Functional Study of Lipoxygenase-Mediated Resistance against Fusarium verticillioides and Aspergillus flavus Infection in Maize
Abstract
1. Introduction
2. Results
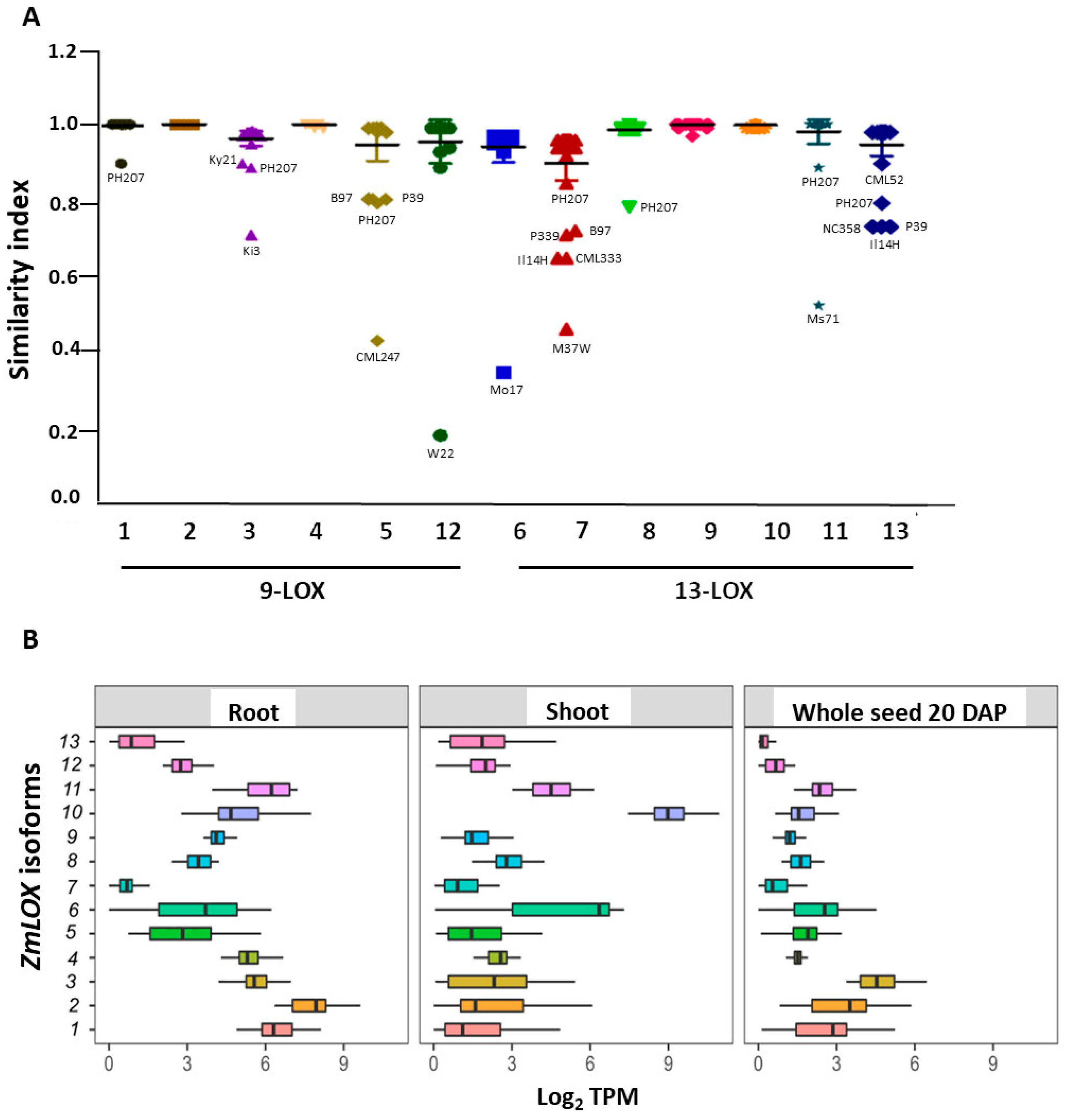
2.1. Overview of the LOX Family in Maize Pan-Genome and Pan-Transcriptome
2.2. Evaluation of Fungal Growth and Mycotoxin Content
2.3. Expression Analysis of Maize Genes Involved in Oxylipin and Jasmonate Synthesis
2.3.1. Modulation of LOX and JA-Related Genes in Response to Fusarium verticillioides
2.3.2. Modulation of LOX and JA-Related Genes in Response to Aspergillus flavus
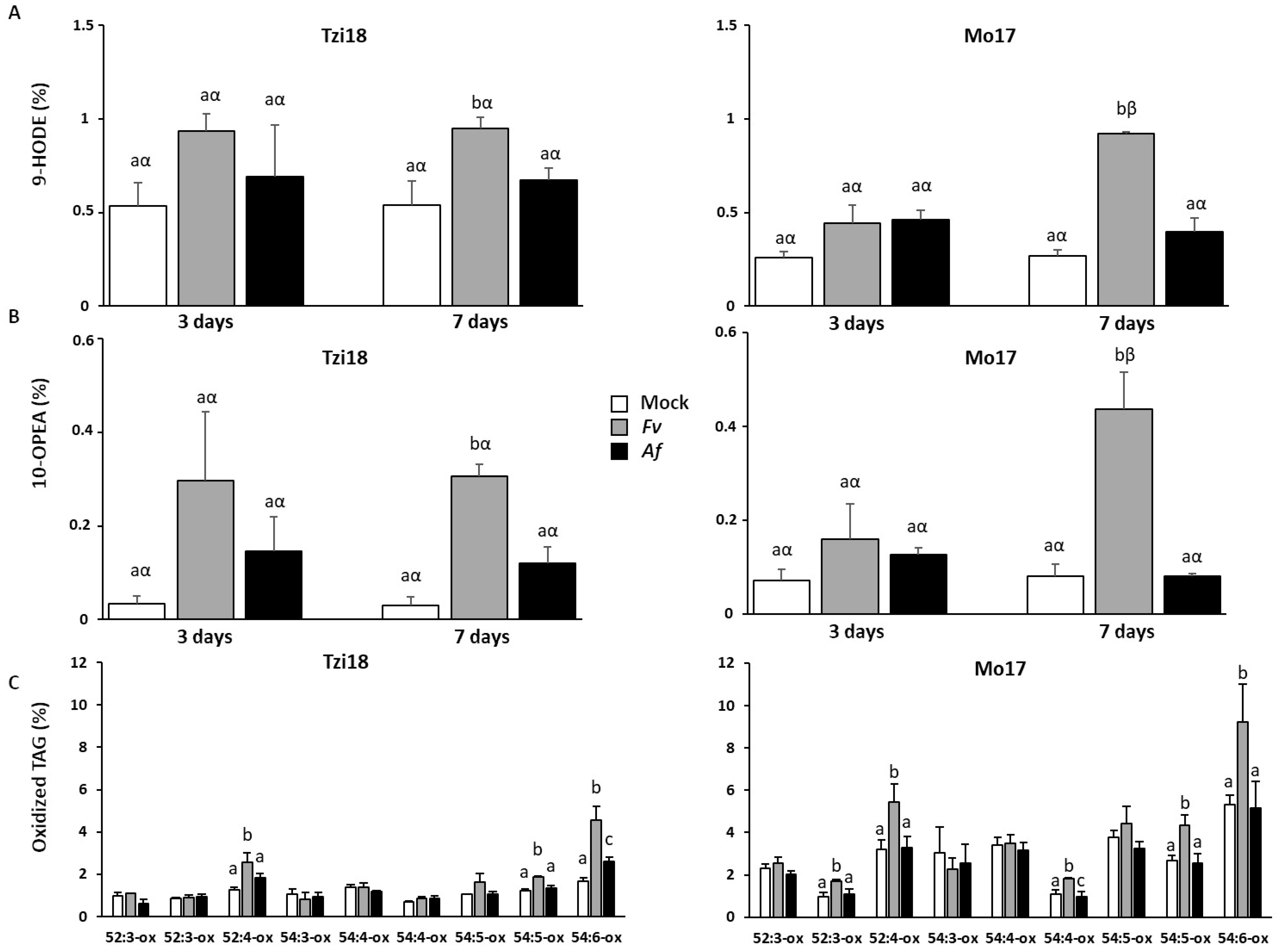
2.4. Analysis of Maize Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis of the Maize Pan-Genome and Pan-Transcriptome of LOX Isoforms
4.2. Description of the Maize Inbred Lines and UFMulox4 Mutant
4.3. Set-Up of Inoculation Assay
4.4. Analysis of Mycotoxin Content
4.5. RNA Extraction and Real-Time RT-qPCR Gene Expression Analysis
4.6. Lipid Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Logrieco, A.; Battilani, P.; Leggieri, M.C.; Jiang, Y.; Haesaert, G.; Lanubile, A.; Mahuku, G.; Mesterhazy, A.; Ortega-Beltran, A.; Pasti, M.; et al. Perspectives on global mycotoxin issues and management from the MycoKey Maize Working Group. Plant Dis. 2021, 105, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, M.C.; Lanubile, A.; Dall’Asta, C.; Pietri, A.; Battilani, P. The impact of seasonal weather variation on mycotoxins: Maize crop in 2014 in northern Italy as a case study. World Mycotoxin J. 2020, 13, 25–36. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Toscano, P.; Battilani, P. Predicted aflatoxin b1 increase in europe due to climate change: Actions and reactions at global level. Toxins 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Nji, Q.N.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mwanza, M. Six main contributing factors to high levels of mycotoxin contamination in African foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G. Crop management practices to minimize the risk of mycotoxins contamination in temperate−zone maize. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; Wiley Blackwell: New Delhi, India, 2014; pp. 59–77. [Google Scholar]
