Promising Application of D-Amino Acids toward Clinical Therapy
Abstract
1. Introduction
2. The Treatment of Diseases Relating to Nervous System with D-AAs
2.1. Schizophrenia and Post-Traumatic Stress Disorder
2.2. Parkinson’s Disease and Seizure
2.3. Improving Emotion, Motot, Preference, Memory, and Cognitive Ability
2.4. Acoustic and Visual Protection
2.5. The Analgesic Effect and Protective Action of D-AAs on Nervous System
3. D-AAs Protect Tissue/Organ against Injury
3.1. Acute Kidney Injury (AKI)
3.2. Reproduction
3.3. Hypertension, Gastric, Colitis
4. D-AAs Protect Normal Cell from Radiation or Chemotherapy and Inhibits the Growth of Tumor Cell or Contamination Cell
5. D-AAs Inhibit the Formation of Biofilm and Enhance Antibiotic Effect
6. Incorporating D-AAs into Drugs Afford Stronger Biostability and Efficiency
7. The Source and Administration of D-AAs for Trials Using, as well as Safety
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Genchi, G. An overview on d-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Cava, F.; Lam, H.; de Pedro, M.A.; Waldor, M.K. Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell. Mol. Life Sci. 2011, 68, 817–831. [Google Scholar] [CrossRef]
- Satoh, S.; Esashi, Y. D-Amino-acid-stimulated ethylene production in seed tissues. Planta 1980, 149, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 2013, 42, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, nutrition, and microbiology of D-amine acids. J. Agric. Food Chem. 1999, 47, 3457–3479. [Google Scholar] [CrossRef]
- Miyamoto, T.; Homma, H. D-Amino acid metabolism in bacteria. J. Biochem. 2021, 170, 5–13. [Google Scholar] [CrossRef]
- Radkov, A.D.; Moe, L.A. Bacterial synthesis of D-amino acids. Appl. Microbiol. Biotechnol. 2014, 98, 5363–5374. [Google Scholar] [CrossRef]
- Lam, H.; Oh, D.-C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-Amino Acids Govern Stationary Phase Cell Wall Remodeling in Bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef]
- Horcajo, P.; de Pedro, M.A.; Cava, F. Peptidoglycan Plasticity in Bacteria: Stress-Induced Peptidoglycan Editing by Noncanonical D-Amino Acids. Microb. Drug Resist. 2012, 18, 306–313. [Google Scholar] [CrossRef]
- Kolukisaoglu, U. D-Amino Acids in Plants: Sources, Metabolism, and Functions. Int. J. Mol. Sci. 2020, 21, 5421. [Google Scholar] [CrossRef]
- Vranova, V.; Zahradnickova, H.; Janous, D.; Skene, K.R.; Matharu, A.S.; Rejsek, K.; Formanek, P. The significance of D-amino acids in soil, fate and utilization by microbes and plants: Review and identification of knowledge gaps. Plant Soil 2012, 354, 21–39. [Google Scholar] [CrossRef]
- Chieffi Baccari, G.; Falvo, S.; Santillo, A.; Di Giacomo Russo, F.; Di Fiore, M.M. d-Amino acids in mammalian endocrine tissues. Amino Acids 2020, 52, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Hamase, K.; Morikawa, A.; Zaitsu, K. D-Amino acids in mammals and their diagnostic value. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2002, 781, 73–91. [Google Scholar] [CrossRef]
- Ollivaux, C.; Soyez, D.; Toullec, J.-Y. Biogenesis of D-amino acid containing peptides/proteins: Where, when and how? J. Pept. Sci. 2014, 20, 595–612. [Google Scholar] [CrossRef]
- Kreil, G. D-Amino acids in animal peptides. Annu. Rev. Biochem. 1997, 66, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Sato, T.; Enomoto, N.; Ishii, Y.; Sasaki, K.; Yamada, T. High concentrations of D-amino acids in human gastric juice. Amino Acids 2007, 32, 137–140. [Google Scholar] [CrossRef]
- Fisher, G.H.; D’Aniello, A.; Vetere, A.; Padula, L.; Cusano, G.P.; Man, E.H. Free D-aspartate and D-alanine in normal and Alzheimer brain. Brain Res. Bull. 1991, 26, 983–985. [Google Scholar] [CrossRef]
- Kimura, T.; Hamase, K.; Miyoshi, Y.; Yamamoto, R.; Yasuda, K.; Mita, M.; Rakugi, H.; Hayashi, T.; Isaka, Y. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci. Rep. 2016, 6, 26137. [Google Scholar] [CrossRef]
- Tsai, G.; Yang, P.; Chung, L.-C.; Lange, N.; Coyle, J.T. D-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 1998, 44, 1081–1089. [Google Scholar] [CrossRef]
- Hin, N.; Duvall, B.; Berry, J.F.; Ferraris, D.V.; Rais, R.; Alt, J.; Rojas, C.; Slusher, B.S.; Tsukamoto, T. D-Amino acid oxidase inhibitors based on the 5-hydroxy-1,2,4-triazin-6(1H)-one scaffold. Bioorg. Med. Chem. Lett. 2016, 26, 2088–2091. [Google Scholar] [CrossRef]
- Franchini, L.; Carrano, N.; Di Luca, M.; Gardoni, F. Synaptic GluN2A-Containing NMDA Receptors: From Physiology to Pathological Synaptic Plasticity. Int. J. Mol. Sci. 2020, 21, 1538. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Oka, T. Free d-aspartate and d-serine in the mammalian brain and periphery. Prog. Neurobiol. 1997, 52, 325–353. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Lin, C.-H.; Lane, H.-Y. D-Amino Acids and pLG72 in Alzheimer’s Disease and Schizophrenia. Int. J. Mol. Sci. 2021, 22, 10917. [Google Scholar] [CrossRef]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409–13414. [Google Scholar] [CrossRef] [PubMed]
- Panatier, A.; Theodosis, D.T.; Mothet, J.-P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H.R. Glia-Derived d-Serine Controls NMDA Receptor Activity and Synaptic Memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef]
