New Insight into Assembled Fe3O4@PEI@Ag Structure as Acceptable Agent with Enzymatic and Photothermal Properties
Abstract
:1. Introduction
2. Results and Discussion
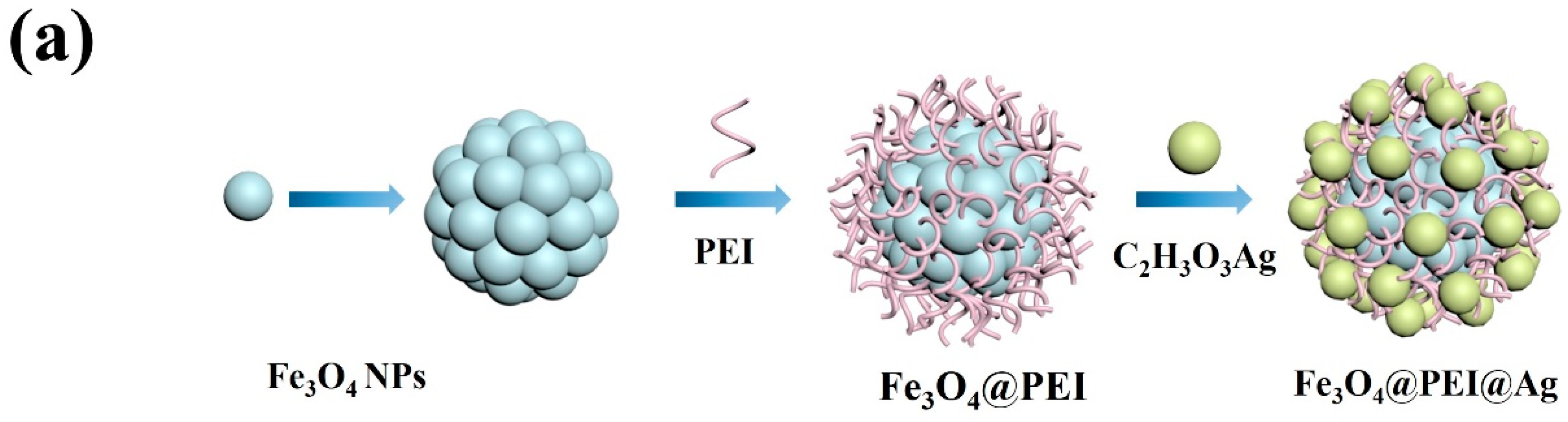
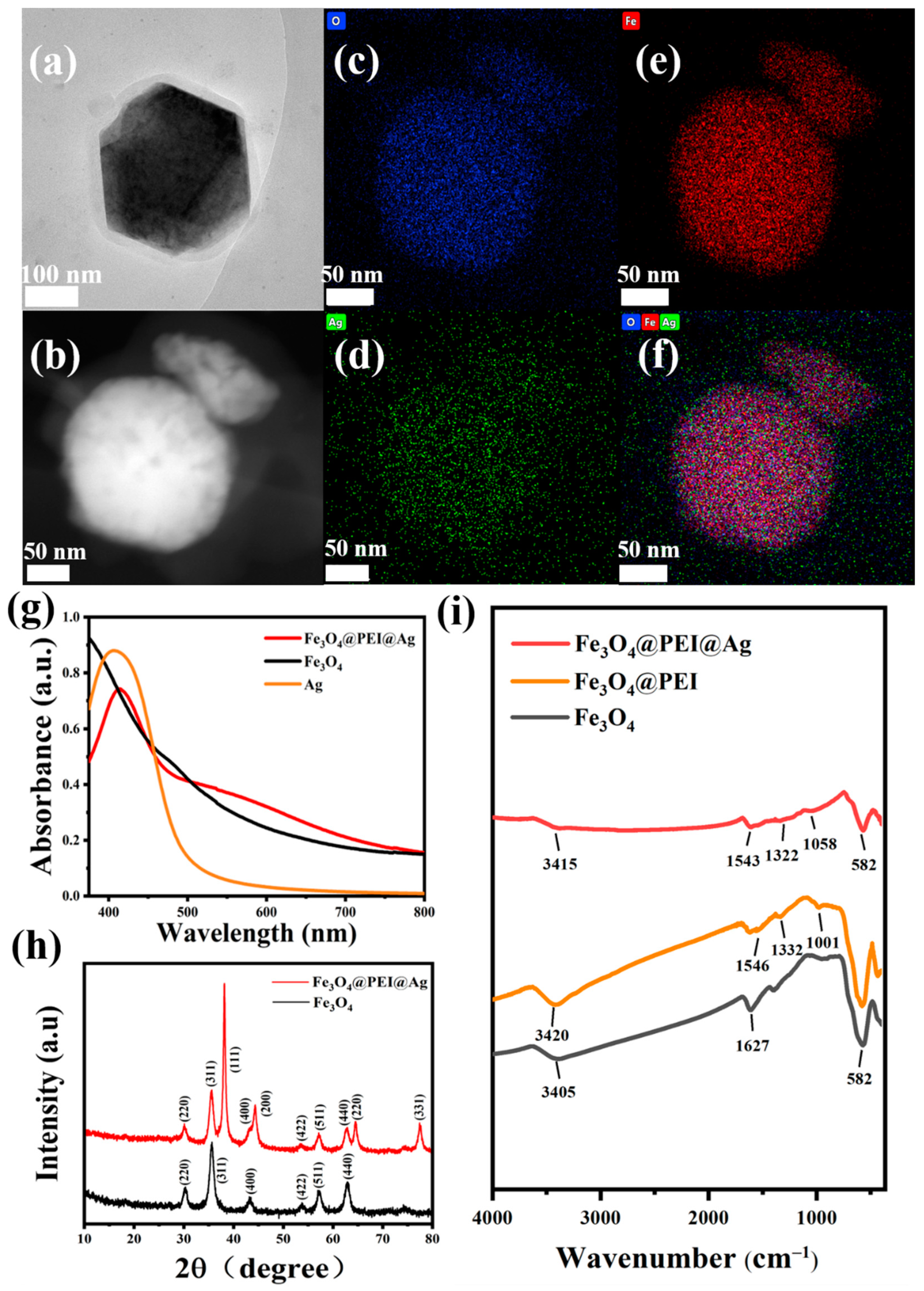
2.1. The Synthesis Work of Fe3O4@PEI@Ag, Fe3O4@PEI@Ag@ICG, and Relative Structural Characterizations
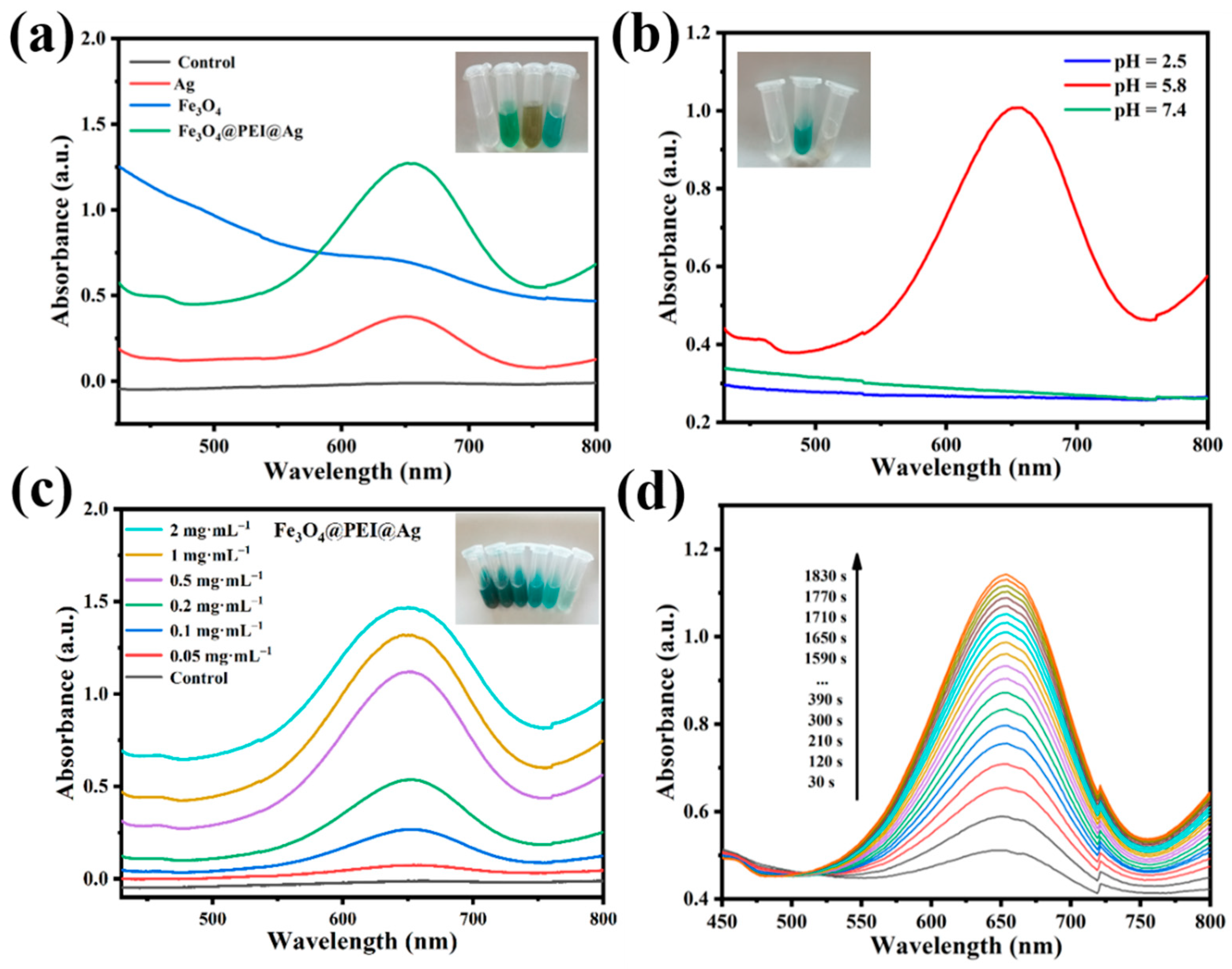
2.2. Peroxidase-like Activity of Assembled Fe3O4@PEI@Ag Structure
