Experimental Crossing Confirms Reproductive Isolation between Cryptic Species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal
Abstract
1. Introduction
- According to the biological species concept, we will define the biological species (or simply species) as the multitude of actually or potentially interbreeding populations, which are reproductively isolated from other such groups [9,10]. Thus, the main criterion for defining a biological species is the presence of a reproductive barrier. The mechanisms of reproductive isolation fall into two groups, prezygotic (mate discrimination, timing of mating and gamete release, fertilization barriers, etc.) and postzygotic (hybrid inviability or sterility) [11,12]. As a species may comprise potentially (not actually) interbreeding populations by definition, then geographic isolation does not qualify as a reproductive barrier [10].
- Then, we will use the term morphological species, or morphospecies, for the set of individuals with indistinguishable morphological traits. Usually, it is impossible to say if there are any reproductive barriers between such individuals if they are sampled in different locations.
- Finally, we suggest using the term barcoding species for taxonomic entities separated on the basis of at least one phylogenetic marker sequence with at least one species delimitation method. This is mostly equivalent to the term molecular operational taxonomic unit (MOTU) or genospecies [13]. If there are particular sequence-based (or allozyme-based) clusters but species delimitation did not separate them, was not applied or was not applicable, we will call them barcoding lineages or allozyme lineages, respectively. Sequence-based delimitation indicates that there is some degree of separation, but may not necessarily mean genetic incompatibility.
- If one morphological species accommodates several barcoding species, we will term these genetically diverse but morphologically indistinguishable entities cryptic species, as suggested in [9]. Similarly, if several barcoding lineages are contained within a morphological species, it is logical to call them cryptic lineages. If a morphological difference is found after closer examination, the lineages or species become pseudocryptic (e.g., [14]). It is worth noting that cryptic lineages or species may occur both sympatrically and allopatrically, and they may originate either in the process of diversification or by convergent evolution of close (but not necessarily sister) groups [9,15].
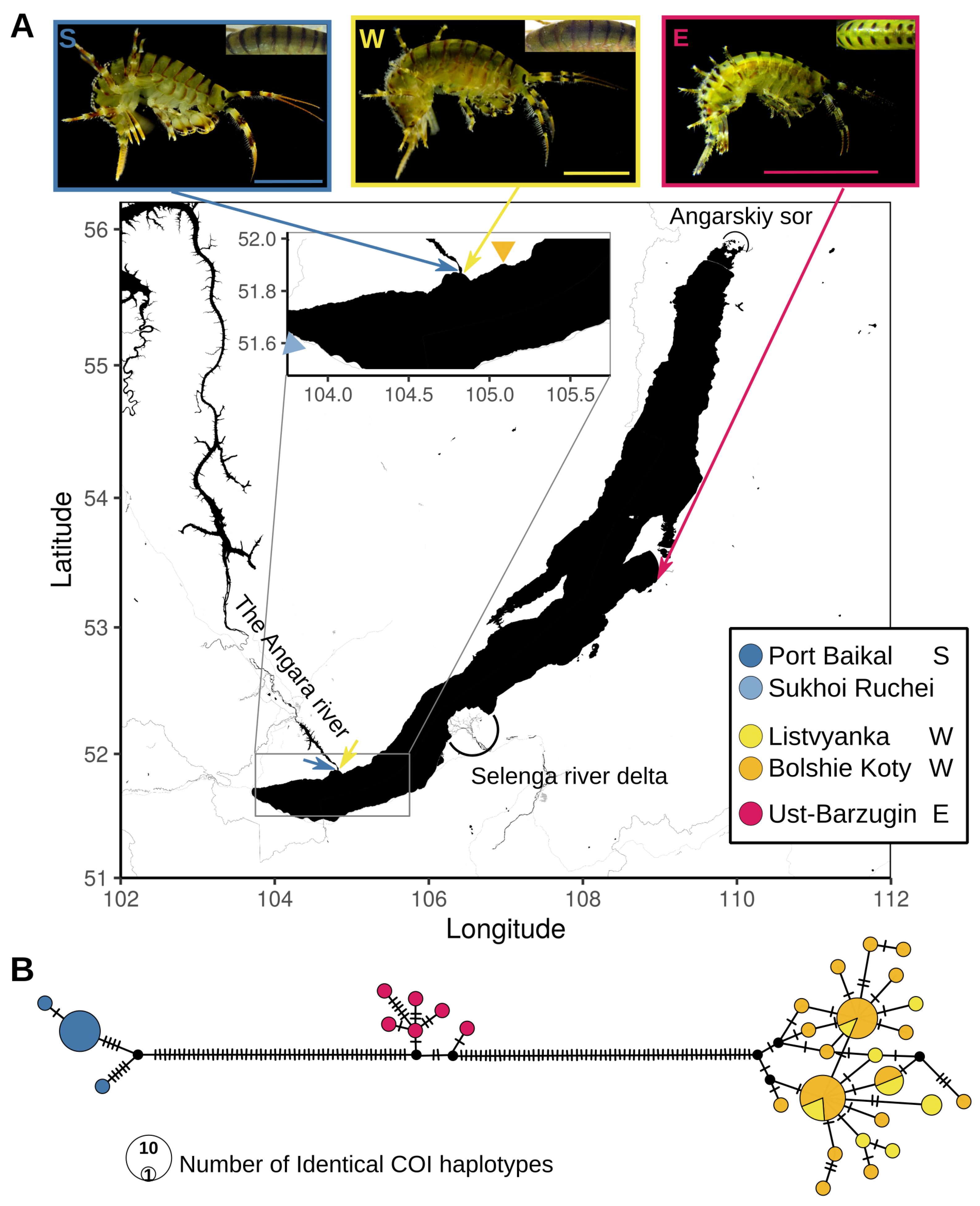
2. Results
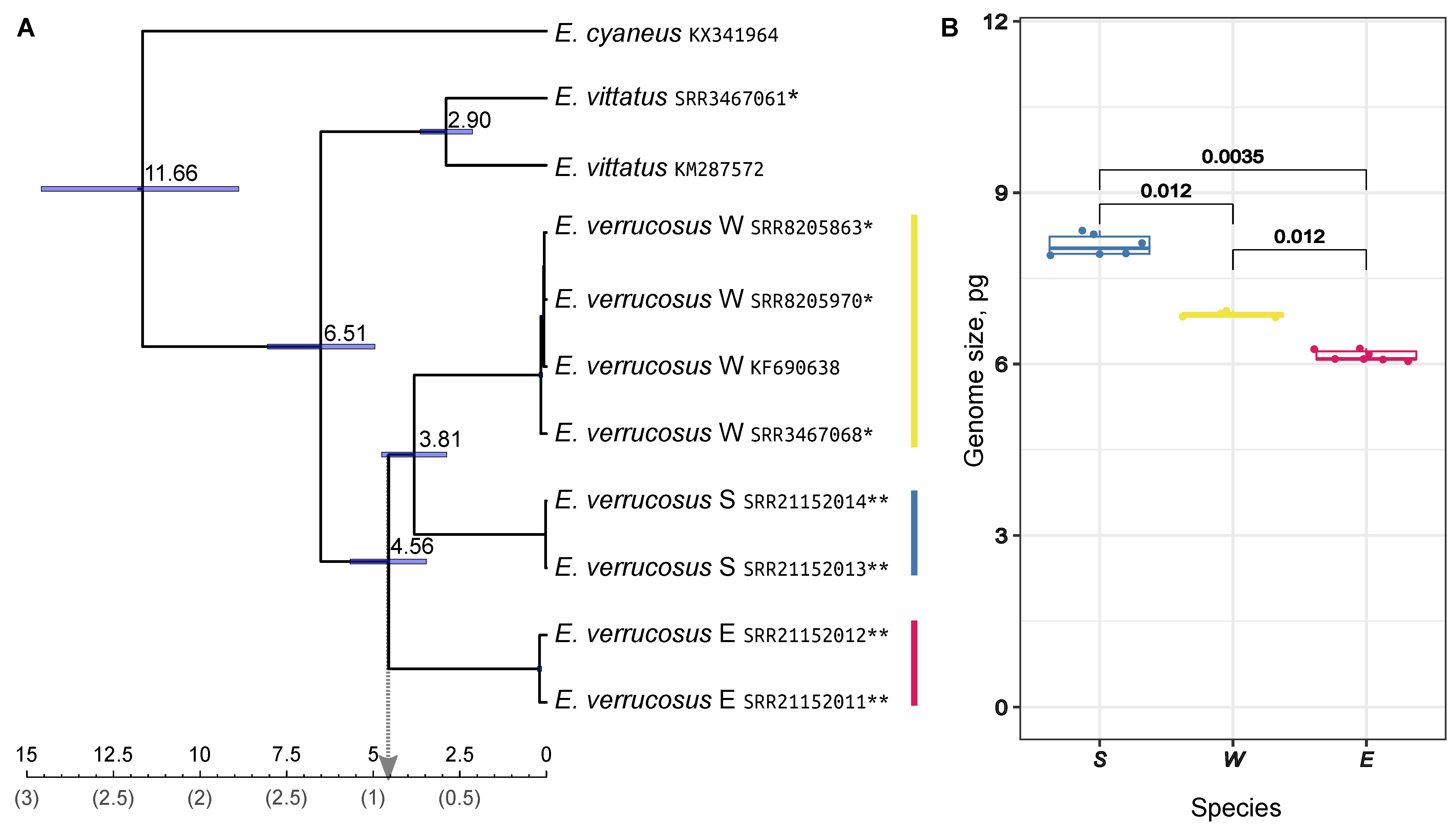
2.1. Molecular Phylogeny and Difference in Genome Sizes Confirm Deep Genetic Separation between E. verrucosus Species