- Kleckner, N.W.; Dingledine, R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988, 241, 835–837. [Google Scholar] [CrossRef]
- Matsui, T.; Sekiguchi, M.; Hashimoto, A.; Tomita, U.; Nishikawa, T.; Wada, K. Functional comparison of d-serine and glycine in rodents—The effect on cloned nmda receptors and the extracellular concentration. J. Neurochem. 1995, 65, 454–458. [Google Scholar] [CrossRef]
- Coyle, J.T.; Tsai, G.; Goff, D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. In Glutamate and Disorders of Cognition and Motivation; Moghaddam, B., Wolf, M., Eds.; New York Academy of Sciences: New York, NY, USA, 2003; Volume 1003, pp. 318–327. [Google Scholar]
- Balu, D.T. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. Adv. Pharm. 2016, 76, 351–382. [Google Scholar] [CrossRef]
- Coyle, J.T. NMDA Receptor and Schizophrenia: A Brief History. Schizophr. Bull. 2012, 38, 920–926. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Dor-Abarbanel, A.E.; Edelman, S.; Durrant, A.R.; Hashimoto, K.; Javitt, D.C.; Heresco-Levy, U. Behavioral and cognitive effects of the N-methyl-d-aspartate receptor co-agonist d-serine in healthy humans: Initial findings. J. Psychiatr. Res. 2015, 61, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bendikov, I.; Nadri, C.; Amar, S.; Panizzutti, R.; De Miranda, J.; Wolosker, H.; Agam, G. A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr. Res. 2007, 90, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.E.; Yang, P.; Chang, Y.-C.; Chong, M.-Y. D-Alanine Added to Antipsychotics for the Treatment of Schizophrenia. Biol. Psychiatry 2006, 59, 230–234. [Google Scholar] [CrossRef]
- Ohnuma, T.; Sakai, Y.; Maeshima, H.; Hatano, T.; Hanzawa, R.; Abe, S.; Kida, S.; Shibata, N.; Suzuki, T.; Arai, H. Changes in plasma glycine, l-serine, and d-serine levels in patients with schizophrenia as their clinical symptoms improve: Results from the Juntendo University Schizophrenia Projects (JUSP). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1905–1912. [Google Scholar] [CrossRef]
- Ehlers, A.; Clark, D.M. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther. 2000, 38, 319–345. [Google Scholar] [CrossRef]
- Heresco-Levy, U.; Kremer, I.; Javitt, D.C.; Goichman, R.; Reshef, A.; Blanaru, M.; Cohen, T. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 2002, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Heresco-Levy, U.; Vass, A.; Bloch, B.; Wolosker, H.; Dumin, E.; Balan, L.; Deutsch, L.; Kremer, I. Pilot controlled trial of d-serine for the treatment of post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 2009, 12, 1275–1282. [Google Scholar] [CrossRef]
- Gelfin, E.; Kaufman, Y.; Korn-Lubetzki, I.; Bloch, B.; Kremer, I.; Javitt, D.C.; Heresco-Levy, U. D-serine adjuvant treatment alleviates behavioural and motor symptoms in Parkinson’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 543–549. [Google Scholar] [CrossRef]
- Beesley, S.; Sullenberger, T.; Ailani, R.; D’Orio, C.; Crockett, M.S.; Kumar, S.S. d-Serine Intervention In The Medial Entorhinal Area Alters TLE-Related Pathology In CA1 Hippocampus Via The Temporoammonic Pathway. Neuroscience 2021, 453, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.; Sullenberger, T.; Crotty, K.; Ailani, R.; D’Orio, C.; Evans, K.; Ogunkunle, E.O.; Roper, M.G.; Kumar, S.S. D-serine mitigates cell loss associated with temporal lobe epilepsy. Nat. Commun. 2020, 11, 4966. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Santos, P.; O’Riordan, K.J.; Stafstrom, C.E.; Marie Hardwick, J. Potent anti-seizure effects of D-leucine. Neurobiol. Dis. 2015, 82, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Avellar, M.; Scoriels, L.; Madeira, C.; Vargas-Lopes, C.; Marques, P.; Dantas, C.; Manhaes, A.C.; Leite, H.; Panizzutti, R. The effect of D-serine administration on cognition and mood in older adults. Oncotarget 2016, 7, 11881–11888. [Google Scholar] [CrossRef] [PubMed]
- Guercio, G.D.; Bevictori, L.; Vargas-Lopes, C.; Madeira, C.; Oliveira, A.; Carvalho, V.F.; d’Avila, J.C.; Panizzutti, R. d-serine prevents cognitive deficits induced by acute stress. Neuropharmacology 2014, 86, 1–8. [Google Scholar] [CrossRef]
- Fujita, Y.; Ishima, T.; Hashimoto, K. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci. Rep. 2016, 6, 37261. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Yang, C.; Jia, H.; Gu, Z.; Tu, X.; Tian, S.; Liu, J.; Li, G.; Ma, Y. D-serine Ameliorates Motor and Cognitive Impairments in β-amyloid 1-42 Injected Mice by Inhibiting JNK Signaling Pathway. J. Chem. Neuroanat. 2020, 109, 101852. [Google Scholar] [CrossRef]
- Bo, J.-Z.; Xue, L.; Li, S.; Yin, J.-W.; Li, Z.-Y.; Wang, X.; Wang, J.-F.; Zhang, Y.-S. D-serine reduces memory impairment and neuronal damage induced by chronic lead exposure. Neural Regen. Res. 2021, 16, 836–841. [Google Scholar] [CrossRef]
