maculatus). International Journal of Molecular Sciences, 23(18), 10905. https://doi.org/10.3390/ijms231810905