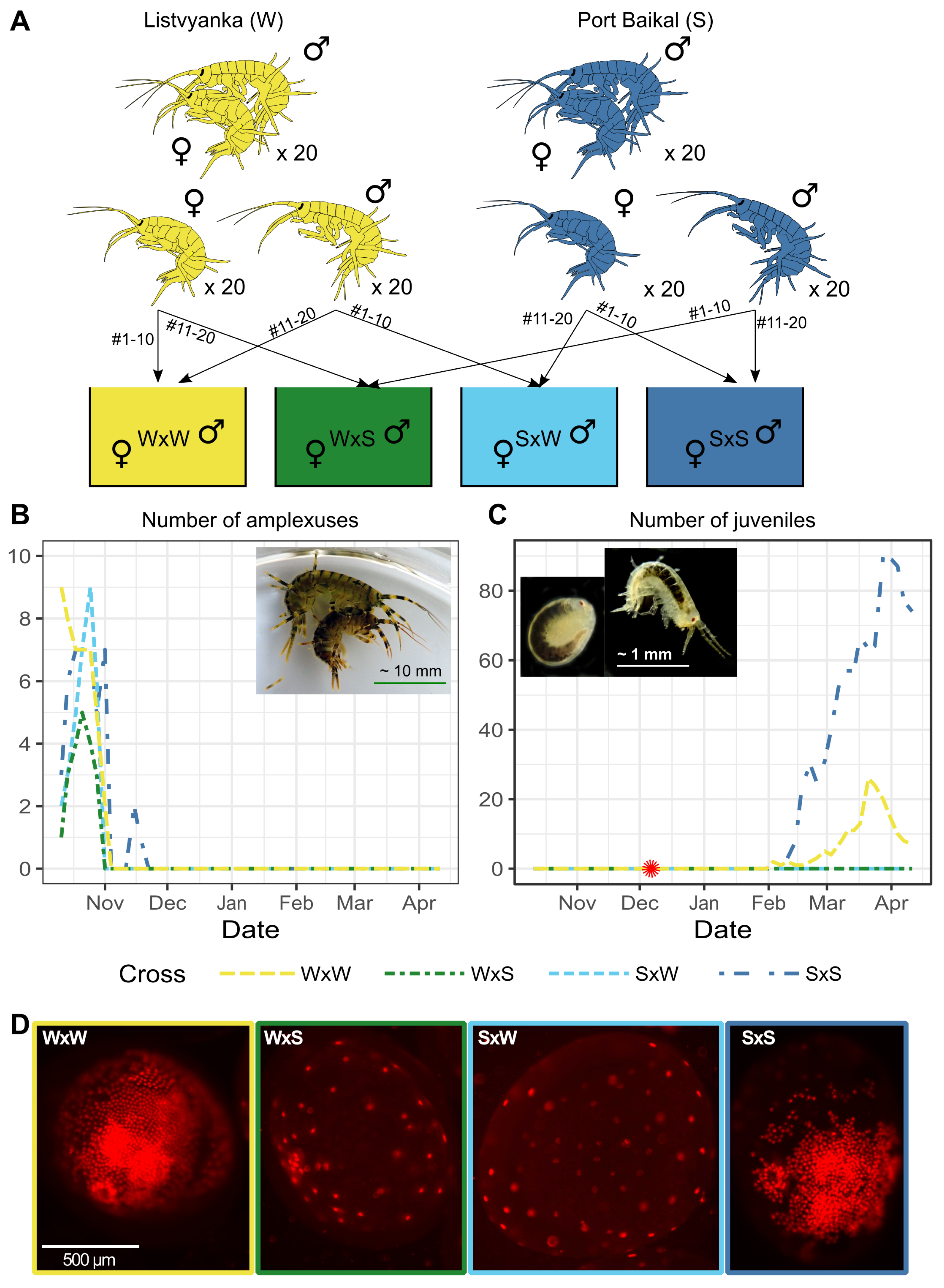
2.2. There Is a Reproductive Barrier between the Western and Southern Species
3. Discussion
4. Materials and Methods
4.1. Animal Sampling
4.2. RNA Sequencing and Phylogenetic Analysis
4.3. Flow Cytometry
4.4. Crossing Experiment
4.5. Data Analysis and Figure Preparation
5. Conclusions
- In this work, we found genome size differences for three (pseudo)cryptic species within the Baikal amphipod E. verrucosus. The genome size differences may be a consequence rather than a trigger of speciation, but they additionally confirm the genetic separation within the studied species.
- We also showed that at least the two species that are morphologically indistinguishable and have adjacent ranges are separated by a post-zygotic reproductive barrier. The presence of a post-zygotic barrier without an absolute pre-zygotic barrier is slightly unusual, as it would be detrimental to the fitness due to the energy invested in reproductive effort. However, it is explainable, as these species are separated with a geographic barrier, and thus this loss of fitness does not occur and is not selected against. The factors that determine the incompatibility are an interesting target for future work, as these could provide a new insight into the speciation mechanisms.