- Prokudina, O.I.; Alekhina, T.A. Effect of D-serine on Anxiety-like Behavior and Spatial Learning Ability in GC Rats Selected for the Predisposition to Catatonic Reactions. J. Evol. Biochem. Physiol. 2021, 57, 1267–1276. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, Z.; Zou, K.; Huang, Z.; Liu, L.; Yang, Y.; Wang, W. d-Serine produces antidepressant-like effects in mice through suppression of BDNF signaling pathway and regulation of synaptic adaptations in the nucleus accumbens. Mol. Med. 2021, 27, 127. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, F.; Yang, S.; Fang, P.; Deng, Z.-F.; Xiao, J.-L.; Hu, Z.-L.; Chen, J.-G. SKF83959 Produces Antidepressant Effects in a Chronic Social Defeat Stress Model of Depression through BDNF-TrkB Pathway. Int. J. Neuropsychopharmacol. 2015, 18, pyu096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atsushi, H.; Toru, N.; Takae, O.; Kiyohisa, T. D-Alanine inhibits methamphetamine-induced hyperactivity in rats. Eur. J. Pharmacol. 1991, 202, 105–107. [Google Scholar] [CrossRef]
- Sasaki, T.; Yasoshima, Y.; Matsui, S.; Yokota-Hashimoto, H.; Kobayashi, M.; Kitamura, T. Intraperitoneal injection of d-serine inhibits high-fat diet intake and preference in male mice. Appetite 2017, 118, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kinoshita, Y.; Matsui, S.; Kakuta, S.; Yokota-Hashimoto, H.; Kinoshita, K.; Iwasaki, Y.; Kinoshita, T.; Yada, T.; Amano, N.; et al. N-methyl-D-aspartate receptor coagonist D-serine suppresses intake of high-preference food. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R561–R575. [Google Scholar] [CrossRef]
- Semenza, E.R.; Harraz, M.M.; Abramson, E.; Malla, A.P.; Vasavda, C.; Gadalla, M.M.; Kornberg, M.D.; Snyder, S.H.; Roychaudhuri, R. D-cysteine is an endogenous regulator of neural progenitor cell dynamics in the mammalian brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2110610118. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Lin, Y.-J.; Lu, P.-C.; Tain, Y.-L. Early Supplementation of D-Cysteine or L-Cysteine Prevents Hypertension and Kidney Damage in Spontaneously Hypertensive Rats Exposed to High-Salt Intake. Mol. Nutr. Food Res. 2018, 62, 1700596. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Tripatara, P.; Patel, N.S.A.; Collino, M.; Gallicchio, M.; Kieswich, J.; Castiglia, S.; Benetti, E.; Stewart, K.N.; Brown, P.A.J.; Yaqoob, M.M.; et al. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Investig. 2008, 88, 1038–1048. [Google Scholar] [CrossRef]
- Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 2015, 439, 212–218. [Google Scholar] [CrossRef]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. Faseb J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef] [PubMed]
- Tripatara, P.; Patel, N.S.A.; Brancaleone, V.; Renshaw, D.; Rocha, J.; Sepodes, B.; Mota-Filipe, H.; Perretti, M.; Thiemermann, C. Characterisation of cystathionine gamma-lyase/hydrogen sulphide pathway in ischaemia/reperfusion injury of the mouse kidney: An in vivo study. Eur. J. Pharmacol. 2009, 606, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Sato, M.; Konno, A.; Hirai, H.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H. d-Cysteine promotes dendritic development in primary cultured cerebellar Purkinje cells via hydrogen sulfide production. Mol. Cell. Neurosci. 2018, 93, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Kimura, H. Production of hydrogen sulfide from d-cysteine and its therapeutic potential. Front. Endocrinol. 2013, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.K.M.; Araújo, T.S.L.; Sousa, N.A.; Sousa, F.B.M.; Nogueira, K.M.; Nicolau, L.A.D.; Medeiros, J.V.R. Evidence that d-cysteine protects mice from gastric damage via hydrogen sulfide produced by d-amino acid oxidase. Nitric Oxide 2017, 64, 1–6. [Google Scholar] [CrossRef]
- Ohta, T.; Morikawa, Y.; Sato, M.; Konno, A.; Hirai, H.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H.; Seki, T. Therapeutic potential of d-cysteine against in vitro and in vivo models of spinocerebellar ataxia. Exp. Neurol. 2021, 343, 113791. [Google Scholar] [CrossRef]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef]
- Ohinata, Y.; Yamasoba, T.; Schacht, J.; Miller, J.M. Glutathione limits noise-induced hearing loss. Hear. Res. 2000, 146, 28–34. [Google Scholar] [CrossRef]
- Campbell, K.; Claussen, A.; Meech, R.; Verhulst, S.; Fox, D.; Hughes, L. d-methionine (d-met) significantly rescues noise-induced hearing loss: Timing studies. Hear. Res. 2011, 282, 138–144. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J.; et al. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997, 18, 559–571. [Google Scholar] [PubMed]
- Campbell, K.C.M.; Rybak, L.P.; Meech, R.P.; Hughes, L. D-Methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear. Res. 1996, 102, 90–98. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Meech, R.P.; Klemens, J.J.; Gerberi, M.T.; Dyrstad, S.S.W.; Larsen, D.L.; Mitchell, D.L.; El-Azizi, M.; Verhulst, S.J.; Hughes, L.F. Prevention of noise- and drug-induced hearing loss with d-methionine. Hear. Res. 2007, 226, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Schacht, J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear. Res. 2000, 142, 34–40. [Google Scholar] [CrossRef]