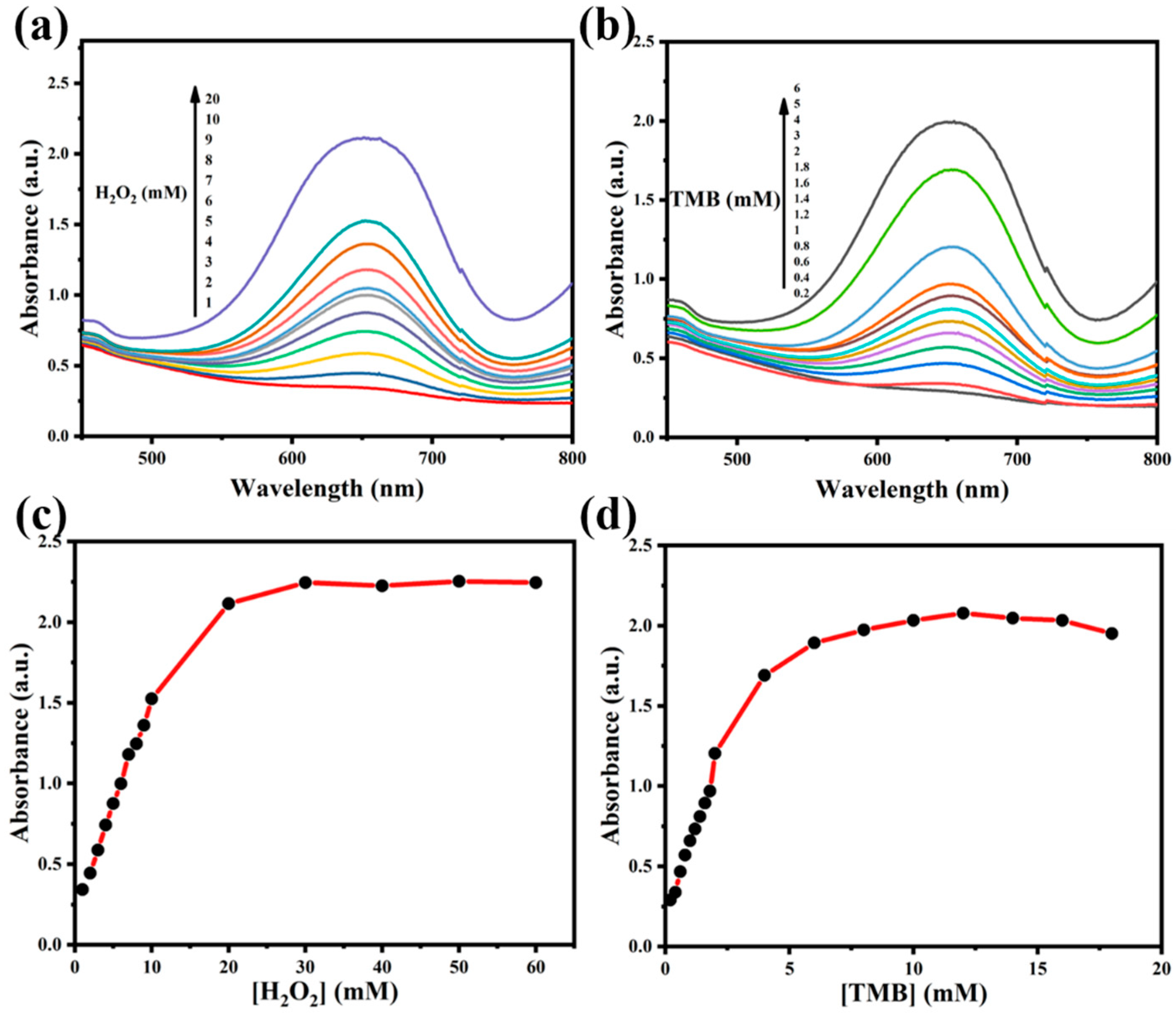
2.3. Steady-State Kinetic Assay of Fe3O4@PEI@Ag Structure
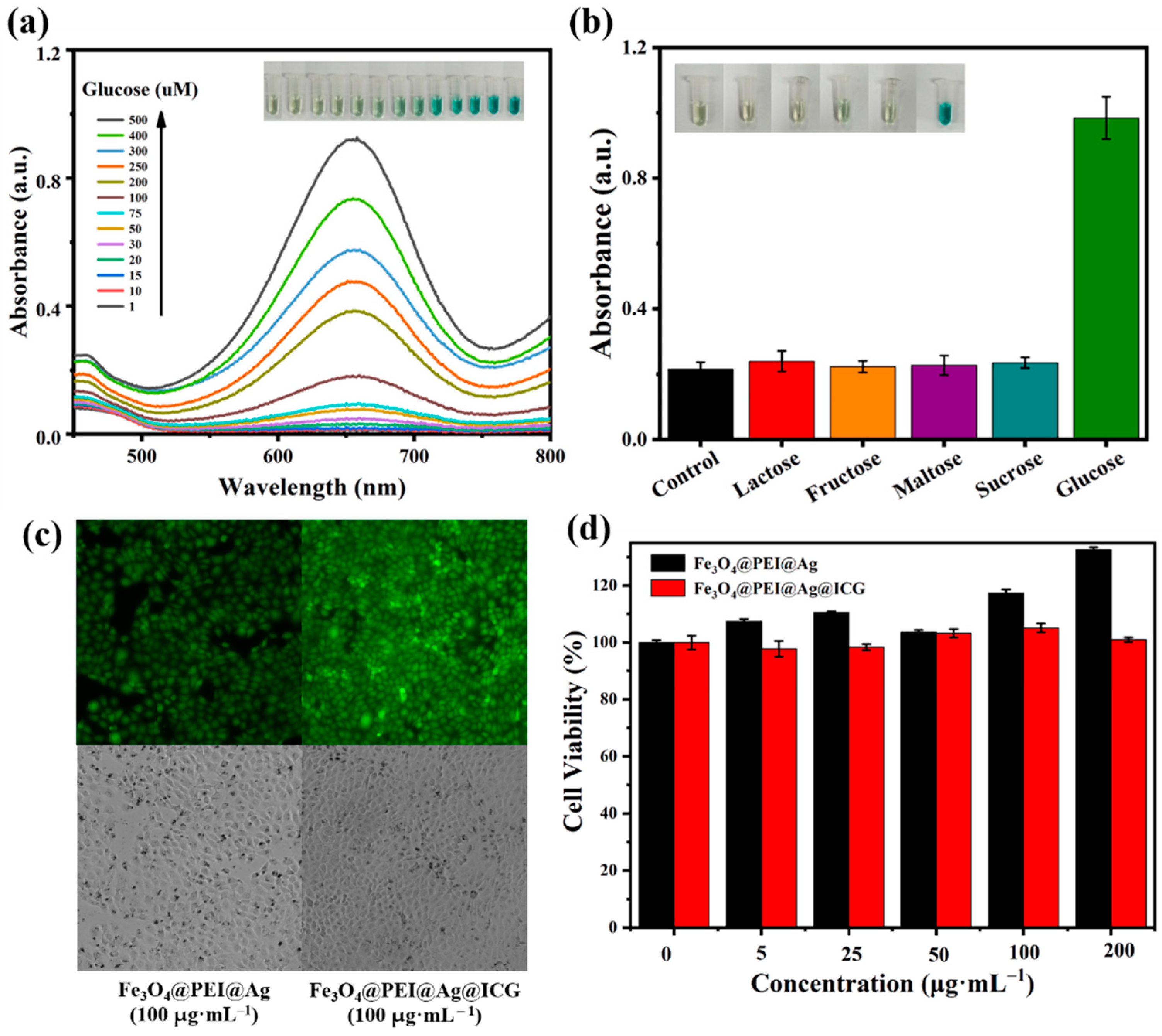
2.4. Colorimetric Detection of Glucose and Cytotoxicity Measurement of Fe3O4@PEI@Ag and Fe3O4@PEI@Ag@ICG
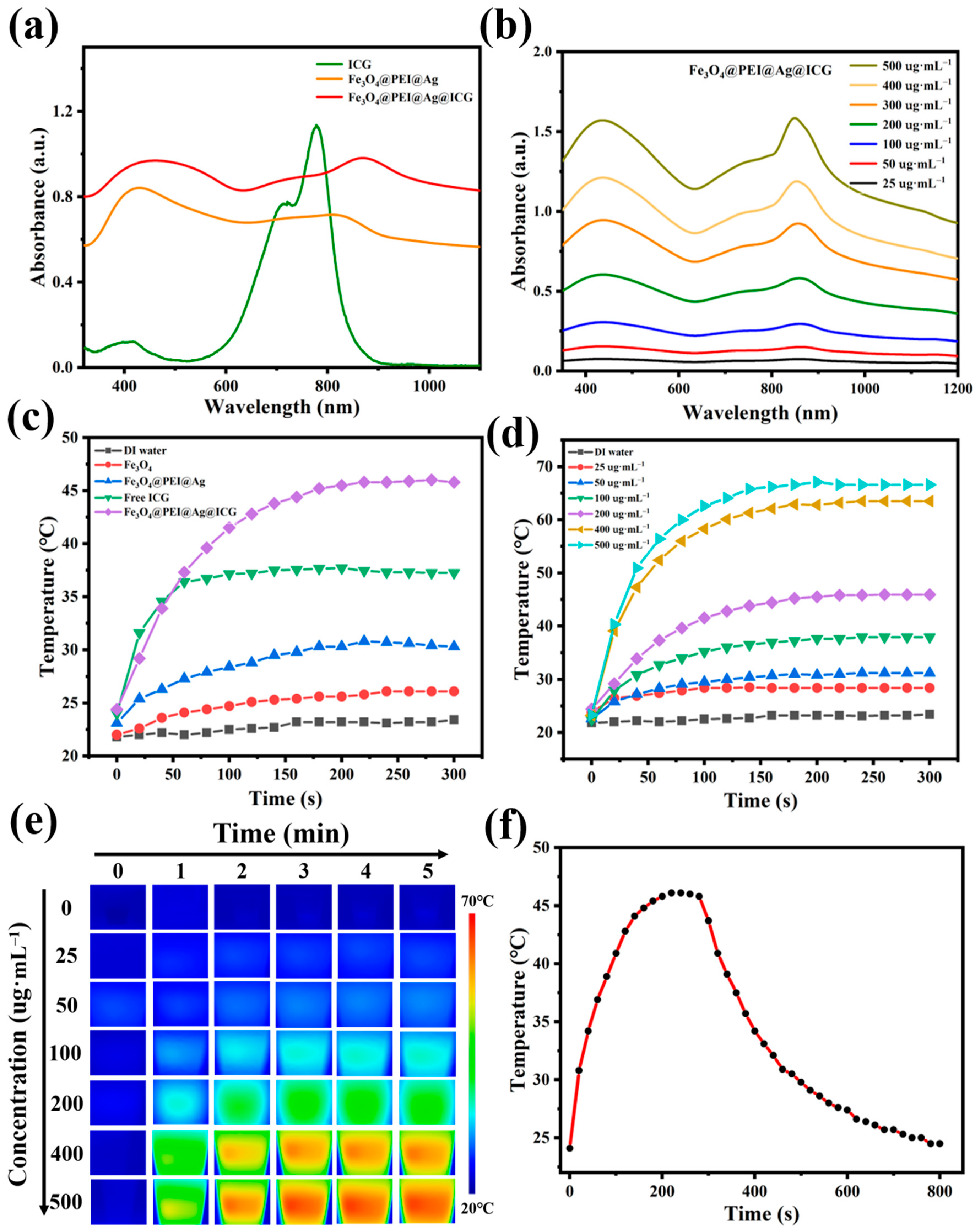
2.5. Photothermal Property of Fe3O4@PEI@Ag@ICG Structure
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fe3O4 NPs
3.3. Preparation of Fe3O4@PEI@Ag and Fe3O4@PEI@Ag@ICG Structures
3.4. Characterizations
3.5. Peroxidase-Like Activity Characterization
3.6. Colorimetric Detection of Glucose and Cytotoxicity Measurement of Fe3O4@PEI@Ag and Fe3O4@PEI@Ag@ICG Structures
- (a)
- A total of 100 μL of GOx and 100 μL glucose with different concentrations in 300 μL Phosphate-buffered Saline (pH 7.4) were incubated at 37 °C for 15 min;
- (b)
- A total of 300 μL phosphate buffer (pH 5.8), 100 μL TMB, 100 μL Fe3O4@PEI@Ag aqueous solutions were added to the above glucose reaction solution (0.5 mL);
- (c)
- The mixed solution was incubated at 37 °C for 15 min;
- (d)
- UV-vis detection was performed to observe the absorption value of the reaction products at 652 nm. In the control experiments, 10 mM lactose, 10 mM fructose, 10 mM maltose, and 10 mM Sucrose were used instead of glucose, respectively.
