Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Structure
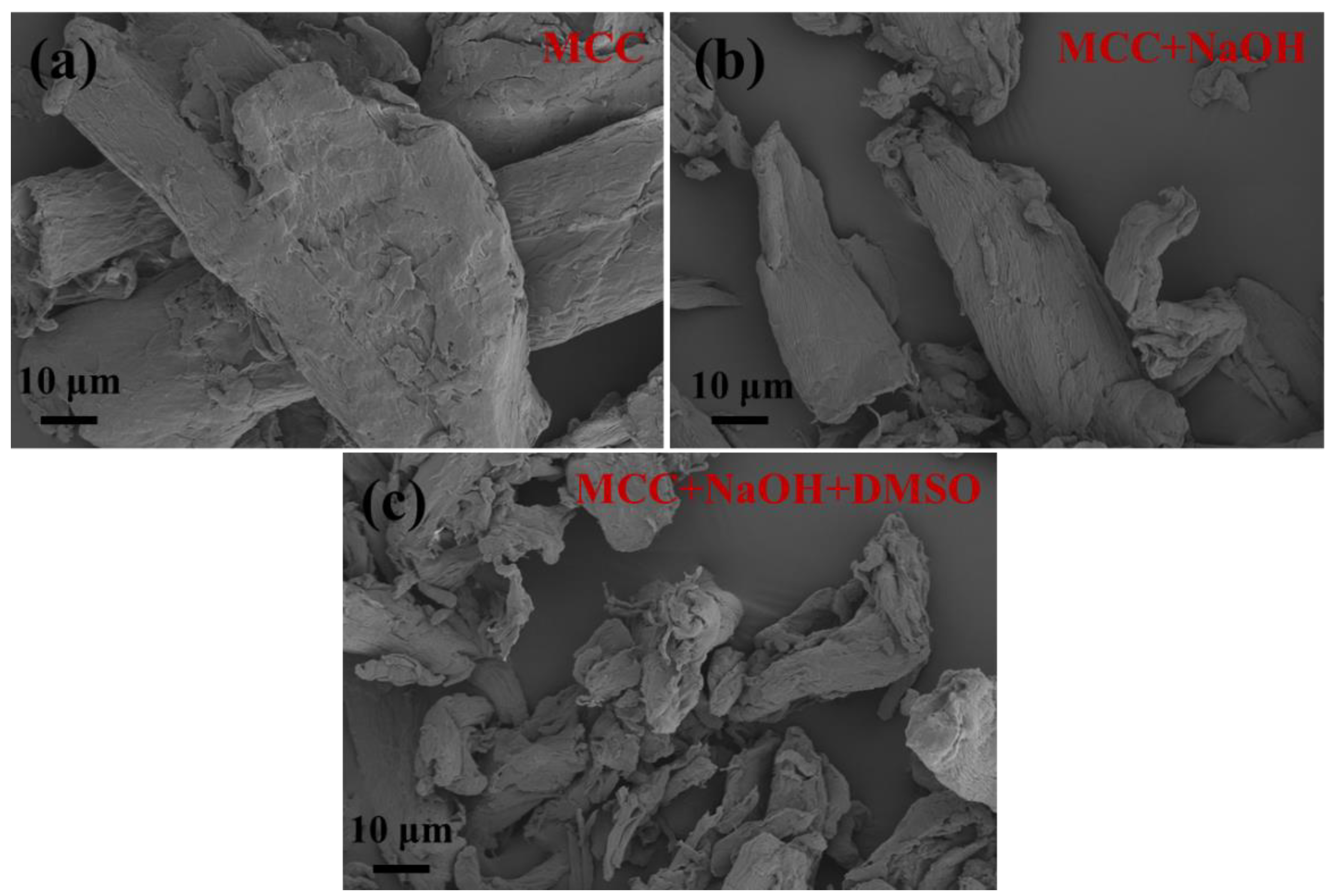
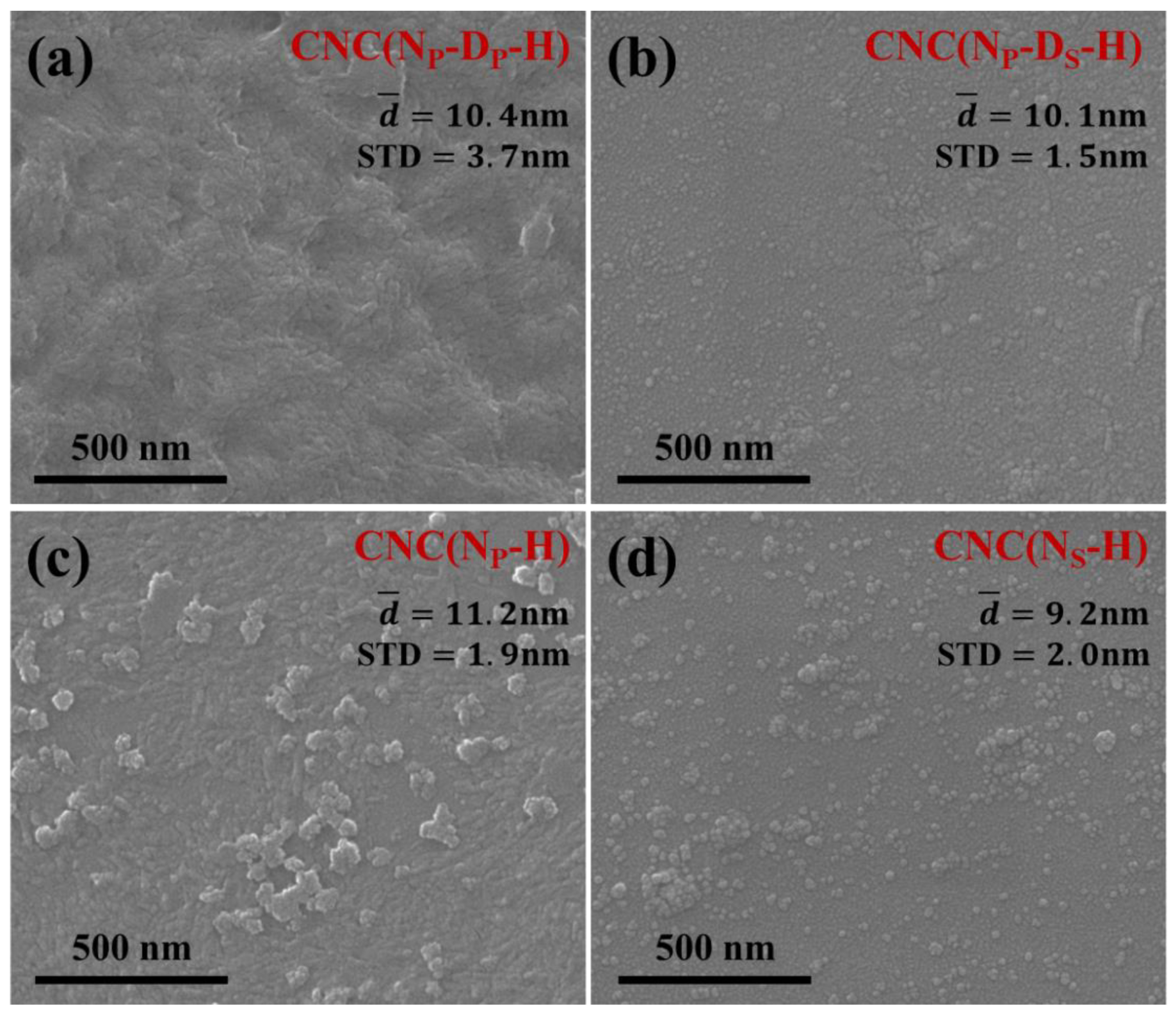
2.2. Micromorphology
2.3. Crystallinity
2.4. Thermal Stability
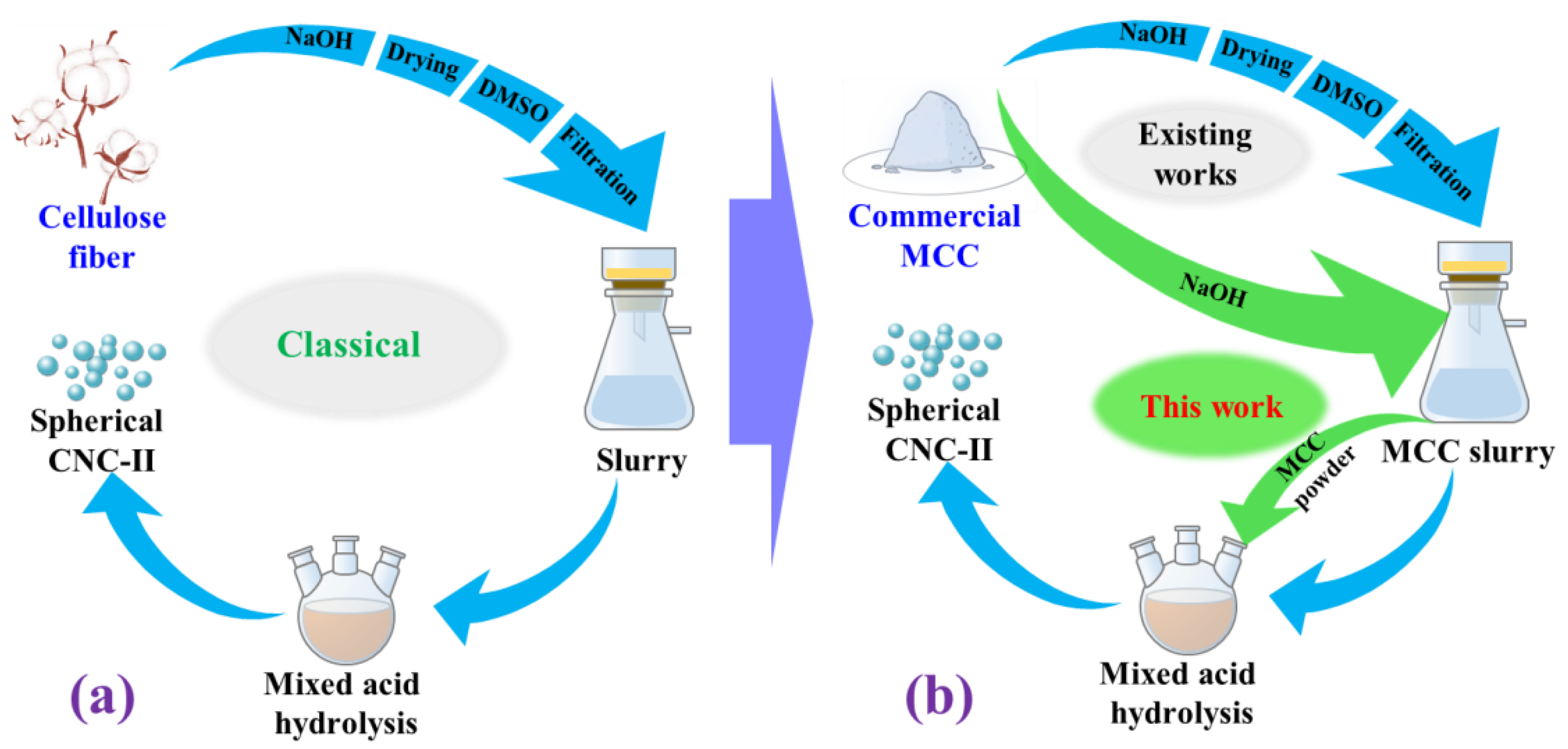
3. Materials and Methods
3.1. Materials
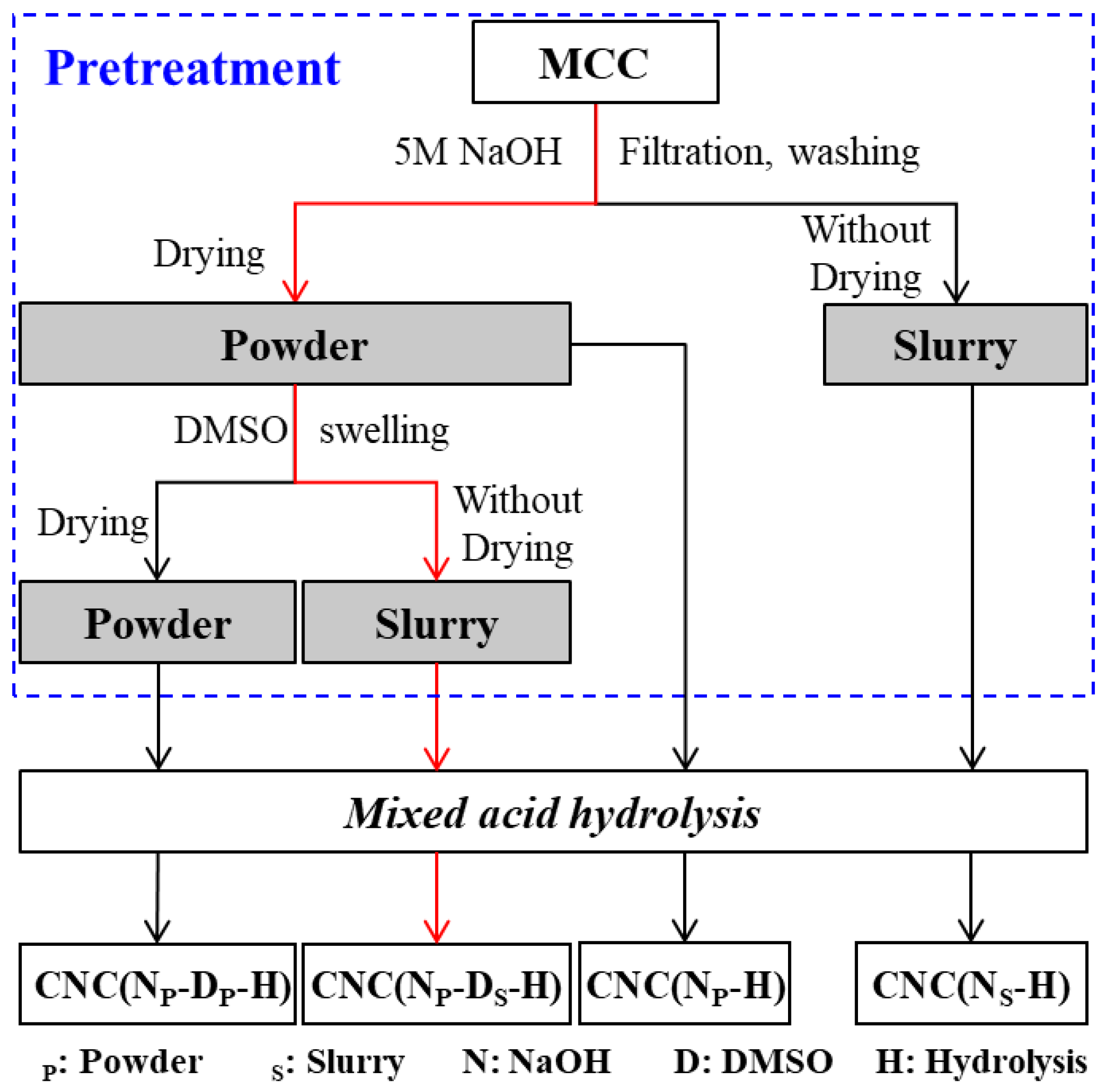
3.2. Pretreatment of MCC
3.2.1. Alkaline Treatment of MCC
3.2.2. Swelling Treatment of Alkaline Treated MCC
3.3. Mixed Acid Hydrolysis
3.4. Characterization
3.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.4.2. Morphological Investigation
3.4.3. X-ray Diffraction (XRD) Analysis
3.4.4. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.T.; Chen, X.Q. Preparation and characterization of spherical cellulose nanocrystals with high purity by the composite enzymolysis of pulp fibers. Bioresource Technol. 2019, 291, 121842. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Haras, M.R.; Kao, N.; Ward, L. Single-step heterogeneous catalysis production of highly monodisperse spherical nanocrystalline cellulose. Int. J. Biol. Macromol. 2020, 154, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Elder, T.J.; Pu, Y.; Ragauskas, A.J. Facile synthesis of spherical cellulose nanoparticles. Carbohyd. Polym. 2007, 69, 607–611. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Pickering emulsions stabilized by spherical cellulose nanocrystals. Carbohyd. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef]