- Sarrocco, S.; Mauro, A.; Battilani, P. Use of competitive filamentous fungi as an alternative approach for mycotoxin risk reduction in staple cereals: State of art and future perspectives. Toxins 2019, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Marino, T.P.; Wenndt, A.J.; Fouts, J.Q.; Holland, J.B.; Nelson, R.J. Dissecting symptomatology and fumonisin contamination produced by Fusarium verticillioides in maize ears. Phytopathology 2018, 108, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Lanubile, A.; Pasini, L.; Lo Pinto, M.; Battilani, P.; Prandini, A.; Marocco, A. Evaluation of broad spectrum sources of resistance to Fusarium verticillioides and advanced maize breeding lines. World Mycotoxin J. 2010, 4, 43–51. [Google Scholar] [CrossRef]
- Stagnati, L.; Martino, M.; Battilani, P.; Busconi, M.; Lanubile, A.; Marocco, A. Development of early maturity maize hybrids for resistance to fusarium and aspergillus ear rots and their associated mycotoxins. World Mycotoxin J. 2020, 13, 459–471. [Google Scholar] [CrossRef]
- Warburton, M.L.; Williams, W.P.; Windham, G.L.; Murray, S.C.; Xu, W.; Hawkins, L.K.; Duran, J.F. Phenotypic and genetic characterization of a maize association mapping panel developed for the identification of new sources of resistance to Aspergillus flavus and aflatoxin accumulation. Crop Sci. 2013, 53, 2374–2383. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Marocco, A. Breeding maize for resistance to mycotoxins. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; Wiley Blackwell: New Delhi, India, 2014; pp. 37–58. [Google Scholar]
- Maschietto, V.; Colombi, C.; Pirona, R.; Pea, G.; Strozzi, F.; Marocco, A.; Rossini, L.; Lanubile, A. QTL mapping and candidate genes for resistance to Fusarium ear rot and fumonisin contamination in maize. BMC Plant Biol. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Septiani, P.; Lanubile, A.; Stagnati, L.; Busconi, M.; Nelissen, H.; Pè, M.E.; Dell’Acqua, M.; Marocco, A. Unravelling the genetic basis of Fusarium seedling rot resistance in the MAGIC maize population: Novel targets for breeding. Sci. Rep. 2019, 9, 5665. [Google Scholar] [CrossRef] [PubMed]
- Stagnati, L.; Lanubile, A.; Samayoa, L.F.; Bragalanti, M.; Giorni, P.; Busconi, M.; Holland, J.B.; Marocco, A. A genome wide association study reveals markers and genes associated with resistance to Fusarium verticillioides infection of seedlings in a maize diversity panel. G3 Genes Genom. Genet. 2019, 9, 571–579. [Google Scholar] [CrossRef]
- Stagnati, L.; Rahjoo, V.; Samayoa, L.F.; Holland, J.B.; Borrelli, V.M.G.; Busconi, M.; Lanubile, A.; Marocco, A. A genome-wide association study to understand the effect of Fusarium verticillioides infection on seedlings of a maize diversity panel. G3 Genes Genom. Genet. 2020, 10, 1685–1696. [Google Scholar] [CrossRef]