3.7. The Photothermal Effect Measurement under NIR-Light Activations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Romine, A.M.; Rubel, C.Z.; Engle, K.M.; Shi, B.F. Transition-Metal-Catalyzed, Coordination-Assisted Functionalization of Nonactivated C(sp(3))-H Bonds. Chem. Rev. 2021, 121, 14957–15074. [Google Scholar] [CrossRef]
- Quintana, C.; Cifuentes, M.P.; Humphrey, M.G. Transition metal complex/gold nanoparticle hybrid materials. Chem. Soc. Rev. 2020, 49, 2316–2341. [Google Scholar] [CrossRef]
- Zhang, X.P.; Chandra, A.; Lee, Y.M.; Cao, R.; Ray, K.; Nam, W. Transition metal-mediated O-O bond formation and activation in chemistry and biology. Chem. Soc. Rev. 2021, 50, 4804–4811. [Google Scholar] [CrossRef]
- van de L’Isle, M.O.N.; Ortega-Liebana, M.C.; Unciti-Broceta, A. Transition metal catalysts for the bioorthogonal synthesis of bioactive agents. Curr. Opin. Chem. Biol. 2021, 61, 32–42. [Google Scholar] [CrossRef]
- Klein, S.; Stiegler, L.M.S.; Harreiss, C.; Distel, L.V.R.; Neuhuber, W.; Spiecker, E.; Hirsch, A.; Kryschi, C. Understanding the Role of Surface Charge in Cellular Uptake and X-ray-Induced ROS Enhancing of Au-Fe3O4 Nanoheterodimers. ACS Appl. Bio Mater. 2018, 1, 2002–2011. [Google Scholar] [CrossRef]
- Luo, Y.; Fu, Y.; Huang, Z.; Li, M. Transition metals and metal complexes in autophagy and diseases. J. Cell Physiol. 2021, 236, 7144–7158. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Liu, W.; Guo, J.; Chen, C.; Ni, P.; Jiang, Y.; Zhang, C.; Wang, B.; Lu, Y. Ultrathin PdCu alloy nanosheet-assembled 3D nanoflowers with high peroxidase-like activity toward colorimetric glucose detection. Mikrochim. Acta 2021, 188, 114. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Chen, Z.W.; Yin, J.J.; Zhou, Y.T.; Zhang, Y.; Song, L.; Song, M.J.; Hu, S.L.; Gu, N. Dual Enzyme-like Activities of Iron Oxide Nanoparticles and Their Implication for Diminishing Cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef]
- Kang, X.; Sun, T.; Zhang, L.; Zhou, C.; Xu, Z.; Du, M.; Xiao, S.; Liu, Y.; Gong, M.; Zhang, D. Synergistic Theranostics of Magnetic Resonance Imaging and Photothermal Therapy of Breast Cancer Based on the Janus Nanostructures Fe3O4-Aushell-PEG. Int. J. Nanomed. 2021, 16, 6383–6394. [Google Scholar] [CrossRef]
- Dutta, B.; Nema, A.; Shetake, N.G.; Gupta, J.; Barick, K.C.; Lawande, M.A.; Pandey, B.N.; Priyadarsini, I.K.; Hassan, P.A. Glutamic acid-coated Fe3O4 nanoparticles for tumor-targeted imaging and therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110915. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy. Colloids Surf. B Biointerfaces 2019, 174, 252–259. [Google Scholar]
- Klein, S.; Harreiss, C.; Menter, C.; Hummer, J.; Distel, L.V.R.; Meyer, K.; Hock, R.; Kryschi, C. NOBF4-Functionalized Au-Fe3O4 Nanoheterodimers for Radiation Therapy: Synergy Effect Due to Simultaneous Reactive Oxygen and Nitrogen Species Formation. ACS Appl. Mater. Inter. 2018, 10, 17071–17080. [Google Scholar] [CrossRef]
- Li, J.; Xu, Q.; Wei, X.; Hao, Z. Electrogenerated chemiluminescence immunosensor for Bacillus thuringiensis Cry1Ac based on Fe3O4@Au nanoparticles. J. Agric. Food Chem. 2013, 61, 1435–1440. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Q.; Shi, M.; Yin, C.; Zhao, Z.; Shen, K.; Qiu, Y.; Xiao, Y.; Zhao, Y.; Yang, X.; et al. Fe3O4@TiO2-Laden Neutrophils Activate Innate Immunity via Photosensitive Reactive Oxygen Species Release. Nano Lett. 2020, 20, 261–271. [Google Scholar] [CrossRef]
- Nosrati, H.; Baghdadchi, Y.; Abbasi, R.; Barsbay, M.; Ghaffarlou, M.; Abhari, F.; Mohammadi, A.; Kavetskyy, T.; Bochani, S.; Rezaeejam, H.; et al. Iron oxide and gold bimetallic radiosensitizers for synchronous tumor chemoradiation therapy in 4T1 breast cancer murine model. J. Mater. Chem. B 2021, 9, 4510–4522. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.J.; Qi, J.X.; Yao, Q.; Oderinde, O.; Yao, C.; Song, W.; Shu, W.X.; Chen, P.; Wang, Y.H. Improvement of the surface wettability of silicone hydrogel films by self-assembled hydroxypropyltrimethyl ammonium chloride chitosan mixed colloids. Coll. Surf. A 2018, 558, 422–428. [Google Scholar] [CrossRef]
- He, H.; Sun, D.W.; Pu, H.; Huang, L. Bridging Fe3O4@Au nanoflowers and Au@Ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B1. Food Chem. 2020, 324, 126832. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mammeri, F.; Ammar, S.; Nguyen, T.B.N.; Nguyen, T.N.; Nghiem, T.H.L.; Thuy, N.T.; Ho, T.A. Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application. Nanomaterials 2021, 11, 1288. [Google Scholar] [CrossRef]