- Xu, J.T.; Chen, X.Q.; Shen, W.H.; Li, Z. Spherical vs. rod-like cellulose nanocrystals from enzymolysis: A comparative study as reinforcing agents on polyvinyl alcohol. Carbohyd. Polym. 2021, 256, 117493. [Google Scholar] [CrossRef]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2020, 166, 288–296. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Zhang, W.B.; You, X.Y.; Chauhan, I.; Mohanty, P.; Li, X.P. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its application as rheology modifier and 3D bioprinting. Int. J. Biol. Macromol. 2021, 166, 1586–1616. [Google Scholar] [CrossRef]
- Chen, R.; Ma, Z.H.; Sun, D.Y.; Wang, X.; Han, Y. Cellulose I nanocrystals (CNCs I) prepared in mildly acidic lithium bromide trihydrate (MALBTH) and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2022, 201, 59–66. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Deng, F.J.; Liang, J.; Huang, Q.; Dou, J.B.; Liu, M.Y.; Cao, Q.Y.; Zhang, X.Y.; Wei, Y. Direct grafting of cellulose nanocrystals with poly(ionic liquids) via Gamma-ray irradiation and their utilization for adsorptive removal of CR. Int. J. Biol. Macromol. 2022, 194, 1029–1037. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Anand Kishore, K. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Li, X.F.; Ding, E.Y.; Li, G.K. A method of preparing spherical nano-crystal cellulose with mixed crystalline forms of cellulose I and II. Chin. J. Polym. Sci. 2001, 19, 291–296. [Google Scholar]
- Lu, F.F.; Yu, H.Y.; Yan, C.F.; Yao, J.M. Polylactic acid nanocomposite films with spherical nanocelluloses as efficient nucleation agents: Effects on crystallization, mechanical and thermal properties. RSC Adv. 2016, 6, 46008–46018. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhang, H.; Abdalkarim, S.Y.H.; Yang, L.; Zhu, J.; Gu, J.; Yao, J. Interfacial compatible poly (ethylene glycol) chains modified cellulose nanosphere as bifunctional reinforcements in green polylatic acid for food packagings. J. Taiwan Inst. Chem. 2019, 95, 583–593. [Google Scholar] [CrossRef]
- Yan, C.F.; Yu, H.Y.; Yao, J.M. One-step extraction and functionalization of cellulose nanospheres from Lyocell fibers with cellulose II crystal structure. Cellulose 2015, 22, 3773–3788. [Google Scholar] [CrossRef]
- Meyabadi, T.F.; Dadashian, F.; Sadeghi, G.M.M.; Asl, H.E.Z. Spherical cellulose nanoparticles preparation from waste cotton using a green method. Powder Technol. 2014, 261, 232–240. [Google Scholar] [CrossRef]
- Beaumont, M.; Nypelö, T.; König, J.; Zirbs, R.; Opietnik, M.; Potthast, A.; Rosenau, T. Synthesis of redispersible spherical cellulose II nanoparticles decorated with carboxylate groups. Green Chem. 2016, 18, 1465–1468. [Google Scholar] [CrossRef]
- Chen, X.Q.; Deng, X.Y.; Shen, W.H.; Jia, M.Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohyd. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef]
- Lam, N.T.; Saewong, W.; Sukyai, P. Effect of varying hydrolysis time on extraction of spherical bacterial cellulose nanocrystals as a reinforcing agent for poly(vinyl alcohol) composites. J. Polym. Res. 2017, 24, 71. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.Y.; Liu, Y.N.; Qin, Y.F.; Li, T.; Chen, L.; Zhu, M.F. Efficient extraction of carboxylated spherical cellulose nanocrystals with narrow distribution through hydrolysis of Lyocell fibers by using ammonium persulfate as an oxidant. J. Mater. Chem. A 2014, 2, 251–258. [Google Scholar] [CrossRef]