- Lockwood, D.S.; Ding, D.L.; Wang, J.; Salvi, R.J. D-methionine attenuates inner hair cell loss in carboplatin-treated chinchillas. Audiol. Neuro-Otol. 2000, 5, 263–266. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Martin, S.M.; Meech, R.P.; Hargrove, T.L.; Verhulst, S.J.; Fox, D.J. D-methionine (D-met) significantly reduces kanamycin-induced ototoxicity in pigmented guinea pigs. Int. J. Audiol. 2016, 55, 273–278. [Google Scholar] [CrossRef]
- Campbell, K.C.; Rehemtulla, A.; Sunkara, P.; Hamstra, D.; Buhnerkempe, M.; Ross, B. Oral D-methionine protects against cisplatin-induced hearing loss in humans: Phase 2 randomized clinical trial in India. Int. J. Audiol. 2021, 61, 621–631. [Google Scholar] [CrossRef]
- Campbell, K.; Cosenza, N.; Meech, R.; Buhnerkempe, M.; Qin, J.; Rybak, L.; Fox, D. Preloaded D-methionine protects from steady state and impulse noise-induced hearing loss and induces long-term cochlear and endogenous antioxidant effects. PLoS ONE 2021, 16, e0261049. [Google Scholar] [CrossRef]
- Fox, D.J.; Cooper, M.D.; Speil, C.A.; Roberts, M.H.; Yanik, S.C.; Meech, R.P.; Hargrove, T.L.; Verhulst, S.J.; Rybak, L.P.; Campbell, K.C.M. d-Methionine reduces tobramycin-induced ototoxicity without antimicrobial interference in animal models. J. Cyst. Fibros. 2016, 15, 518–530. [Google Scholar] [CrossRef]
- Vogt, W. Oxidation of methionyl residues in proteins—Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Meech, R.P.; Rybak, L.P.; Hughes, L.F. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: Mechanisms of otoprotection. J. Am. Acad. Audiol. 2003, 14, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-W.; Liu, S.-H.; Young, Y.-H.; Lin-Shiau, S.-Y. D-Methionine attenuated cisplatin-induced vestibulotoxicity through altering ATPase activities and oxidative stress in guinea pigs. Toxicol. Appl. Pharmacol. 2006, 215, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.W.; Liu, S.H.; Hsu, C.J.; Lin-Shiau, S.Y. Correlation of increased activities of Na+, K+-ATPase and Ca2+-ATPase with the reversal of cisplatin ototoxicity induced by D-methionine in guinea pigs. Hear. Res. 2005, 205, 102–109. [Google Scholar] [CrossRef]
- Staubli, U.; Rangel-Diaz, N.; Alcantara, M.; Li, Y.-X.; Yang, J.-Y.; Zhang, K.-M.; Foster, A.C. Restoration of visual performance by d-serine in models of inner and outer retinal dysfunction assessed using sweep VEP measurements in the conscious rat and rabbit. Vis. Res. 2016, 127, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xiong, W.; Yang, G.; Kojic, L.; Wang, Y.T.; Cynader, M. The regulatory role of long-term depression in juvenile and adult mouse ocular dominance plasticity. Sci. Rep. 2011, 1, 203. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.R.; Esguerra, M.; Kim, P.M.; Newman, E.A.; Snyder, S.H.; Zahs, K.R.; Miller, R.F. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 6789–6794. [Google Scholar] [CrossRef]
- Gustafson, E.C.; Stevens, E.R.; Wolosker, H.; Miller, R.F. Endogenous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J. Neurophysiol. 2007, 98, 122–130. [Google Scholar] [CrossRef]
- Jacquet, Y.F. The NMDA receptor: Central role in pain inhibition in rat periaqueductal gray. Eur. J. Pharmacol. 1988, 154, 271–276. [Google Scholar] [CrossRef]
- Ito, M.; Yoshikawa, M.; Ito, K.; Matsuda, M.; Jin, X.L.; Takahashi, S.; Kobayashi, H.; Suzuki, T. Antinociceptive effect of intracerebroventricular administration of D-serine on formalin-induced pain. J. Anesth. 2014, 28, 228–234. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; He, Z.; Zhang, Q.; Yu, S. Intracerebroventricular infusion of D-serine decreases nociceptive behaviors induced by electrical stimulation of the dura mater of rat. Neurol. Res. 2019, 41, 204–207. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Ito, K.; Maeda, M.; Akahori, K.; Takahashi, S.; Jin, X.L.; Matsuda, M.; Suzuki, T.; Oka, T.; Kobayashi, H.; et al. Activation of supraspinal NMDA receptors by both D-serine alone or in combination with morphine leads to the potentiation of antinociception in tail-flick test of rats. Eur. J. Pharmacol. 2007, 565, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Andoh, H.; Ito, K.; Suzuki, T.; Kawaguchi, M.; Kobayashi, H.; Oka, T.; Hashimoto, A. Acute treatment with morphine augments the expression of serine racemase and d-amino acid oxidase mRNAs in rat brain. Eur. J. Pharmacol. 2005, 525, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Luongo, L.; Guida, F.; Marabese, I.; Romano, R.; Iannotta, M.; Rossi, F.; D’Aniello, A.; Stella, L.; Marmo, F.; et al. D-Aspartate drinking solution alleviates pain and cognitive impairment in neuropathic mice. Amino Acids 2016, 48, 1553–1567. [Google Scholar] [CrossRef]
- Boccella, S.; Vacca, V.; Errico, F.; Marinelli, S.; Squillace, M.; Guida, F.; Di Maio, A.; Vitucci, D.; Palazzo, E.; De Novellis, V.; et al. D-Aspartate Modulates Nociceptive-Specific Neuron Activity and Pain Threshold in Inflammatory and Neuropathic Pain Condition in Mice. Biomed Res. Int. 2015, 2015, 905906. [Google Scholar] [CrossRef]