- Cao, A.; de la Fuente, M.; Gesteiro, N.; Santiago, R.; Malvar, R.A.; Butrón, A. Genomics and pathways involved in maize resistance to Fusarium ear rot and kernel contamination with fumonisins. Front. Plant Sci. 2022, 13, 866478. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Williams, W.P.; Warburton, M.; Windham, G.; Xu, W.; Bhattramakki, D. Mapping quantitative trait loci for aflatoxin accumulation resistance in two populations containing resistant maize inbred Mp717. Crop Sci. 2022, 62, 780–791. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The potential of fatty acids and their derivatives as antifungal agents: A review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Sugimoto, K.; Allmann, S.; Kolomiets, M.V. Editorial: Oxylipins: The front line of plant interactions. Front. Plant Sci. 2022, 13, 878765. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant Lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant lipoxygenases and their role in plant physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The Lipoxygenase Pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Borrego, E.J.; Kolomiets, M.V. Synthesis and functions of jasmonates in maize. Plants 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A.; et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef]
- Tolley, J.P.; Nagashima, Y.; Gorman, Z.; Kolomiets, M.V.; Koiwa, H. Isoform-specific subcellular localization of Zea mays lipoxygenases and oxo-phytodienoate reductase 2. Plant Gene 2018, 13, 36–41. [Google Scholar] [CrossRef]
- Gao, X.; Stumpe, M.; Feussner, I.; Kolomiets, M. A novel plastidial lipoxygenase of maize (Zea mays) ZmLOX6 encodes for a fatty acid hydroperoxide lyase and is uniquely regulated by phytohormones and pathogen infection. Planta 2008, 227, 491–503. [Google Scholar] [CrossRef]
- Christensen, S.A.; Huffaker, A.; Kaplan, F.; Sims, J.; Ziemann, S.; Doehlemann, G.; Ji, L.; Schmitz, R.J.; Kolomiets, M.V.; Alborn, H.T.; et al. Maize death acids, 9-lipoxygenase-derived cyclopente(a)nones, display activity as cytotoxic phytoalexins and transcriptional mediators. Proc. Natl. Acad. Sci. USA 2015, 112, 11407–11412. [Google Scholar] [CrossRef]
- Christensen, S.A.; Huffaker, A.; Hunter, C.T.; Alborn, H.T.; Schmelz, E.A. A maize death acid, 10-oxo-11-phytoenoic acid, is the predominant cyclopentenone signal present during multiple stress and developmental conditions. Plant Signal. Behav. 2016, 11, e1120395. [Google Scholar] [CrossRef]
