Epidemiology of Breast Cancer in Mexican Women with Obesity as a Risk Factor
Abstract
:1. Introduction
2. Results
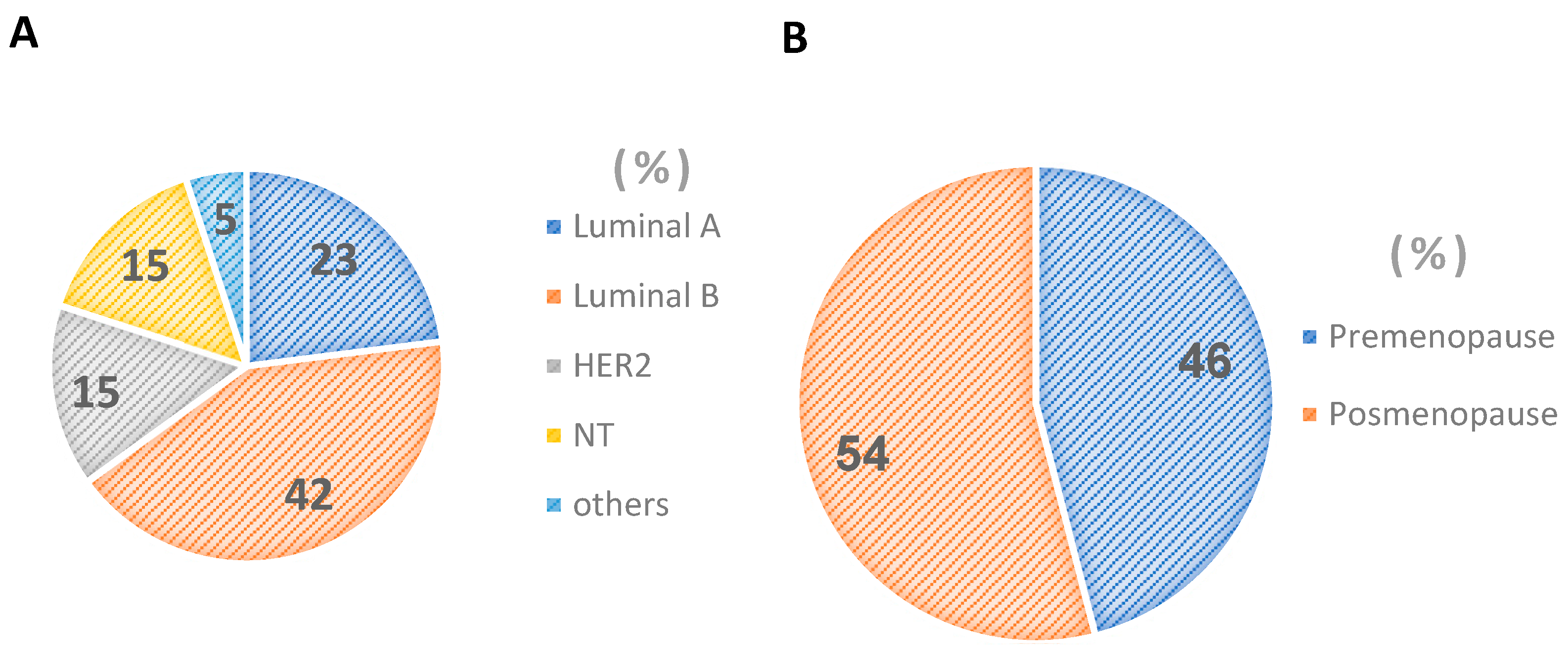
2.1. Women under and over 55 Years of Age
2.2. Types of Breast Cancer in Mexican Women
2.3. Percentage Stage Clinical and Tumor Grade Breast Cancer in Mexican Women
2.4. Characteristics Diagnostic Start and End of Treatment without Recurrence and with Recurrence of Cancer in Mexican Women
2.5. Percentage under and over 55 Years of Age without Recurrence and with Recurrence of Cancer in Mexican Women
2.6. Type of Breast Cancer in Mexican Women without Recurrence and with Recurrence
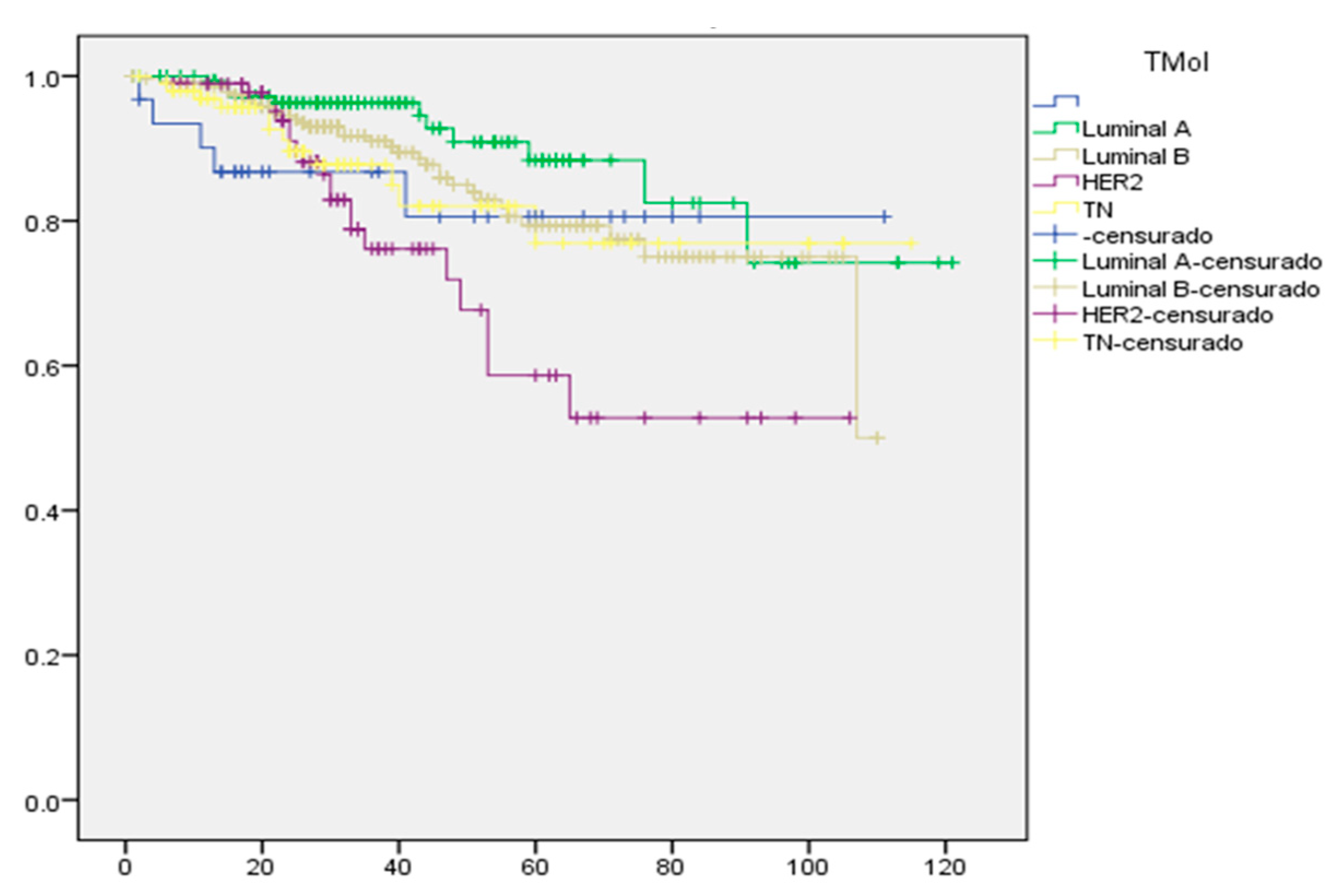
2.7. Kaplan–Meier Survival Curve for Mexican Women with Breast Cancer
3. Discussion
4. Materials and Methods
4.1. Study Type
4.2. Population
4.3. Data
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Histological Types | Women < 55 (%) | Women > 55 (%) | All Population (%) |
|---|---|---|---|
| LA | 33 | 39 | 35 |
| LB | 28 | 20 | 25 |
| HER2 | 17 | 21 | 19 |
| TN | 21 | 20 | 21 |
References
- Zimta, A.A.; Tigu, A.B.; Muntean, M.; Cenariu, D.; Slaby, O.; Berindan-Neagoe, I. Molecular Links between Central Obesity and Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5364. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 26 March 2021).