- Taken together, these data indicate that the previously applied barcoding approach indeed effectively indicated the separate biological species within E. verrucosus. These results provide new data for investigating genome evolution within relatively short times and also highlight the need for precise tracking of the sample origin in any studies in this morphospecies. In the case of the regions where the particular species is unknown (such as the Angara-Yenisei river system), it is highly desirable to determine the barcoding lineage for monitoring studies.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COI or cox1 | Cytochrome c oxidase subunit I |
| MRCA | Most recent common ancestor |
| Mya | Million years ago |
| PI | Propidium iodide |
| Tya | Thousand years ago |
| W, S, E | Western, southern, and eastern, respectively |
References
- Schön, I.; Martens, K. Adaptive, pre-adaptive and non-adaptive components of radiations in ancient lakes: A review. Org. Divers. Evol. 2004, 4, 137–156. [Google Scholar] [CrossRef]
- Hampton, S.E.; McGowan, S.; Ozersky, T.; Virdis, S.G.P.; Vu, T.T.; Spanbauer, T.L.; Kraemer, B.M.; Swann, G.; Mackay, A.W.; Powers, S.M.; et al. Recent ecological change in ancient lakes. Limnol. Oceanogr. 2018, 63, 2277–2304. [Google Scholar] [CrossRef]
- Cristescu, M.E.; Adamowicz, S.J.; Vaillant, J.J.; Haffner, D.G. Ancient lakes revisited: From the ecology to the genetics of speciation. Mol. Ecol. 2010, 19, 4837–4851. [Google Scholar] [CrossRef] [PubMed]
- Kozhova, O.M.; Izmest’eva, L.R. (Eds.) Lake Baikal: Evolution and Biodiversity; Backhyus Publishers: Leiden, The Netherlands, 1998. [Google Scholar]
- Mats, V.D.; Shcherbakov, D.Y.; Efimova, I.M. Late Cretaceous-Cenozoic history of the Lake Baikal depression and formation of its unique biodiversity. Stratigr. Geol. Correl. 2011, 19, 404. [Google Scholar] [CrossRef]
- Timoshkin, O.A. Lake Baikal: Diversity of fauna, problems of its immiscibility and origin, ecology and “exotic” communities. In Index of Animal Species Inhabiting Lake Baikal and Its Catchment Area; Nauka: Novosibirsk, Russia, 2001; Volume 1, pp. 74–113. [Google Scholar]
- Takhteev, V. On the current state of taxonomy of the Baikal Lake amphipods (Crustacea: Amphipoda) and the typological ways of constructing their system. Arthropoda Sel. 2019, 28, 374–402. [Google Scholar] [CrossRef]
- Hou, Z.; Sket, B. A review of Gammaridae (Crustacea: Amphipoda): The family extent, its evolutionary history, and taxonomic redefinition of genera. Zool. J. Linn. Soc. 2016, 176, 323–348. [Google Scholar] [CrossRef]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Mayr, E. What is a species, and what is not? Philos. Sci. 1996, 63, 262–277. [Google Scholar] [CrossRef]
- Coyne, J.A. Genetics and speciation. Nature 1992, 355, 511–515. [Google Scholar] [CrossRef]
- Palumbi, S.R. Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Syst. 1994, 25, 547–572. [Google Scholar] [CrossRef]
- Blaxter, M.L. The promise of a DNA taxonomy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Lajus, D.; Sukhikh, N.; Alekseev, V. Cryptic or pseudocryptic: Can morphological methods inform copepod taxonomy? An analysis of publications and a case study of the Eurytemora affinis species complex. Ecol. Evol. 2015, 5, 2374–2385. [Google Scholar] [CrossRef] [PubMed]
- Fišer, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 27, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Delić, T.; Trontelj, P.; Rendoš, M.; Fišer, C. The importance of naming cryptic species and the conservation of endemic subterranean amphipods. Sci. Rep. 2017, 7, 3391. [Google Scholar] [CrossRef]
- Rocha-Olivares, A.; Fleeger, J.W.; Foltz, D.W. Differential tolerance among cryptic species: A potential cause of pollutant-related reductions in genetic diversity. Environ. Toxicol. Chem. 2004, 23, 2132–2137. [Google Scholar] [CrossRef]
- Feckler, A.; Thielsch, A.; Schwenk, K.; Schulz, R.; Bundschuh, M. Differences in the sensitivity among cryptic lineages of the Gammarus fossarum complex. Sci. Total Environ. 2012, 439, 158–164. [Google Scholar] [CrossRef]
- Galipaud, M.; Bollache, L.; Lagrue, C. Variations in infection levels and parasite-induced mortality among sympatric cryptic lineages of native amphipods and a congeneric invasive species: Are native hosts always losing? Int. J. Parasitol. Parasites Wildl. 2017, 6, 439–447. [Google Scholar] [CrossRef]
- Lagrue, C.; Wattier, R.; Galipaud, M.; Gauthey, Z.; Rullmann, J.P.; Dubreuil, C.; Rigaud, T.; Bollache, L. Confrontation of cryptic diversity and mate discrimination within Gammarus pulex and Gammarus fossarum species complexes. Freshw. Biol. 2014, 59, 2555–2570. [Google Scholar] [CrossRef]