- Ogorodnikova, A.V.; Gorina, S.S.; Mukhtarova, L.S.; Mukhitova, F.K.; Toporkova, Y.Y.; Hamberg, M.; Grechkin, A.N. Stereospecific biosynthesis of (9S,13S)-10-oxo-phytoenoic acid in young maize roots. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 1262–1270. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Wang, F.; Huang, P.C.; Wang, Y.; Ruan, X.; Ma, L.; Li, X.; Kolomiets, M.V.; Gao, X. Transcriptome and oxylipin profiling joint analysis reveals opposite roles of 9-oxylipins and jasmonic acid in maize resistance to Gibberella stalk rot. Front. Plant Sci. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Gao, X.; Shim, W.B.; Göbel, C.; Kunze, S.; Feussner, I.; Meeley, R.; Balint-Kurti, P.; Kolomiets, M. Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol. Plant Microbe Interact. 2007, 20, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Göbel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the lipoxygenase ZmLOX3 increases susceptibility of maize to Aspergillus spp. Mol. Plant Microbe Interact. 2009, 2, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Maschietto, V.; Marocco, A.; Malachova, A.; Lanubile, A. Resistance to Fusarium verticillioides and fumonisin accumulation in maize inbred lines involves an earlier and enhanced expression of lipoxygenase (LOX) genes. J. Plant Physiol. 2015, 188, 9–18. [Google Scholar] [CrossRef]
- Ogunola, O.F.; Hawkins, L.K.; Mylroie, E.; Kolomiets, M.V.; Borrego, E.; Tang, J.D.; Williams, W.P.; Warburton, M.L. Characterization of the maize lipoxygenase gene family in relation to aflatoxin accumulation resistance. PLoS ONE 2017, 12, e0181265. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; Battilani, P.; Marocco, A. Infection with toxigenic and atoxigenic strains of Aspergillus flavus induces different transcriptional signatures in maize kernels. J. Plant Interact. 2017, 12, 21–30. [Google Scholar] [CrossRef][Green Version]
- Lanubile, A.; Borrelli, V.M.; Soccio, M.; Giorni, P.; Stagnati, L.; Busconi, M.; Marocco, A. Loss of ZmLIPOXYGENASE4 decreases Fusarium verticillioides resistance in maize seedlings. Genes 2021, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Lanubile, A.; Scala, V.; Reverberi, M.; Gregori, R.; Falavigna, C.; Dall’asta, C.; Park, Y.S.; Bennett, J.; Borrego, E.J.; et al. Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize. Mol. Plant Pathol. 2018, 19, 2162–2176. [Google Scholar] [CrossRef]
- Woodhouse, M.R.; Cannon, E.K.; Portwood, J.L.; Harper, L.C.; Gardiner, J.M.; Schaeffer, M.L.; Andorf, C.M. A pan-genomic approach to genome databases using maize as a model system. BMC Plant Biol. 2021, 21, 385. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115, Erratum in Science 2012, 337, 1040. [Google Scholar] [CrossRef]