- Ehrenpreis, S. D-phenylalanine and other enkephalinase inhibitors as pharmacological agents: Implications for some important therapeutic application. Acupunct. Electro-Ther. Res. 1982, 7, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Goudarzvand, M.; Panahi, Y.; Yazdani, R.; Miladi, H.; Tahmasebi, S.; Sherafat, A.; Afraei, S.; Abouhamzeh, K.; Jamee, M.; Al-Hussieni, K.J.M.R.; et al. The Effects of D-aspartate on Neurosteroids, Neurosteroid Receptors, and Inflammatory Mediators in Experimental Autoimmune Encephalomyelitis. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 316–325. [Google Scholar] [CrossRef]
- Usiello, A.; Di Fiore, M.M.; De Rosa, A.; Falvo, S.; Errico, F.; Santillo, A.; Nuzzo, T.; Baccari, G.C. New Evidence on the Role of D-Aspartate Metabolism in Regulating Brain and Endocrine System Physiology: From Preclinical Observations to Clinical Applications. Int. J. Mol. Sci. 2020, 21, 8718. [Google Scholar] [CrossRef]
- Errico, F.; Nistico, R.; Napolitano, F.; Mazzola, C.; Astone, D.; Pisapia, T.; Giustizieri, M.; D’Aniello, A.; Mercuri, N.B.; Usiello, A. Increased D-aspartate brain content rescues hippocampal age-related synaptic plasticity deterioration of mice. Neurobiol. Aging 2011, 32, 2229–2243. [Google Scholar] [CrossRef]
- Kawahara, M.; Sadakane, Y.; Koyama, H.; Konoha, K.; Ohkawara, S. D-Histidine and L-histidine attenuate zinc-induced neuronal death in GT1-7 cells. Metallomics 2013, 5, 453–460. [Google Scholar] [CrossRef]
- Yogo, K.; Murayama, C.; Fujisawa, Y.; Maeyama, T.; Hirayama, R.; Ogawa, Y.; Matsumoto, K.-i.; Nakanishi, I.; Yasuda, H.; Ishiyama, H.; et al. Potential Mechanisms for Protective Effect of D-Methionine on Plasmid DNA Damage Induced by Therapeutic Carbon Ions. Radiat. Res. 2020, 193, 513–519. [Google Scholar] [CrossRef]
- Gopal, K.V.; Wu, C.; Shrestha, B.; Campbell, K.C.M.; Moore, E.J.; Gross, G.W. d-Methionine protects against cisplatin-induced neurotoxicity in cortical networks. Neurotoxicol. Teratol. 2012, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut microbiota-derived D-serine protects against acute kidney injury. Jci Insight 2018, 3, e97957. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Nakade, Y.; Kitajima, S.; Nakagawa, S.Y.; Oshima, M.; Sakai, N.; Ogura, H.; Sato, K.; Toyama, T.; Yamamura, Y.; et al. Protective Effect of D-Alanine Against Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2022, 322, F667–F679. [Google Scholar] [CrossRef] [PubMed]
- Hesaka, A.; Tsukamoto, Y.; Nada, S.; Kawamura, M.; Ichimaru, N.; Sakai, S.; Nakane, M.; Mita, M.; Okuzaki, D.; Okada, M.; et al. D-Serine Mediates Cellular Proliferation for Kidney Remodeling. Kidney360 2021, 2, 1611–1624. [Google Scholar] [CrossRef]
- Tsuji, A.; Ikeda, Y.; Murakami, M.; Kitagishi, Y.; Matsuda, S. D-Leucine protects oocytes from chronic psychological stress in mice. Reprod. Med. Biol. 2021, 20, 477–484. [Google Scholar] [CrossRef]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Santillo, A.; Falvo, S.; Longobardi, S.; Baccari, G.C. Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia. Int. J. Mol. Sci. 2016, 17, 1127. [Google Scholar] [CrossRef]
- Macchia, G.; Topo, E.; Mangano, N.; D’Aniello, E.; Boni, R. dl-Aspartic acid administration improves semen quality in rabbit bucks. Anim. Reprod. Sci. 2010, 118, 337–343. [Google Scholar] [CrossRef]
- Raspa, M.; Paoletti, R.; Mahabir, E.; Scavizzi, F. d-aspartate treatment in vitro improves mouse sperm fertility in young B6N mice. Theriogenology 2020, 148, 60–67. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Chieffi, P.; Di Fiore, M.M.; Senese, R.; Baccari, G.C. D-Aspartate Induces Proliferative Pathways in Spermatogonial GC-1 Cells. J. Cell. Physiol. 2016, 231, 490–495. [Google Scholar] [CrossRef]
- Santillo, A.; Venditti, M.; Minucci, S.; Baccari, G.C.; Falvo, S.; Rosati, L.; Di Fiore, M.M. D-Asp upregulates PREP and GluA2/3 expressions and induces p-ERK1/2 and p-Akt in rat testis. Reproduction 2019, 158, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Boni, R.; Tosti, E.; Zagami, M.; Talevi, R. Intracellular calcium and protein tyrosine phosphorylation during the release of bovine sperm adhering to the fallopian tube epithelium in vitro. Reproduction 2005, 129, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Maddocks, S.; Millar, M.; Kerr, J.B.; Saunders, P.T.; McKinnell, C. Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J. Androl. 1992, 13, 172–184. [Google Scholar] [PubMed]
- Haklar, G.; Ulukaya-Durakbasa, C.; Yuksel, M.; Dagli, T.; Yalcin, A.S. Oxygen radicals and nitric oxide in rat mesenteric ischaemia-reperfusion: Modulation by L-arginine and N-G-nitro-L-arginine methyl ester. Clin. Exp. Pharmacol. Physiol. 1998, 25, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Wölkart, G.; Stessel, H.; Brunner, F. In vivo administration of d-arginine: Effects on blood pressure and vascular function in angiotensin II-induced hypertensive rats. Atherosclerosis 2004, 176, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, T.; Onizawa, M.; Saito, C.; Hikichi, R.; Yamada, D.; Minamidate, A.; Mochimaru, T.; Asahara, S.-i.; Kido, Y.; Oshima, S.; et al. Oral administration of d-serine prevents the onset and progression of colitis in mice. J. Gastroenterol. 2021, 56, 732–745. [Google Scholar] [CrossRef]
- Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P.; et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J. Allergy Clin. Immunol. 2017, 139, 1525–1535. [Google Scholar] [CrossRef]