- Mamos, T.; Wattier, R.; Burzyński, A.; Grabowski, M. The legacy of a vanished sea: A high level of diversification within a European freshwater amphipod species complex driven by 15 My of Paratethys regression. Mol. Ecol. 2016, 25, 795–810. [Google Scholar] [CrossRef]
- Katouzian, A.R.; Sari, A.; Macher, J.N.; Weiss, M.; Saboori, A.; Leese, F.; Weigand, A.M. Drastic underestimation of amphipod biodiversity in the endangered Irano-Anatolian and Caucasus biodiversity hotspots. Sci. Rep. 2016, 6, 22507. [Google Scholar] [CrossRef][Green Version]
- Grabowski, M.; Mamos, T.; Bącela-Spychalska, K.; Rewicz, T.; Wattier, R.A. Neogene paleogeography provides context for understanding the origin and spatial distribution of cryptic diversity in a widespread Balkan freshwater amphipod. PeerJ 2017, 5, e3016. [Google Scholar] [CrossRef] [PubMed]
- Alther, R.; Fišer, C.; Altermatt, F. Description of a widely distributed but overlooked amphipod species in the European Alps. Zool. J. Linn. Soc. 2017, 179, 751–766. [Google Scholar] [CrossRef]
- Hupało, K.; Karaouzas, I.; Mamos, T.; Grabowski, M. Molecular data suggest multiple origins and diversification times of freshwater gammarids on the Aegean archipelago. Sci. Rep. 2020, 10, 19813. [Google Scholar] [CrossRef] [PubMed]
- Mamos, T.; Jażdżewski, K.; Čiamporová Zaťovičová, Z.; Čiampor, F.; Grabowski, M. Fuzzy species borders of glacial survivalists in the Carpathian biodiversity hotspot revealed using a multimarker approach. Sci. Rep. 2021, 11, 21629. [Google Scholar] [CrossRef] [PubMed]
- Copilaş-Ciocianu, D.; Rewicz, T.; Sands, A.F.; Palatov, D.; Marin, I.; Arbačiauskas, K.; Hebert, P.D.N.; Grabowski, M.; Audzijonyte, A. A DNA barcode reference library for endemic Ponto-Caspian amphipods. Sci. Rep. 2022, 12, 11332. [Google Scholar] [CrossRef]
- Müller, J. Mitochondrial DNA Variation and the Evolutionary History of Cryptic Gammarus fossarum Types. Mol. Phylogenetics Evol. 2000, 15, 260–268. [Google Scholar] [CrossRef]
- Wattier, R.; Mamos, T.; Copilaş-Ciocianu, D.; Jelić, M.; Ollivier, A.; Chaumot, A.; Danger, M.; Felten, V.; Piscart, C.; Žganec, K.; et al. Continental-scale patterns of hyper-cryptic diversity within the freshwater model taxon Gammarus fossarum (Crustacea, Amphipoda). Sci. Rep. 2020, 10, 16536. [Google Scholar] [CrossRef]
- Hou, Z.; Jin, P.; Liu, H.; Qiao, H.; Sket, B.; Cannizzaro, A.G.; Berg, D.J.; Li, S. Past climate cooling promoted global dispersal of amphipods from Tian Shan montane lakes to circumboreal lakes. Glob. Change Biol. 2022, 28, 3830–3845. [Google Scholar] [CrossRef]
- Hupalo, K.; Copilaș-Ciocianu, D.; Leese, F.; Weiss, M. COI Is Not Always Right: Integrative Taxonomy Reveals Striking Overestimation of Species Diversity in a Meditertranean Freshwater Amphipod; Researchgate: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Kamaltynov, R.; Väinölä, R. Species diversity and speciation in the endemic amphipods of Lake Baikal: Molecular evidence. Crustaceana 1999, 72, 945–956. [Google Scholar] [CrossRef]
- Mashiko, K.; Kamaltynov, R.; Morino, H.; Sherbakov, D.Y. Genetic differentiation among gammarid (Eulimnogammarus cyaneus) populations in Lake Baikal, East Siberia. Arch. Für Hydrobiol. 2000, 249–261. [Google Scholar] [CrossRef]
- Gomanenko, G.V.; Kamaltynov, R.M.; Kuzmenkova, Z.V.; Berenos, K.; Sherbakov, D.Y. Population Structure of the Baikalian Amphipod Gmelinoides fasciatus (Stebbing). Russ. J. Genet. 2005, 41, 907–912. [Google Scholar] [CrossRef]
- Bukin, Y.S.; Petunina, J.V.; Sherbakov, D.Y. The Mechanisms for genetic diversity of Baikal endemic amphipod Gmelinoides fasciatus: Relationships between the population processes and paleoclimatic history of the lake. Russ. J. Genet. 2018, 54, 1059–1068. [Google Scholar] [CrossRef]
- Daneliya, M.E.; Väinölä, R. Five subspecies of the Dorogostaiskia parasitica complex (Dybowsky) (Crustacea: Amphipoda: Acanthogammaridae), epibionts of sponges in Lake Baikal. Hydrobiologia 2014, 739, 95–117. [Google Scholar] [CrossRef]
- Daneliya, M.E.; Kamaltynov, R.M.; Väinölä, R. Phylogeography and systematics of Acanthogammarus s. str., giant amphipod crustaceans from Lake Baikal. Zool. Scr. 2011, 40, 623–637. [Google Scholar] [CrossRef]
- Gurkov, A.; Rivarola-Duarte, L.; Bedulina, D.; Fernández Casas, I.; Michael, H.; Drozdova, P.; Nazarova, A.; Govorukhina, E.; Timofeyev, M.; Stadler, P.F.; et al. Indication of ongoing amphipod speciation in Lake Baikal by genetic structures within endemic species. BMC Evol. Biol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Naumenko, S.A.; Logacheva, M.D.; Popova, N.V.; Klepikova, A.V.; Penin, A.A.; Bazykin, G.A.; Etingova, A.E.; Mugue, N.S.; Kondrashov, A.S.; Yampolsky, L.Y. Transcriptome-based phylogeny of endemic Lake Baikal amphipod species flock: Fast speciation accompanied by frequent episodes of positive selection. Mol. Ecol. 2017, 26, 536–553. [Google Scholar] [CrossRef]