- Azrina, Z.A.Z.; Beg, M.D.H.; Rosli, M.Y.; Ramlib, R.; Junadi, N.; Alam, A.K.M.M. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruitbunch pulp via ultrasound assisted hydrolysis. Carbohyd. Polym. 2017, 162, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Purkait, B.S.; Ray, D.; Sengupta, S.; Kar, T.; Mohanty, A.; Misra, M. Isolation of cellulose nanoparticles from sesame husk. Ind. Eng. Chem. Res. 2011, 50, 871–876. [Google Scholar] [CrossRef]
- Evdokimova, O.L.; Svensson, F.G.; Agafonov, A.V.; Håkansson, S.; Seisenbaeva, G.A.; Kessler, V.G. Hybrid drug delivery patches based on spherical cellulose nanocrystals and colloid titania-synthesis and antibacterial properties. Nanomaterials 2018, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, T.; Wang, W.Y.; Sagis, L.M.C.; Yuan, Q.P.; Lei, X.G.; Stuart, M.A.C.; Li, D.; Bao, C.; Bai, J.; et al. Corncob cellulose nanosphere as an eco-friendly detergent. Nat. Sustain. 2020, 3, 448–458. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Nassar, M.A. Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohyd. Polym. 2010, 79, 694–699. [Google Scholar] [CrossRef]
- Bai, H.J.; Chen, J.H.; Zhou, X.Y.; Hu, C.Z. Single and binary adsorption of dyes from aqueous solutions using functionalized microcrystalline cellulose from cotton fiber. Korean J. Chem. Eng. 2020, 37, 1926–1932. [Google Scholar] [CrossRef]