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B.M.; Hamase, K.; Waldor, M.K. Interplay between microbial D-amino acids and host D-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 2016, 1, 16125. [Google Scholar] [CrossRef]
- Shennan, D.B.; Thomson, J. Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol. Rep. 2008, 20, 885–889. [Google Scholar] [CrossRef]
- Sasamura, T.; Matsuda, A.; Kokuba, Y. Nutritional effects of a D-methionine-containing solution on AH109A hepatoma-bearing rats. Biosci. Biotechnol. Biochem. 1998, 62, 2418–2420. [Google Scholar] [CrossRef]
- Sasamura, T.; Matsuda, A.; Kokuba, Y. Effects of D-methionine-containing solution on tumor cell growth in vitro. Arzneim.-Forsch.-Drug Res. 1999, 49, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, T.; Matsuda, A.; Kokuba, Y. Tumor growth inhibition and nutritional effect of D-amino acid solution in AH109A hepatoma-bearing rats. J. Nutr. Sci. Vitaminol. 1998, 44, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Frauli, M.; Ludwig, H. Inhibition of fibroblast proliferation in a culture of human endometrial stromal cells using a medium containing D-valine. Arch. Gynecol. Obstet. 1987, 241, 87–96. [Google Scholar] [CrossRef]
- Hongpaisan, J. Inhibition of proliferation of contaminating fibroblasts by D-valine in cultures of smooth muscle cells from human myometrium. Cell Biol. Int. 2000, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Lee, K.C.; Eisbruch, A.; Sunkara, P.; Borgonha, S.; Phillip, B.; Campbell, K.C.M.; Ross, B.D.; Rehemtulla, A. Double-blind placebo-controlled multicenter phase II trial to evaluate D-methionine in preventing/reducing oral mucositis induced by radiation and chemotherapy for head and neck cancer. Head Neck-J. Sci. Spec. Head Neck 2018, 40, 1375–1388. [Google Scholar] [CrossRef]
- Vuyyuri, S.B.; Hamstra, D.A.; Khanna, D.; Hamilton, C.A.; Markwart, S.M.; Campbell, K.C.M.; Sunkara, P.; Ross, B.D.; Rehemtulla, A. Evaluation of D-methionine as a novel oral radiation protector for prevention of mucositis. Clin. Cancer Res. 2008, 14, 2161–2170. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Meech, R.P.; Rybak, L.P.; Hughes, L.F. D-Methionine protects against cisplatin damage to the stria vascularis. Hear. Res. 1999, 138, 13–28. [Google Scholar] [CrossRef]
- Cloven, N.G.; Re, A.; McHale, M.T.; Burger, R.A.; DiSaia, P.J.; Rose, G.S.; Campbell, K.C.M.; Fan, H. Evaluation of D-methionine as a cytoprotectant in cisplatin treatment of an animal model for ovarian cancer. Anticancer Res. 2000, 20, 4205–4209. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Zhang, N.; Wen, D.; Liu, C.; Li, Q. Inhibition of biofilm formation by d-tyrosine: Effect of bacterial type and d-tyrosine concentration. Water Res. 2016, 92, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, J.; Jiang, J.; Liang, C.; Feng, Y. D-tyrosine affects aggregation behavior of Pantoea agglomerans. J. Basic Microbiol. 2017, 57, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-Amino Acids Trigger Biofilm Disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Leiman, S.A.; May, J.M.; Lebar, M.D.; Kahne, D.; Kolter, R.; Losick, R. D-Amino Acids Indirectly Inhibit Biofilm Formation in Bacillus subtilis by Interfering with Protein Synthesis. J. Bacteriol. 2013, 195, 5391–5395. [Google Scholar] [CrossRef]
- Iwata, Y.; Sakai, N.; Yoneda, I.; Senda, Y.; Sakai-Takemori, Y.; Oshima, M.; Nakagawa-Yoneda, S.; Ogura, H.; Sato, K.; Minami, T.; et al. D-Serine inhibits the attachment and biofilm formation of methicillin-resistant Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2021, 537, 50–56. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Zhou, X.; Hu, H.; Zhang, K.; Du, X.; Peng, X.; Ren, B.; Cheng, L.; Li, M. Effect of D-cysteine on dual-species biofilms of Streptococcus mutans and Streptococcus sanguinis. Sci. Rep. 2019, 9, 6689. [Google Scholar] [CrossRef]
- Ghosh, S.; Qureshi, A.; Purohit, H.J. D-Tryptophan governs biofilm formation rates and bacterial interaction in P-mendocina and S-aureus. J. Biosci. 2019, 44, 3. [Google Scholar] [CrossRef]
- Qi, H.; Li, B.S.; Wang, H.L.; Cai, Q.; Quan, X.; Cui, Y.X.; Meng, W.Y. Effects of D-valine on periodontal or peri-implant pathogens: Porphyromonas gingivalis biofilm. J. Periodontol. 2018, 89, 303–314. [Google Scholar] [CrossRef]
- Li, E.; Wu, J.; Wang, P.; Zhang, D. D-Phenylalanine inhibits biofilm development of a marine microbe, Pseudoalteromonas sp. SC2014. Fems Microbiol. Lett. 2016, 363, fnw198. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Mitigation of a nitrate reducing Pseudomonas aeruginosa biofilm and anaerobic biocorrosion using ciprofloxacin enhanced by D-tyrosine. Sci. Rep. 2017, 7, 6946. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Akers, K.S.; Romano, D.R.; Woodbury, R.L.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. D-Amino Acids Enhance the Activity of Antimicrobials against Biofilms of Clinical Wound Isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 4353–4361. [Google Scholar] [CrossRef]
