The Perfect Match: Assessment of Sample Collection Efficiency for Immunological and Molecular Findings in Different Types of Fabrics
Abstract
:1. Introduction
2. Results
2.1. Immunochromatographic Tests Results
2.2. DNA Quantification
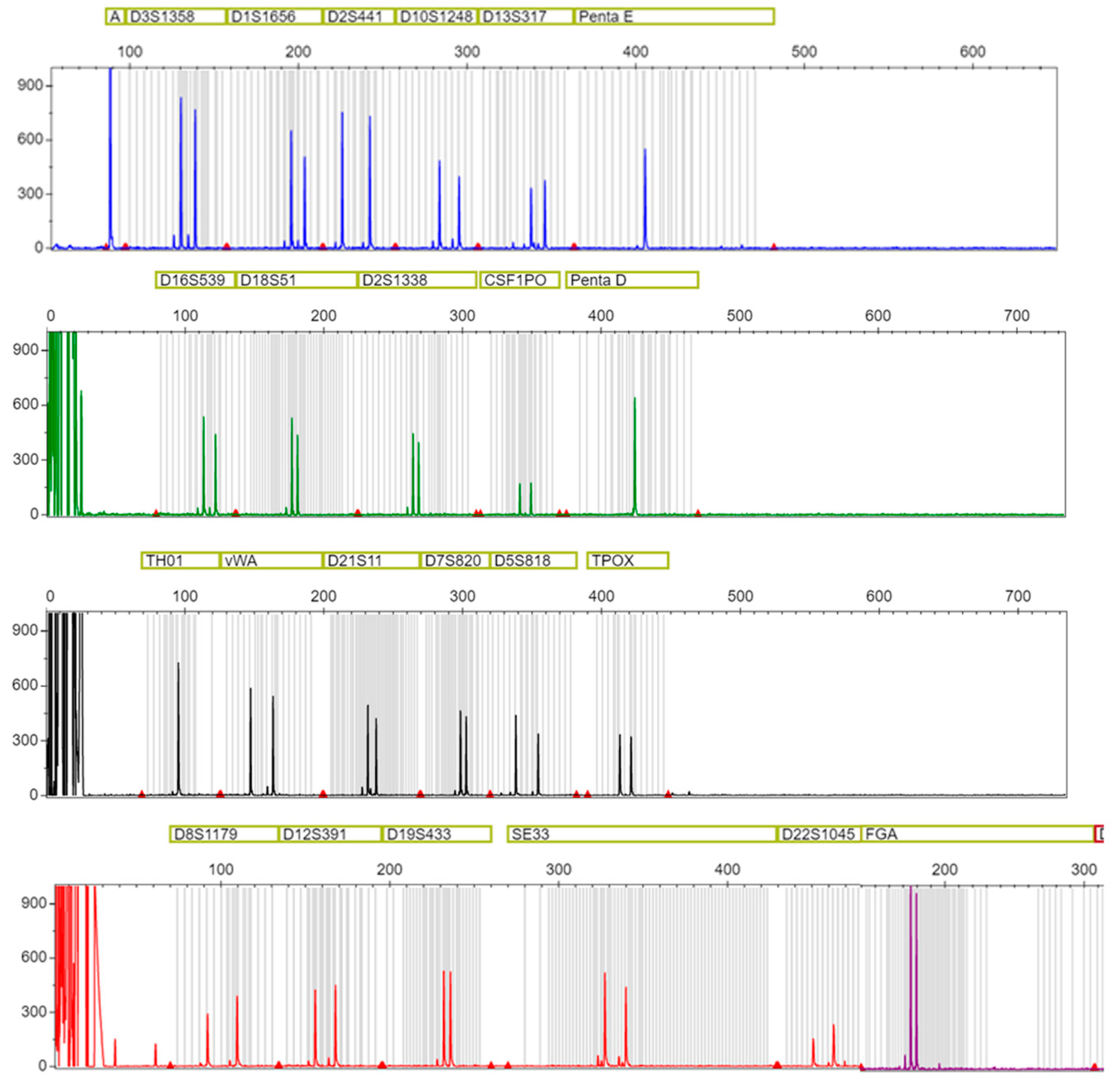
2.3. DNA Profiling
2.4. mtDNA Sequencing
3. Discussion
4. Material and Methods
4.1. Saliva Samples, Fabrics, and Swabs
4.2. Experiment
4.3. DNA Extraction
4.4. DNA Quantification
4.5. Nuclear DNA Profiling
4.6. Mitochondrial DNA (mtDNA) Sequencing
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtkotter, H.; Schwender, K.; Wiegand, P.; Peiffer, H.; Vennemann, M. Improving body fluid identification in forensic trace evidence-construction of an immunochromatographic test array to rapidly detect up to five body fluids simultaneously. Int. J. Leg. Med. 2018, 132, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Virkler, K.; Lednev, I.K. Analysis of body fluids for forensic purposes: From laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci. Int. 2009, 188, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pandeshwar, P.; Das, R. Role of oral fluids in DNA investigations. J. Forensic Leg. Med. 2014, 22, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, P.H.; Kipps, A.E. The significance of amylase in forensic investigations of body fluids. Forensic Sci. 1975, 6, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, T.; Watanabe, K.; Fujinami, Y.; Sakurada, K. Applicability of ELISA detection of statherin for forensic identification of saliva. Int. J. Leg. Med. 2010, 124, 493–498. [Google Scholar] [CrossRef]
- Chauncey, H.H.; Henrigues, B.L.; Tanzer, J.M. Comparative Enzyme Activity of Saliva from the Sheep, Hog, Dog, Rabbit, Rat, and Human. Arch. Oral Biol. 1963, 8, 615–627. [Google Scholar] [CrossRef]