- Karami, B.; Hoseini, S.J.; Eskandari, K.; Ghasemi, A.; Nasrabadi, H. Synthesis of xanthene derivatives by employing Fe3O4 nanoparticles as an effective and magnetically recoverable catalyst in water. Catal. Sci. Technol. 2012, 2, 331–338. [Google Scholar] [CrossRef]
- Chen, R.; Sun, Y.; Huo, B.; Mao, Z.; Wang, X.; Li, S.; Lu, R.; Li, S.; Liang, J.; Gao, Z. Development of Fe3O4@Au nanoparticles coupled to Au@Ag core-shell nanoparticles for the sensitive detection of zearalenone. Anal. Chim. Acta 2021, 1180, 338888. [Google Scholar] [CrossRef]
- Wang, C.W.; Gu, B.; Liu, Q.Q.; Pang, Y.F.; Xiao, R.; Wang, S.Q. Combined use of vancomycin-modified Ag-coated magnetic nanoparticles and secondary enhanced nanoparticles for rapid surface-enhanced Raman scattering detection of bacteria. Int. J. Nanomed. 2018, 13, 1159–1178. [Google Scholar] [CrossRef]
- Ye, Y.; Mao, S.; He, S.; Xu, X.; Cao, X.; Wei, Z.; Gunasekaran, S. Ultrasensitive electrochemical genosensor for detection of CaMV35S gene with Fe3O4-Au@Ag nanoprobe. Talanta 2020, 206, 120205. [Google Scholar] [CrossRef]
- Najafipour, A.; Gharieh, A.; Fassihi, A.; Sadeghi-Aliabadi, H.; Mahdavian, A.R. MTX-Loaded Dual Thermoresponsive and pH-Responsive Magnetic Hydrogel Nanocomposite Particles for Combined Controlled Drug Delivery and Hyperthermia Therapy of Cancer. Mol. Pharm. 2021, 18, 275–284. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Sun, S. Dumbbell-like Au-Fe3O4 nanoparticles for target-specific platin delivery. J. Am. Chem. Soc. 2009, 131, 4216–4217. [Google Scholar] [CrossRef]
- Wang, X.; Cao, W.; Qin, L.; Lin, T.; Chen, W.; Lin, S.; Yao, J.; Zhao, X.; Zhou, M.; Hang, C.; et al. Boosting the Peroxidase-Like Activity of Nanostructured Nickel by Inducing Its 3+ Oxidation State in LaNiO3 Perovskite and Its Application for Biomedical Assays. Theranostics 2017, 7, 2277–2286. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Yang, J.; Jiang, Y.; He, N. Peroxidase-like activity of mesoporous silica encapsulated Pt nanoparticle and its application in colorimetric immunoassay. Anal. Chim. Acta 2015, 862, 53–63. [Google Scholar] [CrossRef]
- Shi, W.J.; Fan, H.; Ai, S.Y.; Zhu, L.S. Honeycomb-like nitrogen-doped porous carbon supporting Pt nanoparticles as enzyme mimic for colorimetric detection of cholesterol. Sens. Actuat. B-Chem. 2015, 221, 1515–1522. [Google Scholar] [CrossRef]
- Zhou, N.A.; Zou, S.Y.; Zou, L.; Shen, R.D.; Zhou, Y.M.; Ling, L.S. Peroxidase-like activity of palladium nanoparticles on hydrogen-bond supramolecular structures over a broader pH range and their application in glucose sensing. Can. J. Chem. 2019, 97, 317–323. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Sun, S.H. Dumbbell-like Pt-Fe3O4 Nanoparticles and Their Enhanced Catalysis for Oxygen Reduction Reaction. Nano Lett. 2009, 9, 1493–1496. [Google Scholar] [CrossRef]
- Wei, F.; Cui, X.Y.; Wang, Z.; Dong, C.C.; Li, J.D.; Han, X.J. Recoverable peroxidase-like Fe3O4@MoS2-Ag nanozyme with enhanced antibacterial ability. Chem. Eng. J. 2021, 408, 127240. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, Q.Y.; Li, L. Synthesis and electrocatalytic properties of AgxAuy/Fe3O4 composite microspheres and nanoparticles. J. Iran. Chem. Soc. 2021, 18, 1211–1217. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, Q.; Fu, Y.; Hao, M.Z.; Wang, W.C.; Zou, H.; Zhang, L.Q.; Tian, M. A Novel Method for the Preparation of Conductive and Magnetic Fe3O4@Ag Hybrid Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 8431–8438. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, Z.; Cao, H.; Huang, Y. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst 2012, 137, 5560–5564. [Google Scholar] [CrossRef]
- Baghayeri, M.; Veisi, H. Fabrication of a facile electrochemical biosensor for hydrogen peroxide using efficient catalysis of hemoglobin on the porous Pd@Fe3O4-MWCNT nanocomposite. Biosens. Bioelectron. 2015, 74, 190–198. [Google Scholar] [CrossRef]
- Zheng, C.; Ke, W.J.; Yin, T.X.; An, X.Q. Intrinsic peroxidase-like activity and the catalytic mechanism of gold@carbon dots nanocomposites. RSC Adv. 2016, 6, 35280–35286. [Google Scholar] [CrossRef]
- Ke, F.; Wang, L.; Zhu, J. Multifunctional Au-Fe3O4@MOF core-shell nanocomposite catalysts with controllable reactivity and magnetic recyclability. Nanoscale 2015, 7, 1201–1208. [Google Scholar] [CrossRef]
- Chudasama, B.; Vala, A.K.; Andhariya, N.; Upadhyay, R.V.; Mehta, R.V. Enhanced Antibacterial Activity of Bifunctional Fe3O4-Ag Core-Shell Nanostructures. Nano Res. 2009, 2, 955–965. [Google Scholar] [CrossRef]