- Hultgren, K.M.; Jeffery, N.W.; Moran, A.; Gregory, T.R. Latitudinal variation in genome size in crustaceans. Biol. J. Linn. Soc. 2018, 123, 348–359. [Google Scholar] [CrossRef]
- Ritchie, H.; Jamieson, A.J.; Piertney, S.B. Genome size variation in deep-sea amphipods. R. Soc. Open Sci. 2017, 4, 170862. [Google Scholar] [CrossRef]
- Hancock, Z.B.; Hardin, F.O.; Murthy, A.; Hillhouse, A.; Johnston, J.S. Rapid genomic expansion and purging associated with habitat transitions in a clade of beach crustaceans (Amphipoda: Haustoriidae). J. Crustac. Biol. 2021, 41, ruab042. [Google Scholar] [CrossRef]
- Vergilino, R.; Dionne, K.; Nozais, C.; Dufresne, F.; Belzile, C. Genome size differences in Hyalella cryptic species. Genome 2012, 55, 134–139. [Google Scholar] [CrossRef]
- Jeffery, N.W.; Gregory, T.R. Genome size estimates for crustaceans using Feulgen image analysis densitometry of ethanol-preserved tissues. Cytom. Part A 2014, 85, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.W.; Yampolsky, L.; Gregory, T.R. Nuclear DNA content correlates with depth, body size, and diversification rate in amphipod crustaceans from ancient Lake Baikal, Russia. Genome 2017, 60, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bazikalova, A.Y. Amphipods of Lake Baikal (in Russian). Proc. Baikal Limnol. Stn. 1945, 11, 1–440. [Google Scholar]
- Kravtsova, L.; Kamaltynov, R.; Karabanov, E.; Mekhanikova, I.; Sitnikova, T.Y.; Rozhkova, N.; Slugina, Z.; Izhboldina, L.; Weinberg, I.; Akinshina, T.; et al. Macrozoobenthic communities of underwater landscapes in the shallow-water zone of southern Lake Baikal. Hydrobiologia 2004, 522, 193–205. [Google Scholar] [CrossRef]
- Gerstfeldt, G. Ueber einige zum Theil neue Arten Platoden, Anneliden, Myriapoden and Crustaceen Sibirien’s, namentlich, seines ostlichen Theiles und des Amur-Gebietes. Mém prés Acad. imp Sci St.-Pétersbourg 1858, 8, 259–296. [Google Scholar]
- Bazikalova, A. The amphipods of the Angara River (in Russian). Trudy Baikal. Limnol. Inst. SO AN SSSR 1957, 15, 377–378. [Google Scholar]
- Mekhanikova, I. Amphipods (Crustacea, Amphipoda) of Angara River head and Irkutsk reservoir (1978–2008). Zool. Zhurnal 2016, 95, 826–836. [Google Scholar] [CrossRef]
- Andrianova, A.V.; Yakubailik, O.E. Geoinformational Web-System for the Analysis of the Expansion of the Baikal Crustaceans of the Yenisei River. In Information Technologies in the Research of Biodiversity; Springer Proceedings in Earth and Environmental Sciences; Bychkov, I., Voronin, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 125–130. [Google Scholar] [CrossRef]
- Timofeyev, M.A.; Shatilina, Z.M.; Bedulina, D.S.; Menzel, R.; Steinberg, C.E.W. Natural organic matter (NOM) has the potential to modify the multixenobiotic resistance (MXR) activity in freshwater amphipods Eulimnogammarus cyaneus and E. verrucosus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 496–503. [Google Scholar] [CrossRef]
- Rueckert, S.; Simdyanov, T.G.; Aleoshin, V.V.; Leander, B.S. Identification of a Divergent Environmental DNA Sequence Clade Using the Phylogeny of Gregarine Parasites (Apicomplexa) from Crustacean Hosts. PLoS ONE 2011, 6, e18163. [Google Scholar] [CrossRef]
- Shatilina, Z.M.; Wolfgang Riss, H.; Protopopova, M.V.; Trippe, M.; Meyer, E.I.; Pavlichenko, V.V.; Bedulina, D.S.; Axenov-Gribanov, D.V.; Timofeyev, M.A. The role of the heat shock proteins (HSP70 and sHSP) in the thermotolerance of freshwater amphipods from contrasting habitats. J. Therm. Biol. 2011, 36, 142–149. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Natyaganova, A. The first cytogenetic report of the endemic fish leech Baicalobdella torquata (Hirudinida, Piscicolidae) from Lake Baikal. Lauterbornia 2012, 75, 63–70. [Google Scholar]
- Axenov-Gribanov, D.; Bedulina, D.; Shatilina, Z.; Jakob, L.; Vereshchagina, K.; Lubyaga, Y.; Gurkov, A.; Shchapova, E.; Luckenbach, T.; Lucassen, M.; et al. Thermal Preference Ranges Correlate with Stable Signals of Universal Stress Markers in Lake Baikal Endemic and Holarctic Amphipods. PLoS ONE 2016, 11, e0164226. [Google Scholar] [CrossRef] [PubMed]
- Bedulina, D.; Meyer, M.F.; Gurkov, A.; Kondratjeva, E.; Baduev, B.; Gusdorf, R.; Timofeyev, M.A. Intersexual differences of heat shock response between two amphipods (Eulimnogammarus verrucosus and Eulimnogammarus cyaneus) in Lake Baikal. PeerJ 2017, 5, e2864. [Google Scholar] [CrossRef] [PubMed]
- Shchapova, E.; Nazarova, A.; Gurkov, A.; Borvinskaya, E.; Rzhechitskiy, Y.; Dmitriev, I.; Meglinski, I.; Timofeyev, M. Application of PEG-covered non-biodegradable polyelectrolyte microcapsules in the crustacean circulatory system on the example of the amphipod Eulimnogammarus verrucosus. Polymers 2019, 11, 1246. [Google Scholar] [CrossRef]