- Li, Y.; Jia, R.; Al-Mahamedh, H.H.; Xu, D.; Gu, T. Enhanced Biocide Mitigation of Field Biofilm Consortia by a Mixture of D-Amino Acids. Front. Microbiol. 2016, 7, 896. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.V.; Senadheera, S.N.; Ross, N.C.; Reilley, K.A.; Ganno, M.L.; Eans, S.E.; Murray, T.F.; McLaughlin, J.P. Alanine analogues of D-Trp CJ-15,208: Novel opioid activity profiles and prevention of drug- and stress-induced reinstatement of cocaine-seeking behaviour. Br. J. Pharmacol. 2014, 171, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.; Boman, A.; Wahlin, B.; Drain, C.M.; Andreu, D.; Boman, H.G.; Merrifield, R.B. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 1990, 87, 4761–4765. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta Biochim. Et Biophys. Sin. 2017, 49, 916–925. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Chen, S.; Hu, H.; Su, J.; Zou, Y. d-Amino acid mutation of PMI as potent dual peptide inhibitors of p53-MDM2/MDMX interactions. Bioorg. Med. Chem. Lett. 2017, 27, 4678–4681. [Google Scholar] [CrossRef]
- Liang, G.; Yang, Z.; Zhang, R.; Li, L.; Fan, Y.; Kuang, Y.; Gao, Y.; Wang, T.; Lu, W.W.; Xu, B. Supramolecular Hydrogel of a D-Amino Acid Dipeptide for Controlled Drug Release in Vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef]
- Lu, J.; Hathaway, H.J.; Royce, M.E.; Prossnitz, E.R.; Miao, Y. Introduction of D-Phenylalanine enhanced the receptor binding affinities of gonadotropin-releasing hormone peptides. Bioorg. Med. Chem. Lett. 2014, 24, 725–730. [Google Scholar] [CrossRef][Green Version]
- Typas, A.; Banzhaf, M.; Gross, C.A.; Vollmer, W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 2012, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Bjorn, L.O. Peptidoglycan in eukaryotes: Unanswered questions. Phytochemistry 2020, 175, 112370. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Liu, H.; Zhu, J.; Xu, H.; Yao, J. D-Ala(2), D-Leu(5) Enkephalin Inhibits TLR4/NF-kappa B Signaling Pathway and Protects Rat Brains against Focal Ischemia-Reperfusion Injury. Mediat. Inflamm. 2021, 2021, 6661620. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Giuliani, L.; Santi, L. D-Trp-6-LH-RH treatment of advanced prostatic cancer. Lancet 1986, 1, 621. [Google Scholar] [CrossRef]
- Parmar, H.; Phillips, R.H.; Rustin, G.; Lightman, S.L.; Schally, A.V. Therapy of advanced ovarian cancer with D-Trp-6-LH-RH (decapeptyl) microcapsules. Biomed. Pharmacother. Biomed. Pharmacother. 1988, 42, 531–538. [Google Scholar]
- Ma, H.; Zhao, J.; Liu, S.; Xie, D.; Zhang, Z.; Nie, D.; Wen, F.; Yang, Z.; Tang, G. F-18-Trifluoromethylated D-Cysteine as a Promising New PET Tracer for Glioma Imaging: Comparative Analysis With MRI and Histopathology in Orthotopic C6 Models. Front. Oncol. 2021, 11, 645162. [Google Scholar] [CrossRef]
- Huang, T.; Tang, G.; Wang, H.; Nie, D.; Tang, X.; Liang, X.; Hu, K.; Yi, C.; Yao, B.; Tang, C. Synthesis and preliminary biological evaluation of S-C-11-methyl-D-cysteine as a new amino acid PET tracer for cancer imaging. Amino Acids 2015, 47, 719–727. [Google Scholar] [CrossRef]
- Neumann, K.D.; Villanueva-Meyer, J.E.; Mutch, C.A.; Flavell, R.R.; Blecha, J.E.; Kwak, T.; Sriram, R.; VanBrocklin, H.F.; Rosenberg, O.S.; Ohliger, M.A.; et al. Imaging Active Infection in vivo Using D-Amino Acid Derived PET Radiotracers. Sci. Rep. 2017, 7, 7903. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Q.; Zhu, H. Distribution, industrial applications, and enzymatic synthesis of d-amino acids. Appl. Microbiol. Biotechnol. 2015, 99, 3341–3349. [Google Scholar] [CrossRef]
- Olney, J.W. Excitotoxic amino acids and neuropsychiatric disorders. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 47–71. [Google Scholar] [CrossRef]
- Chen, X.-G.; Wang, Y.-H.; Wen, C.-C.; Chen, Y.-H. Overdose of D-serine Induces Movement Disorder and Neuromuscular Changes of Zebrafish Larvae. J. Toxicol. Pathol. 2014, 27, 19–24. [Google Scholar] [CrossRef][Green Version]
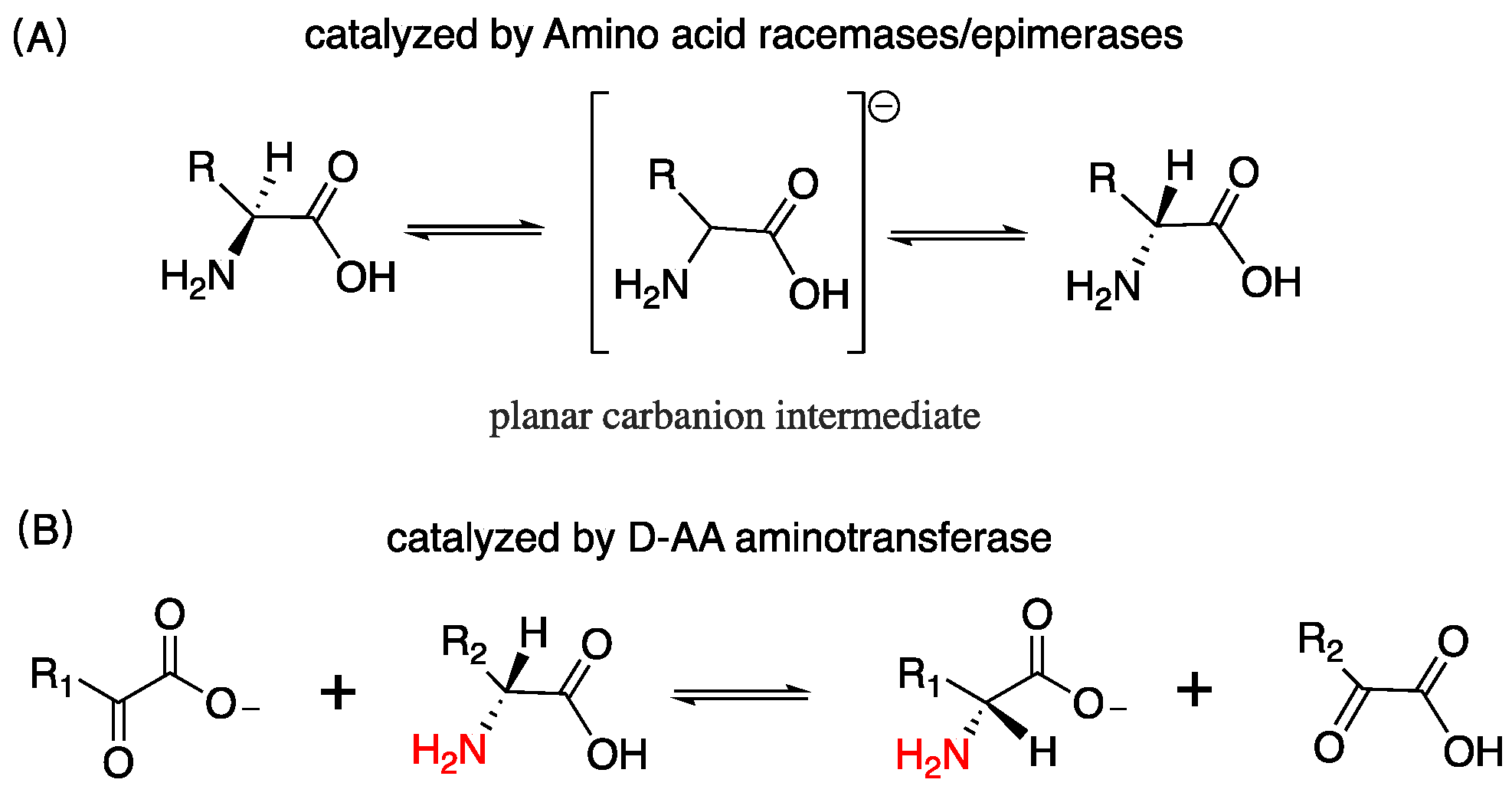
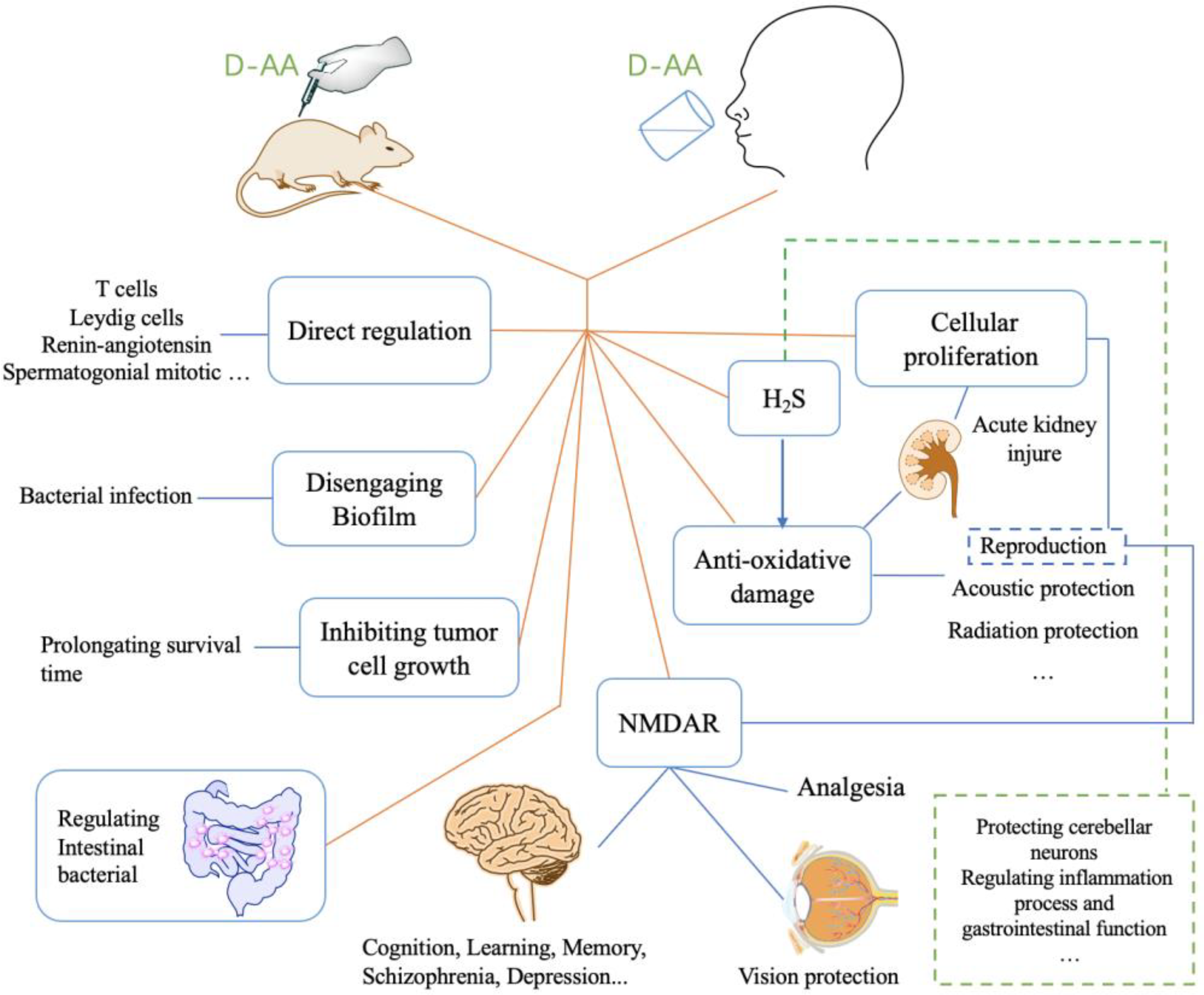

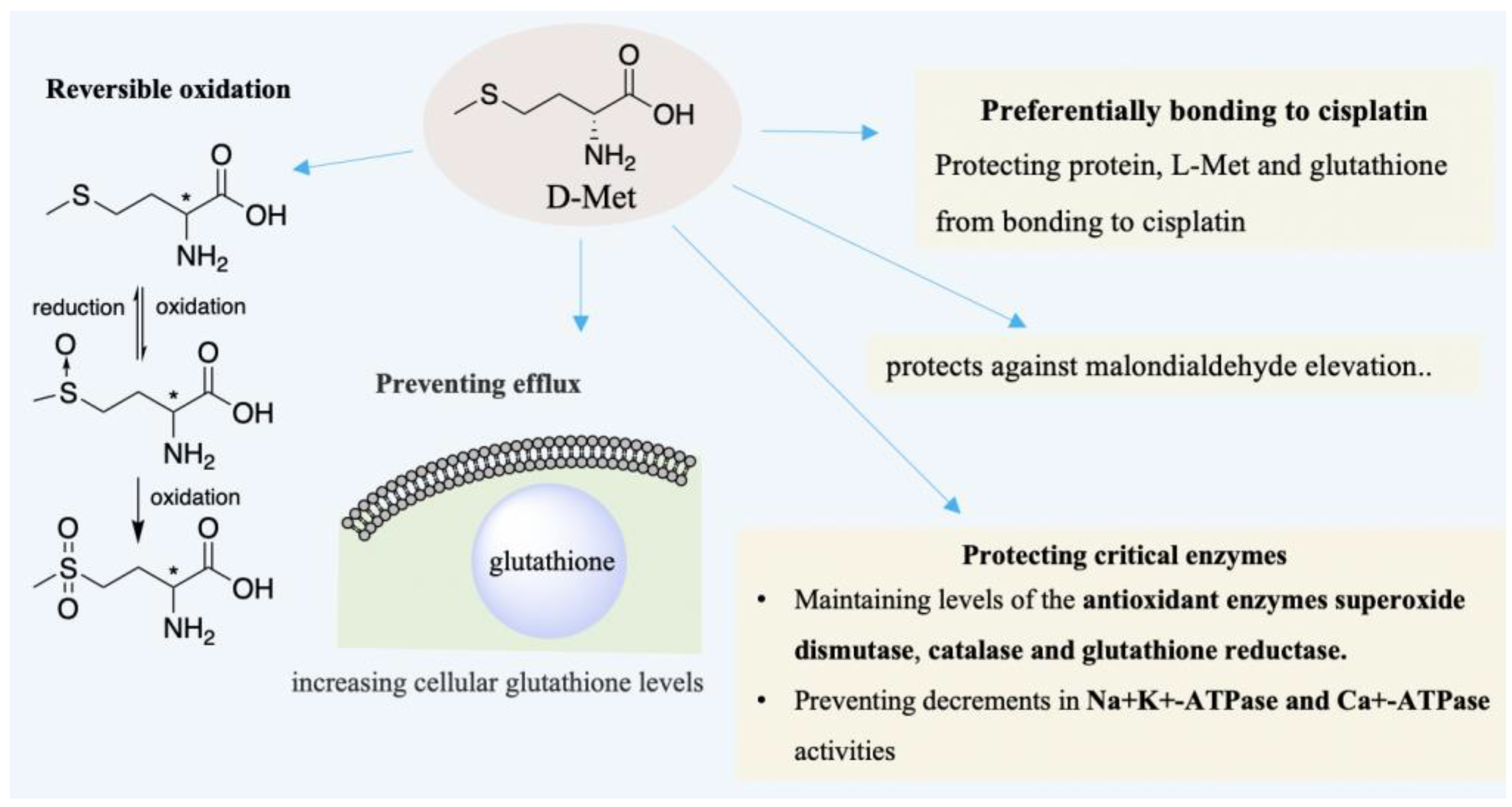
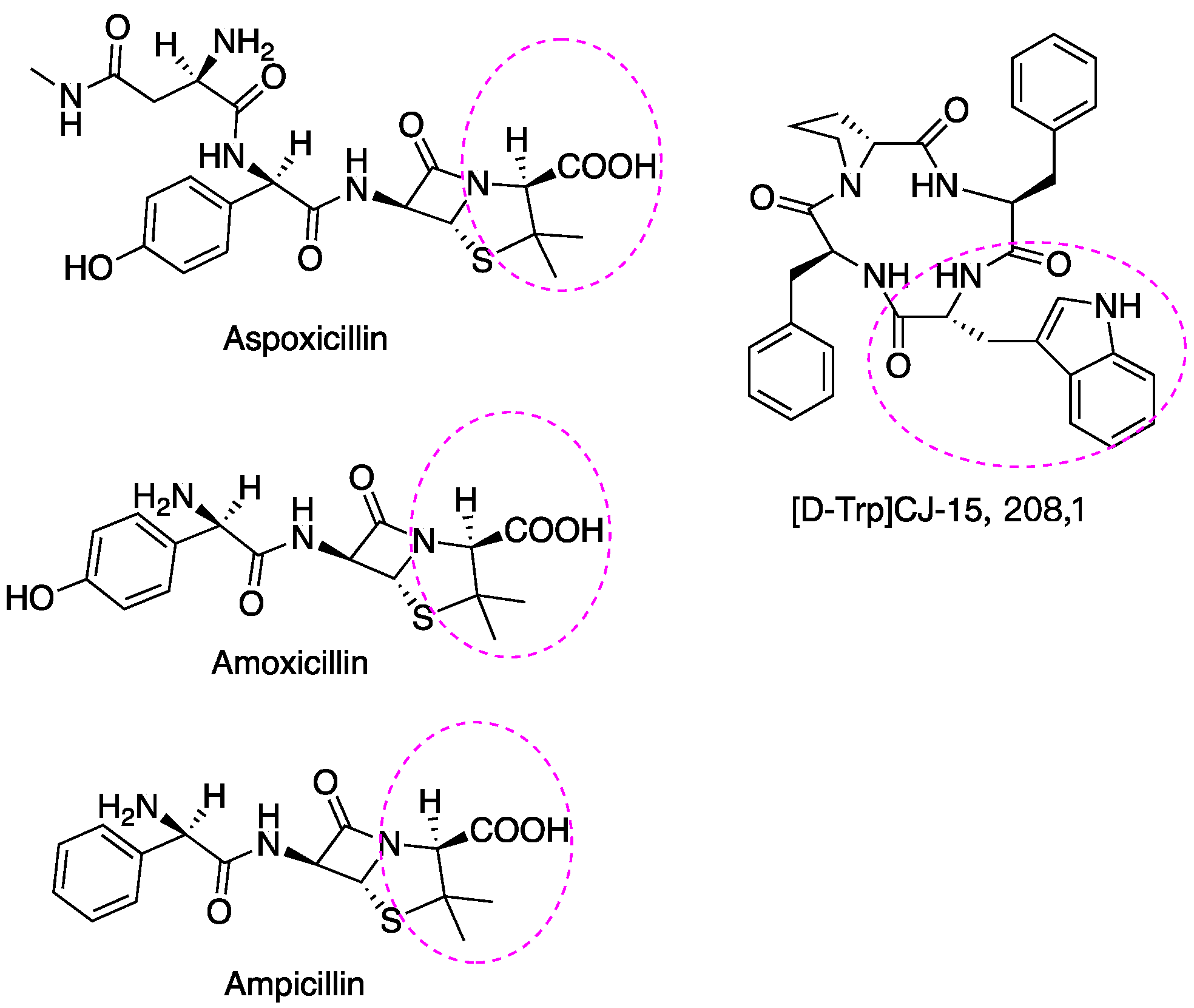
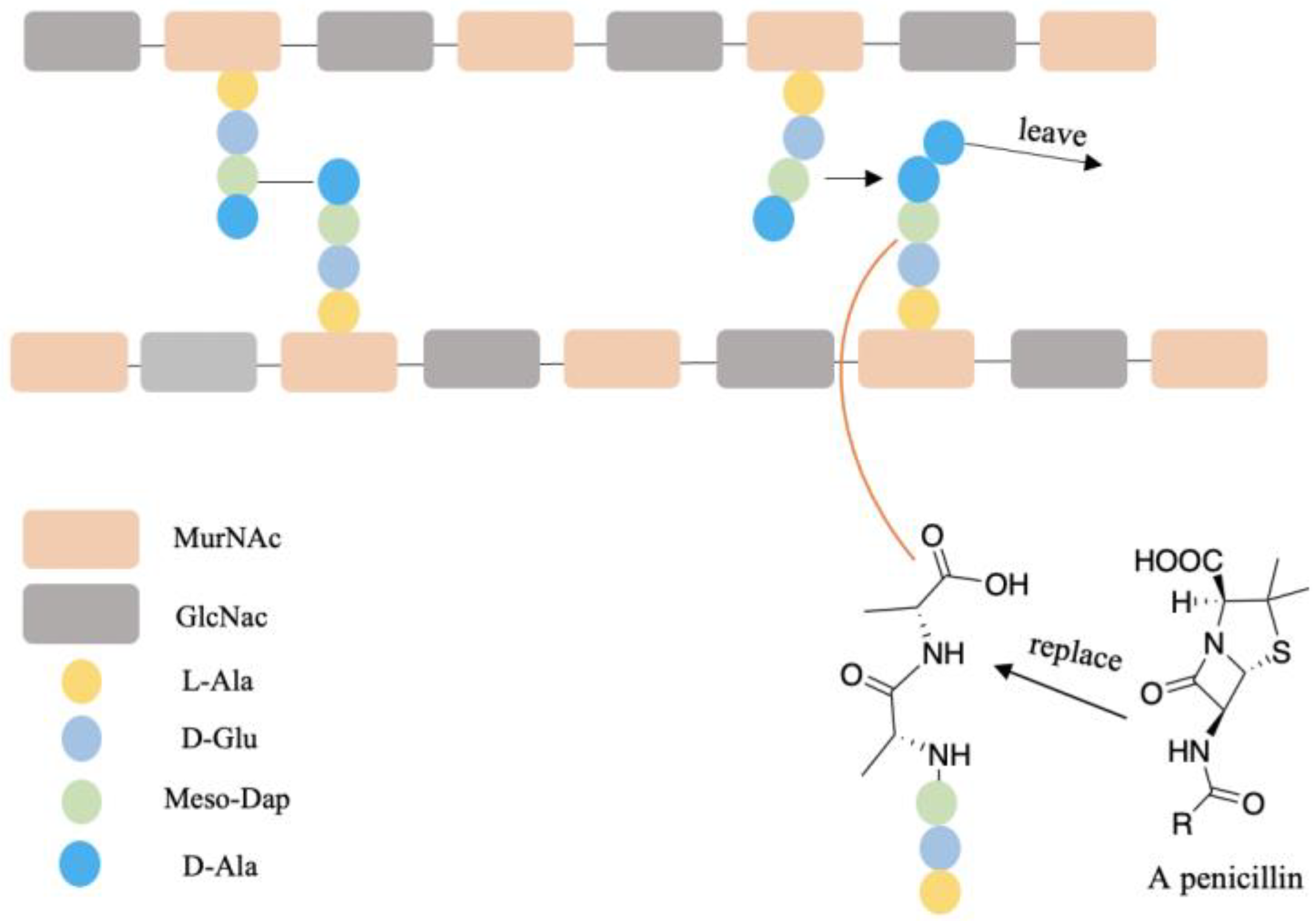
| D-AA | Subject | Diseases/Symptoms | Method | Period | Side Effects | Reference |
|---|---|---|---|---|---|---|
| D-Ala | Human | Schizophrenia | Oral administration, D-Ala 100 mg/kg daily (mixed with orange juice) | 6 weeks | Insomnia, nausea (short-lived and resolved spontaneously) | [36] |
| D-Met | male Chinchillas laniger | Hearing loss induced by noise | Intraperitoneal injection, D-Met saline 200 mg/kg (12 h intervals) after noise exposure | 2 days | - | [70] |
| D-Met | Male Wistar rats | Hearing loss induced by cisplatin | Intraperitoneal injection, D-Met saline at 300 mg/kg | Once (prior to the infusion of CDDP) | - | [73] |
| D-Met | Pigmented male guinea pigs | Hearing loss induced by aminoglycosides | Intraperitoneal injection, D-Met saline twice daily at 200 mg/kg each | 6.5 weeks | - | [75] |
| D-Met | Pigmented guinea pigs | Kanamycin-induced ototoxicity | Intraperitoneal injection, D-met (120, 180, 240, 300, 360, 420, or 480 mg/kg/day) twice per day | 23 days | - | [77] |