- DeSantis, C.E.; Bray, F.; Ferlay, J.; Lortet-Tieulent, J.; Anderson, B.O.; Jemal, A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Unlu, O.; Kiyak, D.; Caka, C.; Yagmur, M.; Yavas, H.G.; Erdogan, F.; Sener, N.; Oguz, B.; Babacan, T.; Altundag, K. Risk factors and histopathological features of breast cancer among women with different menopausal status and age at diagnosis. J. BUON 2017, 22, 184–191. [Google Scholar] [PubMed]
- Maldonado-Vega, M.; Calderón-Salinas, J.V. El tejido adiposo y la respuesta de macrófagos en el proceso inflamatorio y resistencia a la insulina. Rev. Edu. Bioq. 2022, 41, 3–17. [Google Scholar]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of randomized clinical trials from the Women’s Health Initiative. Oncol. JAMA 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Atoum, M.F.; Alzoughool, F.; Al-Hourani, H. Linkage Between Obesity Leptin and Breast Cancer. Breast Cancer 2020, 14, 1178223419898458. [Google Scholar] [CrossRef]
- Grabher, B.J. Breast Cancer: Evaluating Tumor Estrogen Receptor Status with Molecular Imaging to Increase Response to Therapy and Improve Patient Outcomes. J. Nucl. Med. Technol. 2020, 48, 191–201. [Google Scholar] [CrossRef]
- Berger, E.R.; Iyengar, N.M. Consideraciones sobre la obesidad y el equilibrio energético en el cáncer de mama triple negativo. Diario del Cáncer 2021, 27, 17–24. [Google Scholar] [CrossRef]
- Zorzanelli, R.V.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation a role for adaptive immunity in obesity. Circ. Res. 2008, 103, 467–476. [Google Scholar]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunol. J. Cell. Mol. Systems Technol. 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Daham, K.; Kammoun, H.L. Macrophage polarization in obesity and type 2 diabetes: Weighing down our understanding of macrophage function? Front. Immunol. 2014, 28, 1–6. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Heiman, M.; DiMarchi, R. Leptin: Structure, function and biology. Vitam. Horm. 2005, 71, 345–372. [Google Scholar]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef]
- Coppack, S. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Phipps, A.I.; Chlebowski, R.T.; Prentice, R.; McTiernan, A.; Stefanick, M.L.; Wactawski-Wende, J.; Kuller, L.H.; Adams-Campbell, L.L.; Lane, D.; Vitolins, M.; et al. Body Size, Physical Activity, and Risk of Triple-Negative and Estrogen Receptor–Positive Breast Cancer. Multicent. Study 2011, 20, 454–463. [Google Scholar] [CrossRef]
- Byung, C.L.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta 2014, 184, 446–462. [Google Scholar]
- Xia, D.; Song, Y.; Li, C.; Zhang, F.; Wei, M. The change of serum leptin and its relationship with platelet membrane glycoprotein Ib in patients with coronary heart disease. Front. Med. China 2007, 1, 352–355. [Google Scholar] [CrossRef]
- Uchoa, E.T.; Aguilera, G.; Herman, J.P.; Fielder, J.L.; Deak, T.; Cordeiro de Sousa, M.B. Novel aspects of hypothalamic-pituitary-adrenal axis regulation and glucocorticoid actions. J. Neuroendocr. 2014, 26, 557–572. [Google Scholar] [CrossRef]
- Luo, N.; Wang, X.; Fu, Y. Effects of macrophage-specific adiponectin expression on lipid metabolism in vivo. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E180–E186. [Google Scholar] [CrossRef]
- O´Connor, J.C.; Johnson, D.R.; Freund, G.G. Psychneuroimmune implications of type 2 diabetes: Redux. Immunol. Allergy Clin. Am. 2009, 29, 339–358. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Chen, I.C.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Winston, L.A.; Wang, H.; Williams, S.; Lu, Y.S.; Hsueh, T.H.; et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev. Res. 2018, 11, 227–236. [Google Scholar] [CrossRef]
- Calder, P.C.; Namanjeet, A.; Fred, B.; Timo, B. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. l3), S5–S78. [Google Scholar] [CrossRef]
- Zarrati, M.; Aboutaleb, N.; Cheshmazar, E.; Shoormasti, R.S.; Razmpoosh, E.; Nasirinezhad, F. The association of obesity and serum leptin levels with complete blood count and some serum biochemical parameters in Iranian overweight and obese individuals. Med. J. Islamic Repub. Iran. 2009, 33, 72. [Google Scholar] [CrossRef]
- Coats, B.R.; Schoenfelt, K.Q.; Barbosa-Lorenzi, V.C.; Peris, E.; Cui, C.; Hoffman, A.; Zhou, G.; Fernandez, S.; Zhai, L.; Hall, B.A.; et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017, 20, 3149–3161. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. 2022 Indice de Masa Corporal—IMC. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 29 August 2022).