- Yong, C.Y.; Chen, X.Q.; Xiang, Q.; Li, Q.; Xing, X.D. Recyclable magnetite-silver heterodimer nanocomposites with durable antibacterial performance. Bioact. Mater. 2018, 3, 80–86. [Google Scholar] [CrossRef]
- Xue, P.; Yang, R.; Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y. Indocyanine Green-Conjugated Magnetic Prussian Blue Nanoparticles for Synchronous Photothermal/Photodynamic Tumor Therapy. Nano-Micro Lett. 2018, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Zou, P.; Yang, L.L.; Cao, J.; Sun, Y.F.; Han, D.L.; Yang, S.; Wang, Z.; Chen, G.; Wang, B.; et al. A comprehensive study on the synthesis and paramagnetic properties of PEG-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2014, 303, 425–432. [Google Scholar] [CrossRef]
- Leng, W.N.; Pati, P.; Vikesland, P.J. Room temperature seed mediated growth of gold nanoparticles: Mechanistic investigations and life cycle assesment. Environ. Sci. Nano 2015, 2, 440–453. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Wang, J.; Wang, J.; Yu, A.; Zhang, H.; Song, D. Preparation of surface plasmon resonance biosensor based on magnetic core/shell Fe3O4/SiO2 and Fe3O4/Ag/SiO2 nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 484–490. [Google Scholar] [CrossRef]
- Zhang, J.L.; Srivastava, R.S.; Misra, R.D. Core-shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: Synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir 2007, 23, 6342–6351. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Zhang, X.; Luo, L.; He, Y.; Cong, C.; Gao, D. Nanomagnetic liposome-encapsulated parthenolide and indocyanine green for targeting and chemo-photothermal antitumor therapy. Nanomedicine 2020, 15, 871–890. [Google Scholar] [CrossRef]
- Jomma, E.Y.; Ding, S.N. One-Pot Hydrothermal Synthesis of Magnetite Prussian Blue Nano-Composites and Their Application to Fabricate Glucose Biosensor. Sensors 2016, 16, 243. [Google Scholar]
- Wang, S.J.; Chen, C.S.; Chen, L.C. Prussian blue nanoparticles as nanocargoes for delivering DNA drugs to cancer cells. Sci. Technol. Adv. Mat. 2013, 14, 044405. [Google Scholar] [CrossRef]
- Li, T.T.; Geng, Y.; Zhang, H.X.; Wang, J.; Feng, Y.; Chen, Z.Y.; Xie, X.X.; Qin, X.; Li, S.; Wu, C.H.; et al. A versatile nanoplatform for synergistic chemo-photothermal therapy and multimodal imaging against breast cancer. Expert Opin. Drug Deliv. 2020, 17, 725–733. [Google Scholar]
- Xu, Y.; Jian, G.; Peng, Y.; Liu, Z.; Hu, X. Lubricating mechanism of Fe3O4@MoS2 core-shell nanocomposites as oil additives for steel/steel contact. Tribol. Int. 2018, 121, 241–251. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, M.; Qiao, L.; Guo, R. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosens. Bioelectron. 2014, 52, 391–396. [Google Scholar] [CrossRef]
- Meng, X.Q.; Li, D.D.; Chen, L.; He, H.L.; Wang, Q.; Hong, C.Y.; He, J.Y.; Gao, X.F.; Yang, Y.L.; Jiang, B.; et al. High-Performance Self-Cascade Pyrite Nanozymes for Apoptosis-Ferroptosis Synergistic Tumor Therapy. ACS Nano 2021, 15, 5735–5751. [Google Scholar]
- Ma, L.; Zhu, J.; Wu, C.; Li, D.; Tang, X.H.; Zhang, Y.; An, C.H. Three-dimensional MoS2 nanoflowers supported Prussian blue and Au nanoparticles: A peroxidase-mimicking catalyst for the colorimetric detection of hydrogen peroxide and glucose. Spectrochim. Acta A 2021, 259, 119886. [Google Scholar] [CrossRef]
- An, Q.; Sun, C.; Li, D.; Xu, K.; Guo, J.; Wang, C. Peroxidase-like activity of Fe3O4@carbon nanoparticles enhances ascorbic acid-induced oxidative stress and selective damage to PC-3 prostate cancer cells. ACS Appl. Mater. Interfaces 2013, 5, 13248–13257. [Google Scholar]
- Su, L.; Feng, J.; Zhou, X.M.; Ren, C.L.; Li, H.H.; Chen, X.G. Colorimetric Detection of Urine Glucose Based ZnFe2O4 Magnetic Nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef]
- Huang, W.; Lin, T.Y.; Cao, Y.; Lai, X.Y.; Peng, J.; Tu, J.C. Hierarchical NiCo2O4 Hollow Sphere as a Peroxidase Mimetic for Colorimetric Detection of H2O2 and Glucose. Sensors 2017, 17, 217. [Google Scholar]
- Chen, J.; Shu, Y.; Li, H.; Xu, Q.; Hu, X. Nickel metal-organic framework 2D nanosheets with enhanced peroxidase nanozyme activity for colorimetric detection of H2O2. Talanta 2018, 189, 254–261. [Google Scholar] [CrossRef]
- Tan, H.; Li, Q.; Zhou, Z.; Ma, C.; Song, Y.; Xu, F.; Wang, L. A sensitive fluorescent assay for thiamine based on metal-organic frameworks with intrinsic peroxidase-like activity. Anal Chim Acta 2015, 856, 90–95. [Google Scholar]