- Anju, T.R.; Ramamurthy, K.; Dhamodharan, R. Surface modified microcrystalline cellulose from cotton as a potential mineral admixture in cement mortar composite. Cement Concrete Comp. 2016, 74, 147–153. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.L.; Ling, C.; Hou, W.S.; Yan, Z.F. Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method. Waste Manag. 2018, 82, 139–146. [Google Scholar] [CrossRef]
- Hou, W.S.; Ling, C.; Shi, S.; Yan, Z.F. Preparation and characterization ofmicrocrystalline cellulose from waste cotton fabrics by using phosphotungstic acid. Int. J. Biol. Macromol. 2019, 123, 363–368. [Google Scholar] [CrossRef]
- Sun, X.W.; Lu, C.H.; Liu, Y.; Zhang, W.; Zhang, X.X. Melt-processed poly(vinyl alcohol) composites filled with microcrystalline cellulose from waste cotton fabrics. Carbohyd. Polym. 2014, 101, 642–649. [Google Scholar] [CrossRef]
- Rashid, M.; Gafur, M.A.; Sharafat, M.K.; Minami, H.; Miah, M.A.J.; Ahmad, H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohyd. Polym. 2017, 170, 72–79. [Google Scholar] [CrossRef]
- Oh, S.Y.; Dong, I.Y.; Shin, Y.; Hwan, C.K.; Hak, Y.K.; Yong, S.C.; Won, H.P.; Ji, H.Y. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohyd. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassem, I.; Kassab, Z.; Draoui, K.; Achaby, M.E. Ultrasonic-mediated production of carboxylated cellulose nanospheres. J. Environ. Chem. Eng. 2021, 9, 106302. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohyd. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of Lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Bai, H.Y.; Dong, W.F.; Liu, X.Y. All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohyd. Polym. 