- Alisi, A.; Carpio, G.; Oliveira, F.L.; Panera, N.; Nobili, V.; Gaudio, E. The role on tissue macrophage mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediat. Inflamm. 2017, 2017, 8162421. [Google Scholar] [CrossRef] [Green Version]
- CONEVAL. Porcentaje De Población En Situación De Pobreza 2010, Guanajuato. Available online: https://www.coneval.org.mx/coordinacion/entidades/Guanajuato/Paginas/pob_municipal.aspx (accessed on 16 June 2010).

| Characteristics | Women < 55 Years | Women > 55 Years | All Population | |||
|---|---|---|---|---|---|---|
| Diagnostic Start | End of Treatment | Diagnostic Start | End of Treatment | Diagnostic Start | End of Treatment | |
| Age (years) | 46 ± 8 [22–59] | 68 ± 7 [59–90] * | 52 ± 12 [22–90] * | |||
| Weight (Kg) | 69 ± 13 [39–133] | 69 ± 14 [33–115] | 67 ± 13 [34–112] | 65 ± 14 [36–108] | 69 ± 13 [35–133] | 68 ± 14 * [33–116] |
| BMI (kg/m2) | 29 ± 5.1 [17–48] | 28 ± 5.2 [15–49] | 29 ± 5.3 [18–45] | 28 ± 5.6 [17–45] | 29 ± 5.2 [15–48] | 28 ± 5.2 [15–49] |
| Index L/M | 2.74 ± 1.13 [0.01–11] | 3.02 ± 1.25 [0.0–15] | 2.71 ± 1.56 [0.01–10] | 3.38 ± 3.19 [0–33] | 2.73 ± 1.2 [0.01–11] | 3.1 ± 2.1 [0–33] |
| CEA (ng/mL) | 6 ± 34 [0–544] | 26 ± 162 * [1–43] | 3 ± 6 [0–2306] | 5 ± 10 [1–74] | 5 ± 29 [0–544] | 21 ± 141 [0–2306] |
| CA 15-3 (U/mL) | 41 ± 278 [2–4726] | 65 ± 244 * [1–2773] | 35 ± 56 [5–330] | 32 ± 51 [4–292] | 39 ± 243 [2–4723] | 57 ± 214 * [1–2773] |
| Characteristics | Non-Recurrence | Recurrence | All Population | |||
|---|---|---|---|---|---|---|
| Diagnostic Start | End of Treatment | Diagnostic Start | End of Treatment | Diagnostic Start | End of Treatment | |
| Age (years) | 52 ± 12 [22–90] | 52 ± 12 [23–81] * | 52 ± 12 [22–90] | |||
| BMI (kg/m2) | 29 ± 4.5 [17–46] | 29 ± 5.1 * [16–48] | 29 ± 5.6 [17–48] | 28 ± 5.6 * [15–45] | 29 ± 5.2 [17–48] | 28 ± 5.2 * [15–49] |
| Index L/M | 2.6 ±0.9 [0.01–8.3] | 3.0 ± 1.6 * [0.0–15] | 2.9 ± 1.6 [0.01–11] | 3.3 ± 2.8 * [0–33] | 2.7 ± 1.2 [0.009–11] | 3.1 ± 2.1 * [0–33] |
| CEA (ng/mL) | 5 ± 35 [0–544] | 10 ± 58 * [0–627] | 6 ± 12 [0–87] | 41 ± 226 * [0–2306] | 5 ± 30 [0–544] | 21 ± 141 * [0–2306] |
| CA 15-3 (U/mL) | 19 ± 22 [4–251] | 65 ± 244 * [1–2773] | 79 ± 416 [2–4723] | 32 ± 51 * [4–292] | 39 ± 243 * [2–4723] | 57 ± 214 * [1–2773] |
| Women < 55 Years | Women > 55 Years | |||
|---|---|---|---|---|
| Recurrence | No (%) | Yes (%) | No (%) | Yes (%) |
| LA | 75 | 25 | 68 | 31 |
| LB | 70 | 30 | 76 | 23 |
| HER2 | 66 | 34 | 74 | 26 |