| D-Met | Male donryu rats | The survival time of tumor-bearing rats | Intravenous infusion, amino acid mixture 230/kg/day (D-Met, 0.35 g/mL) | 7 days | - | [121] |
| D-Met | Human | Mucositis induced by radiation and chemotherapy | Oral administration, D-Met 100 mg/kg b.i.d. | Twice (before and after radiotherapy) | Nausea, vomiting | [126] |
| D-Ser | Human | Schizophrenia | Oral administration, D-Ser 30 mg/kg daily (mixed with orange juice) | 6 weeks | Insomnia, nausea, diarrhea, constipation (short-lived and resolved spontaneously) | [19] |
| D-Ser | Human | Post-traumatic stress disorder | Oral administration, D-Ser 30 mg/kg daily | 6 weeks | No significant side-effects | [40] |
| D-Ser | Human | Parkinson’s disease | Oral administration, D-Ser 30 mg/kg daily | 6 weeks | No significant side-effects | [41] |
| D-Ser | Adult rats | Temporal Lobe Epilepsy | Cannulation surgery, continuous infusion of D-Ser (100 μM at 0.1 μL/h) | 29 days | - | [43] |
| D-Ser | Older adults | Improving spatial memory, learning and problem solving | Oral administration, D-Ser 30 mg/kg daily (mixed with orange juice) | Approximately 3 months | - | [45] |
| D-Ser | Adult male mice | Cognitive deficits | Intraperitoneal injection, D-Ser saline (1 g/kg or 100 mg/kg) | Once | - | [46] |
| D-Ser | male Sprague-Dawley rats | Memory impairment and neuronal damage induced by chronic lead exposure | Intraperitoneal injection, D-Ser 30 and 60 mg/kg, twice a day | 8 weeks | - | [49] |
| D-Ser | gustatory cortex rats and Wistar rats | Anxiety-like Behavior and Spatial Learning Ability | Intraperitoneal injection, D-Ser 50 mg/kg or 100 mg/kg | Once | - | [50] |
| D-Ser | Adult male mice | Depression | Intranucleus accumbens infusions, D-Ser (2 µg/perside, 5 µg/perside) | 14 days | - | [51] |
| D-Ser | Male mice | High-fat diet intake and preference | Intraperitoneal injection, D-Ser 1, 2, or 4 g/kg body weight | Once | - | [54] |
| D-Ser | Rabbits and rats | Retinal dysfunction | Intraperitoneal injection, D-Ser 600 mg/kg; intravitreal injection D-Ser 12 μmol | Once | - | [85] |
| D-Ser | Male Wistar rats | Antinociception | Intracerebroventricular administration, dose-dependent experiments, D-Ser (10–40,000 nmol); In combination studies, D-serine (10 μmol) | Once | - | [92] |
| D-Ser | Mice | Acute kidney injury | Oral administration D-Ser 20 mM or fecal microbiota transplantation | - | - | [103] |
| D-Ser | Wild-type mice | Colitis | Oral administration, 1.5% (wt/vol) D-Ser diluted into H2O | 7 days | - | [117] |
| D-Cys | Spontaneously Hypertensive Rats | Hypertension and Kidney Damage | Gastric intubation, D-Cys (8 mmol/kg body weight/day) between 4 and 6 weeks of ages | 14 days | - | [57] |
| D-Cys | Mice | Kidney injury from ischaemia-reperfusion | Gastric intubation, 8 mmol/ kg body weight | Once | - | [58] |
| D-Cys | Mice | Gastric damage | Oral administration, D-Cys 100 mg/kg | Once | - | [66] |
| D-Cys | Mice | Spinocerebellar ataxia | Intraperitoneal injection, D-Cys 100 mg/kg | 10 weeks | - | [67] |
| D-Leu | Male mice | Seizure | Intraperitoneal injection, D-Leu 300 mg/kg after kainic acid injected | Once | - | [44] |
| D-Leu | Female mice | Oocyte maturation failure | Oral administration, commercial powder diet containing 0.3% d-Leu | 14 days | - | [106] |
| D-Leu | Male Donryu rats | The survival time of tumor-bearing rats | Intravenous infusion, amino acid mixture 230/kg/day (D-Leu, 1.25 g/mL) | 7 days | - | [123] |
| D-Asp | Spared nerve injury mice | Pain and cognitive impairment | Oral administrate, D-Asp water solution 20 mM | 30 days | - | [94] |
| D-Asp | Male mice | Hippocampal age-related synaptic plasticity deterioration | Oral administrate, D-Asp 20 water solution mM | 3 months or 12 months | - | [99] |
| D-Asp | Sexually mature New Zealand rabbit males | Sperm quality | Oral administration, DL-Asp mixed with food, 1.3 g DL-Asp/head | 14 days | - | [109] |
| D-Arg | Male Sprague-Dawley rats | Blood pressure | Daily subcutaneous injection of 3 mL d-Arg (1 M) | 7 days | - | [116] |
| D-Trp | Female mice | Allergic airway disease | Oral administration, D-Trp 0.9 mg/day per mouse | 3 days | - | [118] |
| D-Val | Male Donryu rats | The survival time of tumor-bearing rats | Intravenous infusion, amino acid mixture 230/kg/day (D-Val, 0.45 g/mL) | 7 days | - | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Hussain, Z.; Zhao, Y. Promising Application of D-Amino Acids toward Clinical Therapy. Int. J. Mol. Sci. 2022, 23, 10794. https://doi.org/10.3390/ijms231810794
Shi Y, Hussain Z, Zhao Y. Promising Application of D-Amino Acids toward Clinical Therapy. International Journal of Molecular Sciences. 2022; 23(18):10794. https://doi.org/10.3390/ijms231810794
Chicago/Turabian StyleShi, Yoahpoing, Zahid Hussain, and Yufen Zhao. 2022. "Promising Application of D-Amino Acids toward Clinical Therapy" International Journal of Molecular Sciences 23, no. 18: 10794. https://doi.org/10.3390/ijms231810794
APA StyleShi, Y., Hussain, Z., & Zhao, Y. (2022). Promising Application of D-Amino Acids toward Clinical Therapy. International Journal of Molecular Sciences, 23(18), 10794. https://doi.org/10.3390/ijms231810794