2018, 179, 297–304. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Ren, R.W.; Chen, X.Q.; Shen, W.H. Preparation and separation of pure spherical cellulose nanocrystals from microcrystalline cellulose by complex enzymatic hydrolysis. Int. J. Biol. Macromol. 2022, 202, 1–10. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.X.; Wang, S.Q.; Yin, Y.F. Effects of ultrasonic treatment during acid hydrolysis on the yield, particle size and structure of cellulose nanocrystals. Carbohyd. Polym. 2016, 135, 248–255. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.Y.; Cheng, R.S. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, X.X.; Tian, D.; Zhou, Z.H.; Lu, C.H. Comparing microcrystalline with spherical nanocrystalline cellulose from waste cotton fabrics. Cellulose 2012, 19, 1189–1198. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.Y.; Cheng, R.S. Preparation and liquid crystalline properties of spherical cellulose nanocrystals. Langmuir 2008, 24, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lourdin, D.; Peixinho, J.; Bréard, J.; Cathala, B.; Leroy, E.; Duchemin, B. Concentration driven cocrystallisation and percolation in all-cellulose nanocomposites. Cellulose 2016, 23, 529–543. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Comrad, C.M., Jr. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Revol, J.F.; Dietrich, A.; Goring, D.A.I. Effect of mercerization on the crystallite size and crystallinity index in cellulose from different sources. Can. J. Chem. 1987, 65, 1724–1725. [Google Scholar] [CrossRef]