| NT | 84 | 16 | 55 | 45 |
| LA | LB | HER2 | TN | |||||
|---|---|---|---|---|---|---|---|---|
| Diagnostic Start | NR | RE | NR | RE | NR | RE | NR | RE |
| L/M | 3 ± 1.1 [0.01–8.3] | 3 ± 1.6 [0.01–10] | 3 ± 1 [0.01–6] | 3 ± 2 * [0.01–11] | 3 ± 1.0 [0.01–5] | 3 ± 1.3 [0.01–7] | 2 ± 1 [0.01–5] | 3 ± 1.7 * [1.35–8] |
| CEA | 2 ± 1.6 [0.2–12] | 4 ± 6 * [0.2–30] | 12 ± 62 [0.2–544] | 9 ± 16 * [0.3–87] | 4 ± 7 [0.2–41] | 5 ± 7 * [0.2–33] | 4 ± 10 [0.2–80] | 3 ± 7 [0.4–40] |
| CA 15-3 | 19 ± 15 [4.7–80] | 32 ± 50 * [3–2263] | 18 ± 22 [5–183] | 210±77 * [2–4723] | 15 ± 8 [6.2–32] | 27 ± 28 * [6.5–107] | 21 ± 32 [4–250] | 48 ± 67 * [5–330] |
| End of treatment | ||||||||
| L/M | 2 ± 1.4 [0.04–9] | 4 ± 4.5 * [0.02–34] | 3 ± 1.8 [0.01–14] | 3 ± 1.5 [2–7] | 3 ± 1.6 [0.03–10] | 3 ± 1.1 [2–5.5] | 3 ± 2.4 [0.01–15] | 3 ± 1.5 [0.16–7] |
| CEA | 2 ±3.4 [0.3–23] | 68 ±334 * [0.7–2306] | 27 ± 105 [0.5–627] | 58 ± 216 * [0.7–267] | 2 ± 2.4 [0.5–15] | 16 ± 51 * [0.5–293] | 8 ± 25.2 [0.20–51] | 4 ± 7.8 * [0.50–43] |
| CA 15-3 | 22 ± 36 [2–255] | 122 ± 360 * [6–2223] | 35 ± 87 [3.3–650] | 243 ± 52 * [6–2772] | 15 ± 15 [1–106] | 32 ± 42 * [4–185.4] | 22 ± 30 [6.3–187] | 59 ± 121 * [0.5–621] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejudo-Arteaga, S.; Guerrero-Ramos, M.Á.; Kuri-Exome, R.; Martínez-Cordero, E.; Farias-Serratos, F.; Maldonado-Vega, M. Epidemiology of Breast Cancer in Mexican Women with Obesity as a Risk Factor. Int. J. Mol. Sci. 2022, 23, 10742. https://doi.org/10.3390/ijms231810742
Cejudo-Arteaga S, Guerrero-Ramos MÁ, Kuri-Exome R, Martínez-Cordero E, Farias-Serratos F, Maldonado-Vega M. Epidemiology of Breast Cancer in Mexican Women with Obesity as a Risk Factor. International Journal of Molecular Sciences. 2022; 23(18):10742. https://doi.org/10.3390/ijms231810742
Chicago/Turabian StyleCejudo-Arteaga, Shaila, Miguel Ángel Guerrero-Ramos, Roberto Kuri-Exome, Erika Martínez-Cordero, Felipe Farias-Serratos, and María Maldonado-Vega. 2022. "Epidemiology of Breast Cancer in Mexican Women with Obesity as a Risk Factor" International Journal of Molecular Sciences 23, no. 18: 10742. https://doi.org/10.3390/ijms231810742
APA StyleCejudo-Arteaga, S., Guerrero-Ramos, M. Á., Kuri-Exome, R., Martínez-Cordero, E., Farias-Serratos, F., & Maldonado-Vega, M. (2022). Epidemiology of Breast Cancer in Mexican Women with Obesity as a Risk Factor. International Journal of Molecular Sciences, 23(18), 10742. https://doi.org/10.3390/ijms231810742