- Li, S.L.; Li, H.; Chen, F.J.; Liu, J.; Zhang, H.L.; Yang, Z.Y.; Wang, B.D. Strong coupled palladium nanoparticles decorated on magnetic graphene nanosheets as enhanced peroxidase mimetics for colorimetric detection of H2O2. Dyes Pigment. 2016, 125, 64–71. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lamontagne, L.K.; Brodsky, C.N.; Chou, L.Y.; Zhuang, J.; Sneed, B.T.; Sheehan, M.K.; Tsung, C.K. The effect of lattice strain on the catalytic properties of Pd nanocrystals. ChemSusChem 2013, 6, 1993–2000. [Google Scholar] [CrossRef]
- Song, Y.J.; Qu, K.G.; Zhao, C.; Ren, J.S.; Qu, X.G. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.N.; Yang, B.C.; Liu, H.; Liu, Z.X.; Zhang, X.; Zheng, X.W.; Liu, Q.Y. FePt-Au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sens. Actuat. B-Chem. 2018, 259, 775–783. [Google Scholar] [CrossRef]
- Mvango, S.; Mashazi, P. Synthesis, characterization of copper oxide-gold nanoalloys and their peroxidase-like activity towards colorimetric detection of hydrogen peroxide and glucose. Mat. Sci. Eng. C-Mater. 2019, 96, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mitra, K.; Singh, R.; Kumari, A.; Sen Gupta, S.K.; Misra, N.; Maiti, P.; Ray, B. Colorimetric detection of hydrogen peroxide and glucose using brominated graphene. Anal. Methods 2017, 9, 6675–6681. [Google Scholar] [CrossRef]
- Ortiz-Gomez, I.; Salinas-Castillo, A.; Garcia, A.G.; Alvarez-Bermejo, J.A.; de Orbe-Paya, I.; Rodriguez-Dieguez, A.; Capitan-Vallvey, L.F. Microfluidic paper-based device for colorimetric determination of glucose based on a metal-organic framework acting as peroxidase mimetic. Microchim. Acta 2018, 185, 47. [Google Scholar] [CrossRef]
- Choleva, T.G.; Gatselou, V.A.; Tsogas, G.Z.; Giokas, D.L. Intrinsic peroxidase-like activity of rhodium nanoparticles, and their application to the colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2018, 185, 22. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Cai, Z.Q.; Liu, K.F.; Li, J.; Zhang, B.Y.; He, J.B. Sensitive colorimetric assay for uric acid and glucose detection based on multilayer-modified paper with smartphone as signal readout. Anal. Bioanal. Chem. 2018, 410, 2647–2655. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Qi, Y.; Guo, S.; Xue, L. Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors 2016, 16, 584. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Milani, A.S.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef]
- Holzer, W.; Mauerer, M.; Penzkofer, A.; Szeimies, R.M.; Abels, C.; Landthaler, M.; Baumler, W. Photostability and thermal stability of indocyanine green. J. Photochem. Photobiol. B 1998, 47, 155–164. [Google Scholar] [CrossRef]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; et al. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Hou, M.; Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y. Calcium-carbonate packaging magnetic polydopamine nanoparticles loaded with indocyanine green for near-infrared induced photothermal/photodynamic therapy. Acta Biomater. 2018, 81, 242–255. [Google Scholar] [CrossRef] [PubMed]


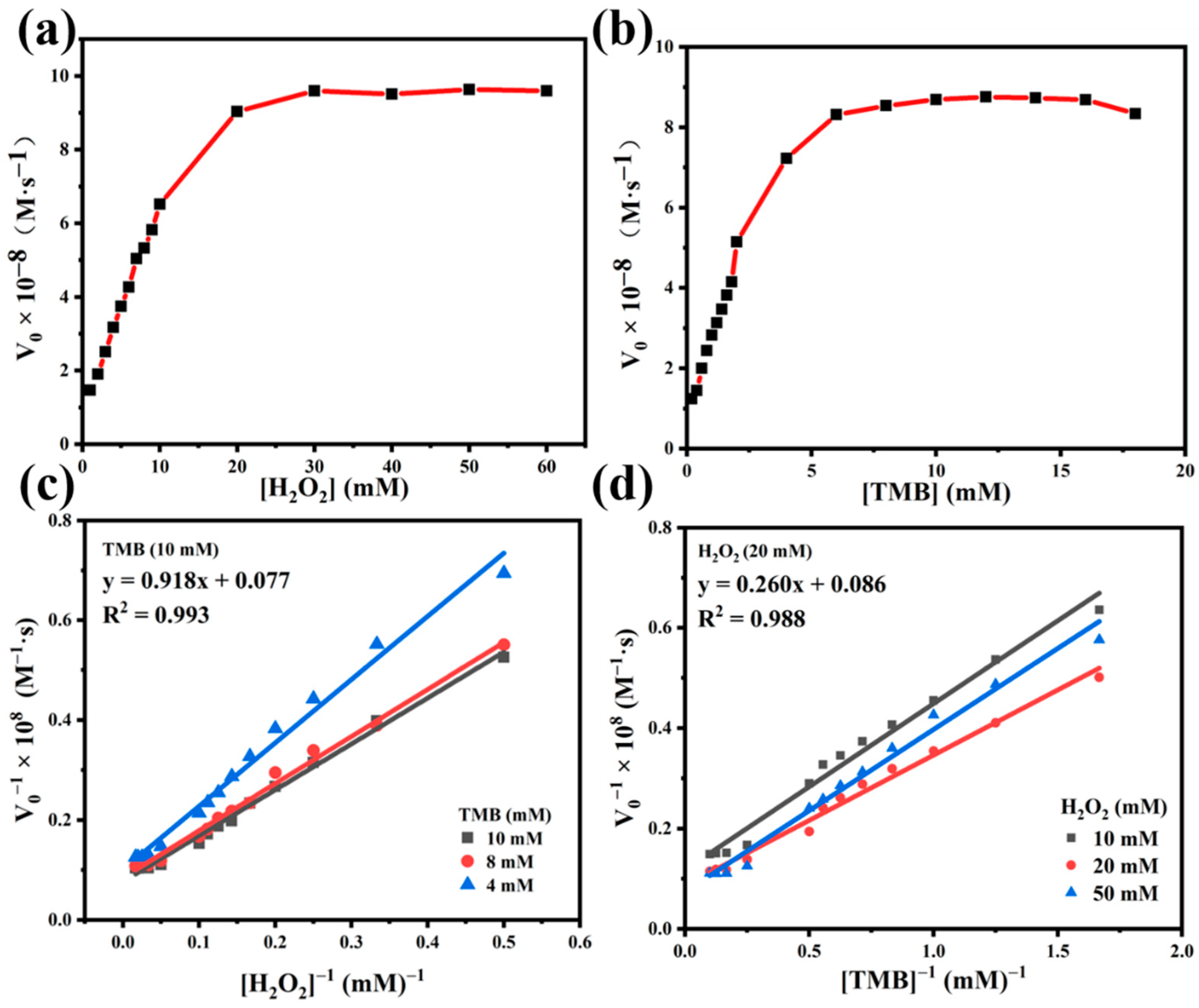
| [S] (mg∙mL−1) | Km (mM) | Kcat (×10−10 mol∙s−1∙mg−1) | Vmax (×10−7 M∙s−1) | ||||
|---|---|---|---|---|---|---|---|
| Fe3O4@PEI@Ag | 0.2 | H2O2 | TMB | H2O2 | TMB | H2O2 | TMB |
| 1.192 | 0.302 | 6.495 | 5.815 | 1.299 | 1.163 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Hu, X.; Yang, Y.; Wu, Q.; He, C.; He, X.; Wang, Z.; Mao, X. New Insight into Assembled Fe3O4@PEI@Ag Structure as Acceptable Agent with Enzymatic and Photothermal Properties. Int. J. Mol. Sci. 2022, 23, 10743. https://doi.org/10.3390/ijms231810743
Wang T, Hu X, Yang Y, Wu Q, He C, He X, Wang Z, Mao X. New Insight into Assembled Fe3O4@PEI@Ag Structure as Acceptable Agent with Enzymatic and Photothermal Properties. International Journal of Molecular Sciences. 2022; 23(18):10743. https://doi.org/10.3390/ijms231810743
Chicago/Turabian StyleWang, Teng, Xi Hu, Yujun Yang, Qing Wu, Chengdian He, Xiong He, Zhenyu Wang, and Xiang Mao. 2022. "New Insight into Assembled Fe3O4@PEI@Ag Structure as Acceptable Agent with Enzymatic and Photothermal Properties" International Journal of Molecular Sciences 23, no. 18: 10743. https://doi.org/10.3390/ijms231810743
APA StyleWang, T., Hu, X., Yang, Y., Wu, Q., He, C., He, X., Wang, Z., & Mao, X. (2022). New Insight into Assembled Fe3O4@PEI@Ag Structure as Acceptable Agent with Enzymatic and Photothermal Properties. International Journal of Molecular Sciences, 23(18), 10743. https://doi.org/10.3390/ijms231810743