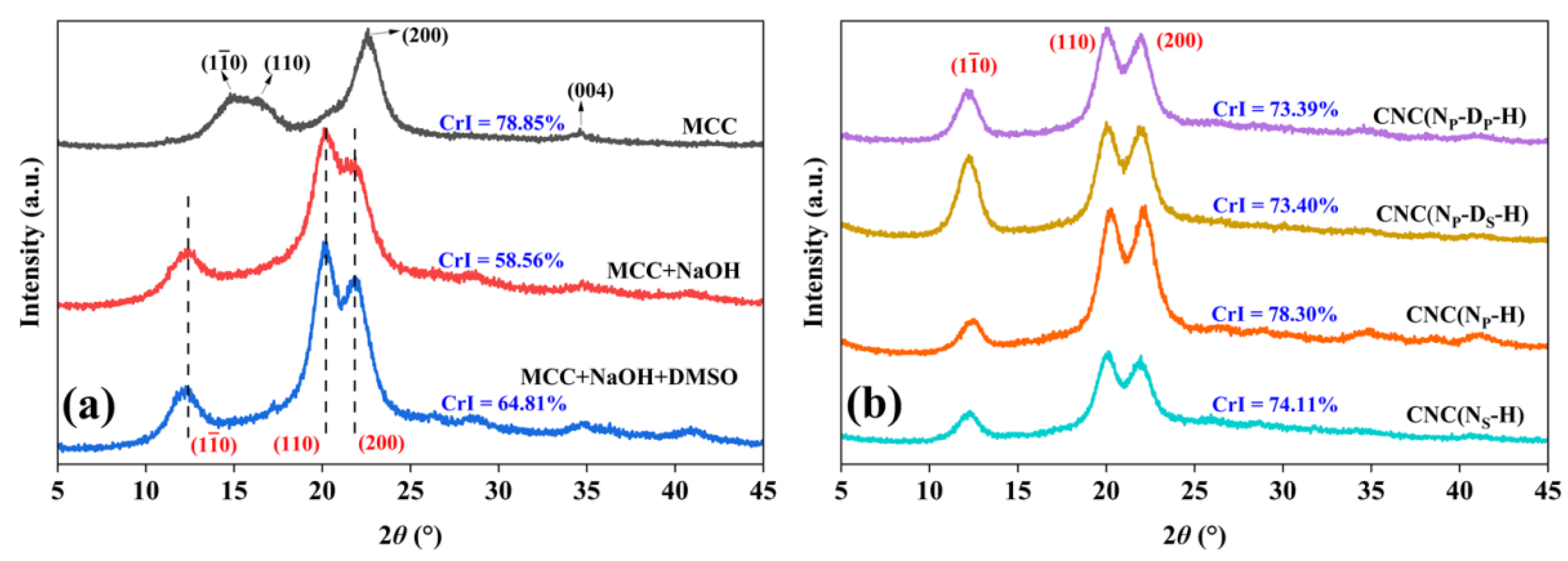

| Sample | CrI (%) | Crystallite Size (D, nm) | ||
|---|---|---|---|---|
| (110) | (200) | |||
| MCC | 78.85 | 4.06 | 3.81 | 5.06 |
| MCC+NaOH | 58.56 | 3.81 | 4.52 | 4.08 |
| MCC+NaOH+DMSO | 64.81 | 4.36 | 5.18 | 4.65 |
| CNC(NP-DP-H) | 73.39 | 7.15 | 5.98 | 5.87 |
| CNC(NP-DS-H) | 73.40 | 7.42 | 6.13 | 5.65 |
| CNC(NP-H) | 78.30 | 7.51 | 6.10 | 5.47 |
| CNC(NS-H) | 74.11 | 7.24 | 5.99 | 5.69 |
| Sample | Initial Weight Loss (%) | T0 (°C) | Main Stage I | Main Stage II | Final Residue (%) | ||
|---|---|---|---|---|---|---|---|
| TMax (°C) | WL (%) | TMax (°C) | WL (%) | ||||
| MCC | 2.45 | 315.1 | 340.1 | 84.62 | – | – | 4.97 |
| MCC+NaOH | 3.34 | 295.5 | 343.0 | 78.22 | – | – | 9.79 |
| MCC+NaOH+DMSO | 3.28 | 299.0 | 344.0 | 75.60 | – | – | 13.41 |
| CNC(NP-DP-H) | 3.70 | 220.5 | 271.2 | 42.56 | 348.7 | 22.94 | 16.72 |
| CNC(NP-DS-H) | 3.67 | 206.3 | 267.8 | 36.96 | 347.8 | 19.82 | 27.60 |
| CNC(NP-H) | 3.77 | 236.3 | 265.8 | 48.18 | 353.3 | 18.81 | 18.97 |
| CNC(NS-H) | 4.95 | 198.1 | 267.6 | 36.37 | 350.1 | 20.29 | 24.86 |
| Raw Material | Pretreatment | Form before Hydrolysis | Mixed Acid Hydrolysis | CrI (%) | T0 (°C) | Particle Size (nm) | Ref. |
|---|---|---|---|---|---|---|---|
| Cotton fiber | NaOH, 75 °C, 4 h; DMSO, 75 °C, 4 h | Slurry | H2SO4:HCl = 3:1; 75 °C, Ultrasonic, 8 h | - | - | 50 | [12] |
| Cellulose fiber | NaOH, 80 °C, 3 h; DMSO, 80 °C, 3 h | Slurry | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 8 h | 82.0 | - | 80 | [3] |