- Lipaeva, P.; Vereshchagina, K.; Drozdova, P.; Jakob, L.; Kondrateva, E.; Lucassen, M.; Bedulina, D.; Timofeyev, M.; Stadler, P.; Luckenbach, T. Different ways to play it cool: Transcriptomic analysis sheds light on different activity patterns of three amphipod species under long-term cold exposure. Mol. Ecol. 2021, 30, 5735–5751. [Google Scholar] [CrossRef]
- Jakob, L.; Vereshchagina, K.P.; Tillmann, A.; Rivarola-Duarte, L.; Axenov-Gribanov, D.V.; Bedulina, D.S.; Gurkov, A.N.; Drozdova, P.; Timofeyev, M.A.; Stadler, P.F.; et al. Thermal reaction norms of key metabolic enzymes reflect divergent physiological and behavioral adaptations of closely related amphipod species. Sci. Rep. 2021, 11, 4562. [Google Scholar] [CrossRef]
- Rivarola-Duarte, L.; Otto, C.; Jühling, F.; Schreiber, S.; Bedulina, D.; Jakob, L.; Gurkov, A.; Axenov-Gribanov, D.; Sahyoun, A.H.; Lucassen, M.; et al. A first Glimpse at the genome of the Baikalian amphipod Eulimnogammarus verrucosus. J. Exp. Zool. Part B Mol. Dev. Evol. 2014, 322, 177–189. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Units of measurement of genome size. In The Evolution of Genome Size; Cavalier-Smith, T., Ed.; John Wiley & Sons: New York, NY, USA, 1985; p. X. [Google Scholar]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Macdonald, K.S., III; Yampolsky, L.; Duffy, J.E. Molecular and morphological evolution of the amphipod radiation of Lake Baikal. Mol. Phylogenetics Evol. 2005, 35, 323–343. [Google Scholar] [CrossRef]
- Drozdova, P.; Rivarola-Duarte, L.; Bedulina, D.; Axenov-Gribanov, D.; Schreiber, S.; Gurkov, A.; Shatilina, Z.; Vereshchagina, K.; Lubyaga, Y.; Madyarova, E.; et al. Comparison between transcriptomic responses to short-term stress exposures of a common Holarctic and endemic Lake Baikal amphipods. BMC Genom. 2019, 20, 712. [Google Scholar] [CrossRef] [PubMed]
- Romanova, E.V.; Aleoshin, V.V.; Kamaltynov, R.M.; Mikhailov, K.V.; Logacheva, M.D.; Sirotinina, E.A.; Gornov, A.Y.; Anikin, A.S.; Sherbakov, D.Y. Evolution of mitochondrial genomes in Baikalian amphipods. BMC Genom. 2016, 17, 1016. [Google Scholar] [CrossRef] [PubMed]
- Romanova, E.V.; Mikhailov, K.V.; Logacheva, M.D.; Kamaltynov, R.M.; Aleoshin, V.V.; Sherbakov, D.Y. The complete mitochondrial genome of Baikalian amphipoda Eulimnogammarus vittatus Dybowsky, 1874. Mitochondrial DNA Part A 2016, 27, 1795–1797. [Google Scholar] [CrossRef]
- Cormier, A.; Wattier, R.; Teixeira, M.; Rigaud, T.; Cordaux, R. The complete mitochondrial genome of Gammarus roeselii (Crustacea, Amphipoda): Insights into mitogenome plasticity and evolution. Hydrobiologia 2018, 825, 197–210. [Google Scholar] [CrossRef]
- Mamos, T.; Grabowski, M.; Rewicz, T.; Bojko, J.; Strapagiel, D.; Burzyński, A. Mitochondrial Genomes, Phylogenetic Associations, and SNP Recovery for the Key Invasive Ponto-Caspian Amphipods in Europe. Int. J. Mol. Sci. 2021, 22, 10300. [Google Scholar] [CrossRef]
- Romanova, E.V.; Bukin, Y.S.; Mikhailov, K.V.; Logacheva, M.D.; Aleoshin, V.V.; Sherbakov, D.Y. The Mitochondrial Genome of a Freshwater Pelagic Amphipod Macrohectopus branickii Is among the Longest in Metazoa. Genes 2021, 12, 2030. [Google Scholar] [CrossRef]
- Mats, V.D.; Perepelova, T.I. A new perspective on evolution of the Baikal Rift. Geosci. Front. 2011, 2, 349–365. [Google Scholar] [CrossRef]
- Copilaş-Ciocianu, D.; Sidorov, D.; Gontcharov, A. Adrift across tectonic plates: Molecular phylogenetics supports the ancient Laurasian origin of old limnic crangonyctid amphipods. Org. Divers. Evol. 2019, 19, 191–207. [Google Scholar] [CrossRef]
- Browne, W.E.; Price, A.L.; Gerberding, M.; Patel, N.H. Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 2005, 42, 124–149. [Google Scholar] [CrossRef]
- Pfenninger, M.; Schwenk, K. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol. Biol. 2007, 7, 121. [Google Scholar] [CrossRef]
- Hessen, D.O.; Daufresne, M.; Leinaas, H.P. Temperature-size relations from the cellular-genomic perspective. Biol. Rev. 2013, 88, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Alfsnes, K.; Leinaas, H.P.; Hessen, D.O. Genome size in arthropods; different roles of phylogeny, habitat and life history in insects and crustaceans. Ecol. Evol. 2017, 7, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Salemaa, H.; Kamaltynov, R. The chromosome numbers of endemic Amphipoda and Isopoda—An evolutionary paradox in the ancient lakes Ohrid and Baikal. In Speciation in Ancient Lakes; Schweizerbart Science Publishers: Stuttgart, Germany, 1994; Volume 44, pp. 247–256. [Google Scholar]