- Hufford, M.B.; Seetharam, A.S.; Woodhouse, M.R.; Chougule, K.M.; Ou, S.; Liu, J.; Ricci, W.A.; Guo, T.; Olson, A.; Qiu, Y.; et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 2021, 373, 655–662. [Google Scholar] [CrossRef]
- Gage, J.L.; Monier, B.; Giri, A.; Buckler, E.S. Ten years of the maize Nested Association Mapping population: Impact, limitations, and future directions. Plant Cell 2020, 32, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.N.; Foerster, J.M.; Johnson, J.M.; Sekhon, R.S.; Muttoni, G.; Vaillancourt, B.; Peñagaricano, F.; Lindquist, E.; Pedraza, M.A.; Barry, K.; et al. Insights into the maize pan-genome and pan-transcriptome. Plant Cell 2014, 26, 121–135. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, G.N.; Murray, S.C.; Isakeit, T.; Park, Y.S.; Yan, Y.; Warburton, M.L.; Kolomiets, M.V. Characterization of genetic diversity and linkage disequilibrium of ZmLOX4 and ZmLOX5 loci in maize. PLoS ONE 2013, 8, e53973. [Google Scholar] [CrossRef]
- Springer, N.M.; Ying, K.; Fu, Y.; Ji, T.; Yeh, C.T.; Jia, Y.; Wu, W.; Richmond, T.; Kitzman, J.; Rosenbaum, H.; et al. Maize inbreds exhibit high levels of Copy Number Variation (CNV) and Presence/Absence Variation (PAV) in genome content. PLoS Genet. 2009, 5, e1000734. [Google Scholar] [CrossRef] [PubMed]
- Darracq, A.; Vitte, C.; Nicolas, S.; Duarte, J.; Pichon, J.P.; Mary-Huard, T.; Chevalier, C.; Bérard, A.; Le Paslier, M.C.; Rogowsky, P.; et al. Sequence analysis of European maize inbred line F2 provides new insights into molecular and chromosomal characteristics of Presence/Absence Variants. BMC Genom. 2018, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhou, Y.; Chen, J.; Shi, J.; Zhao, H.; Zhao, H.; Song, W.; Zhang, M.; Cui, Y.; Dong, X.; et al. Extensive intraspecific gene order and gene structural variations between Mo17 and other maize genomes. Nat. Genet. 2018, 50, 1289–1295. [Google Scholar] [CrossRef]
- Gorman, Z.; Christensen, S.A.; Yan, Y.; He, Y.; Borrego, E.; Kolomiets, M.V. Green leaf volatiles and jasmonic acid enhance susceptibility to anthracnose diseases caused by Colletotrichum graminicola in maize. Mol. Plant Pathol. 2020, 21, 702–715. [Google Scholar] [CrossRef]
- He, Y.; Borrego, E.J.; Gorman, Z.; Huang, P.C.; Kolomiets, M.V. Relative contribution of LOX10, green leaf volatiles and JA to wound-induced local and systemic oxylipin and hormone signature in Zea mays (maize). Phytochemistry 2020, 174, 112334. [Google Scholar] [CrossRef]