| Cellulose fiber | NaOH, 80 °C, 3 h; DMSO, 80 °C, 3 h | Slurry | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 8 h | - | - | 5.9–10.9 | [25] |
| MCC | NaOH, 80 °C, 3 h; DMSO, 80 °C, 3 h | Slurry | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 8 h | 81.3 | - | 60 | [4] |
| MCC | NaOH, 80 °C, 3 h; DMSO, 80 °C, 3 h | Powder | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 2 h | 73.4 | 220.5 | 10.4 | This work |
| MCC | NaOH, 80 °C, 3 h; DMSO, 80 °C, 3 h | Slurry | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 2 h | 73.4 | 206.3 | 10.1 | This work |
| MCC | NaOH, 80 °C, 3 h | Powder | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 2 h | 78.3 | 236.3 | 11.2 | This work |
| MCC | NaOH, 80 °C, 3 h | Slurry | H2SO4:HCl = 3:1; 80 °C, Ultrasonic, 2 h | 74.1 | 198.1 | 9.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Feng, L.; Ding, Z.; Bai, X. Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes. Int. J. Mol. Sci. 2022, 23, 10764. https://doi.org/10.3390/ijms231810764
Zhu P, Feng L, Ding Z, Bai X. Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes. International Journal of Molecular Sciences. 2022; 23(18):10764. https://doi.org/10.3390/ijms231810764
Chicago/Turabian StyleZhu, Peng, Luyao Feng, Zejun Ding, and Xuechun Bai. 2022. "Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes" International Journal of Molecular Sciences 23, no. 18: 10764. https://doi.org/10.3390/ijms231810764
APA StyleZhu, P., Feng, L., Ding, Z., & Bai, X. (2022). Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes. International Journal of Molecular Sciences, 23(18), 10764. https://doi.org/10.3390/ijms231810764