- Brown, W.M.; George, M.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Cothran, R.D.; Stiff, A.R.; Chapman, K.; Wellborn, G.A.; Relyea, R.A. Reproductive interference via interspecific pairing in an amphipod species complex. Behav. Ecol. Sociobiol. 2013, 67, 1357–1367. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Hogg, I.D.; Waas, J.R. Phylogeography and species discrimination in the Paracalliope fluviatilis species complex (Crustacea: Amphipoda): Can morphologically similar heterospecifics identify compatible mates? Biol. J. Linn. Soc. 2010, 99, 196–205. [Google Scholar] [CrossRef][Green Version]
- Alwes, F.; Hinchen, B.; Extavour, C.G. Patterns of cell lineage, movement, and migration from germ layer specification to gastrulation in the amphipod crustacean Parhyale hawaiensis. Dev. Biol. 2011, 359, 110–123. [Google Scholar] [CrossRef]
- Forni, G.; Puccio, G.; Bourguignon, T.; Evans, T.; Mantovani, B.; Rota-Stabelli, O.; Luchetti, A. Complete mitochondrial genomes from transcriptomes: Assessing pros and cons of data mining for assembling new mitogenomes. Sci. Rep. 2019, 9, 14806. [Google Scholar] [CrossRef]
- Allio, R.; Schomaker-Bastos, A.; Romiguier, J.; Prosdocimi, F.; Nabholz, B.; Delsuc, F. MitoFinder: Efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Mol. Ecol. Resour. 2020, 20, 892–905. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. metaSPAdes: A new versatile metagenomic assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; the UGENE team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Nylander, J. catfasta2phyml. 2020. Available online: https://github.com/nylander/catfasta2phyml (accessed on 17 August 2022).
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace Aware Data Structure for Phylogenomic Inference from Supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; Maio, N.D.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree. 2021. Available online: https://github.com/rambaut/figtree (accessed on 10 August 2022).
- DeSalle, R.; Gregory, T.R.; Johnston, J.S. Preparation of samples for comparative studies of arthropod chromosomes: Visualization, In situ hybridization, and genome size estimation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2005; Volume 395, pp. 460–488. [Google Scholar] [CrossRef]
- Picard, C.J.; Johnston, J.S.; Tarone, A.M. Genome sizes of forensically relevant Diptera. J. Med. Entomol. 2012, 49, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.R. Animal Genome Size Database. Available online: http://www.genomesize.com/ (accessed on 1 April 2022).
- Govorukhina, E. Biology of Reproduction, Seasonal and Daily Dynamics of Littoral and Sublittoral Amphipod Species of Lake Baikal. Ph.D. Thesis, Irkutsk State University, Irkutsk, Russia, 2005. [Google Scholar]
- Drozdova, P.; Kizenko, A.; Saranchina, A.; Gurkov, A.; Firulyova, M.; Govorukhina, E.; Timofeyev, M. The diversity of opsins in Lake Baikal amphipods (Amphipoda: Gammaridae). BMC Ecol. Evol. 2021, 21, 81. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 1 April 2022).
- Schauberger, P.; Walker, A. openxlsx: Read, Write and Edit xlsx Files. R Package Version 4.2.5. Available online: https://CRAN.R-project.org/package=openxlsx (accessed on 15 January 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 June 2022).
- Wickham, H.; Seidel, D. scales: Scale Functions for Visualization. R Package Version 1.1.1. Available online: https://CRAN.R-project.org/package=scales (accessed on 5 June 2022).
- Draw Freely|Inkscape. Available online: https://inkscape.org/ (accessed on 10 September 2019).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdova, P.; Saranchina, A.; Madyarova, E.; Gurkov, A.; Timofeyev, M. Experimental Crossing Confirms Reproductive Isolation between Cryptic Species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal. Int. J. Mol. Sci. 2022, 23, 10858. https://doi.org/10.3390/ijms231810858
Drozdova P, Saranchina A, Madyarova E, Gurkov A, Timofeyev M. Experimental Crossing Confirms Reproductive Isolation between Cryptic Species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal. International Journal of Molecular Sciences. 2022; 23(18):10858. https://doi.org/10.3390/ijms231810858
Chicago/Turabian StyleDrozdova, Polina, Alexandra Saranchina, Ekaterina Madyarova, Anton Gurkov, and Maxim Timofeyev. 2022. "Experimental Crossing Confirms Reproductive Isolation between Cryptic Species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal" International Journal of Molecular Sciences 23, no. 18: 10858. https://doi.org/10.3390/ijms231810858
APA StyleDrozdova, P., Saranchina, A., Madyarova, E., Gurkov, A., & Timofeyev, M. (2022). Experimental Crossing Confirms Reproductive Isolation between Cryptic Species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal. International Journal of Molecular Sciences, 23(18), 10858. https://doi.org/10.3390/ijms231810858