- Zernova, O.V.; Lygin, A.V.; Pawlowski, M.L.; Hill, C.B.; Hartman, G.L.; Widholm, J.M.; Lozovaya, V.V. Regulation of plant immunity through modulation of phytoalexin synthesis. Molecules 2014, 19, 7480–7496. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Koprivova, A.; Schuck, S.; Jacoby, R.P.; Klinkhammer, I.; Welter, B.; Leson, L.; Martyn, A.; Nauen, J.; Grabenhorst, N.; Mandelkow, J.F.; et al. Root-specific camalexin biosynthesis controls the plant growth-promoting effects of multiple bacterial strains. Proc. Nat. Acad. Sci. USA 2019, 116, 15735–15744. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; De Leonardis, S.; Battilani, P.; Paciolla, C.; Marocco, A. Defence responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol. Plant Microbe Interact. 2015, 28, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Menkir, A.; Brown, R.L.; Bandyopadhyay, R.; Cleveland, T.E. Registration of six tropical maize germplasm lines with resistance to aflatoxin contamination. J. Plant Reg. 2008, 2, 246–250. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Thuillet, A.C.; Yu, J.; Pressoir, G.; Romero, S.M.; Mitchell, S.E.; Doebley, J.; Kresovich, S.; Goodman, M.M.; Buckler, E.S. Maize association population: A high-resolution platform for quantitative trait locus dissection. Plant J. 2005, 70, 1054–1064. [Google Scholar] [CrossRef]
- Fasihi, V.; Valizadeh, M.; Shiri, M.; Imani, A.A. The survey of maize inbred lines for resistance to Fusarium verticillioides ear rot. J. Appl. Environ. Biol. Sci. 2013, 3, 107–110. [Google Scholar]
- Brown, R.L.; Williams, W.P.; Windham, G.L.; Menkir, A.; Chen, Z.-Y. Evaluation of African-bred maize germplasm lines for resistance to aflatoxin accumulation. Agronomy 2016, 6, 24. [Google Scholar] [CrossRef]
- Park, Y.S. Diverse Functions of the Two Segmentally Duplicated 9-Lipoxygenases ZmLOX4 and ZmLOX5 of Maize. Doctoral Dissertation, Texas A & M University, College Station, TX, USA, 2012. [Google Scholar]
- Lanubile, A.; Giorni, P.; Bertuzzi, T.; Marocco, A.; Battilani, P. Fusarium verticillioides and Aspergillus flavus co-occurrence influences plant and fungal transcriptional profiles in maize kernels and in vitro. Toxins 2021, 13, 680. [Google Scholar] [CrossRef]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubile, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic profile and susceptibility to Fusarium infection of pigmented maize cultivars. Front. Plant Sci. 2018, 9, 1189. [Google Scholar] [CrossRef]
- Lanubile, A.; Logrieco, A.; Battilani, P.; Proctor, R.H.; Marocco, A. Transcriptional changes in developing maize kernels in response to fumonisin-producing and nonproducing strains of Fusarium verticillioides. Plant Sci. 2013, 210, 183–192. [Google Scholar] [CrossRef]
- Manoli, A.; Sturaro, A.; Trevisan, S.; Quaggiotti, S.; Nonis, A. Evaluation of candidate reference genes for qPCR in maize. J. Plant Physiol. 2012, 169, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Meermeyer, M. LinRegInteractive: An R Package for the Interactive Interpretation of Linear Regression Models; Schumpeter Discussion Papers SDP14014; Universitätsbibliothek Wuppertal, University Library: Wuppertal, Germany, 2014. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Package ‘pheatmap’. Bioconductor 2012, 1–6. Available online: http://www2.uaem.mx/r-mirror/web/packages/pheatmap/pheatmap.pdf (accessed on 4 August 2022).
- Pilati, S.; Brazzale, D.; Guella, G.; Milli, A.; Ruberti, C.; Biasioli, F.; Zottini, M.; Moser, C. The onset of grapevine berry ripen-ing is characterized by ROS accumulation and lipoxygenase-mediated membrane peroxidation in the skin. BMC Plant Biol. 2014, 14, 87. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guche, M.D.; Pilati, S.; Trenti, F.; Dalla Costa, L.; Giorni, P.; Guella, G.; Marocco, A.; Lanubile, A. Functional Study of Lipoxygenase-Mediated Resistance against Fusarium verticillioides and Aspergillus flavus Infection in Maize. Int. J. Mol. Sci. 2022, 23, 10894. https://doi.org/10.3390/ijms231810894
Guche MD, Pilati S, Trenti F, Dalla Costa L, Giorni P, Guella G, Marocco A, Lanubile A. Functional Study of Lipoxygenase-Mediated Resistance against Fusarium verticillioides and Aspergillus flavus Infection in Maize. International Journal of Molecular Sciences. 2022; 23(18):10894. https://doi.org/10.3390/ijms231810894
Chicago/Turabian StyleGuche, Mikias Damtew, Stefania Pilati, Francesco Trenti, Lorenza Dalla Costa, Paola Giorni, Graziano Guella, Adriano Marocco, and Alessandra Lanubile. 2022. "Functional Study of Lipoxygenase-Mediated Resistance against Fusarium verticillioides and Aspergillus flavus Infection in Maize" International Journal of Molecular Sciences 23, no. 18: 10894. https://doi.org/10.3390/ijms231810894
APA StyleGuche, M. D., Pilati, S., Trenti, F., Dalla Costa, L., Giorni, P., Guella, G., Marocco, A., & Lanubile, A. (2022). Functional Study of Lipoxygenase-Mediated Resistance against Fusarium verticillioides and Aspergillus flavus Infection in Maize. International Journal of Molecular Sciences, 23(18), 10894. https://doi.org/10.3390/ijms231810894