- Sakurada, K.; Watanabe, K.; Akutsu, T. Current Methods for Body Fluid Identification Related to Sexual Crime: Focusing on Saliva, Semen, and Vaginal Fluid. Diagnostics 2020, 10, 693. [Google Scholar] [CrossRef]
- Virkler, K.; Lednev, I.K. Forensic body fluid identification: The Raman spectroscopic signature of saliva. Analyst 2010, 135, 512–517. [Google Scholar] [CrossRef]
- Li, X.; Ding, Y.; Ling, J.; Yao, W.; Zha, L.; Li, N.; Chang, Y.; Wang, Y.; Cai, J. Bacteria-targeting BSA-stabilized SiC nanoparticles as a fluorescent nanoprobe for forensic identification of saliva. Mikrochim. Acta 2019, 186, 756. [Google Scholar] [CrossRef]
- Conte, J.; Ruddy, A.; Domonoski, L.; Shanahan, A.; Daley, N.; McDevitt, C.; Roca, G. Recovery of DNA from SERATEC() immunochromatographic PSA and saliva test strips. J. Forensic Sci. 2022, 67, 1176–1183. [Google Scholar] [CrossRef]
- Brownlow, R.J.; Dagnall, K.E.; Ames, C.E. A comparison of DNA collection and retrieval from two swab types (cotton and nylon flocked swab) when processed using three QIAGEN extraction methods. J. Forensic Sci. 2012, 57, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.W.Y.; Wong, Y.; Ng, B.K.; Lim, W.S.H.; Rosli, A.R.; Syn, C.K.C. A practical study on direct PCR amplification using the GlobalFiler PCR Amplification Kit on human bloodstains collected with microFLOQ Direct swabs. Forensic Sci. Int. 2019, 300, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Verdon, T.J.; Mitchell, R.J.; van Oorschot, R.A. Swabs as DNA collection devices for sampling different biological materials from different substrates. J. Forensic Sci. 2014, 59, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Cox, J.O.; Rhodes, C.; Lewis, C.; Koroma, M.; Hudson, B.C.; Dawson Cruz, T.; Seashols-Williams, S.J. Comparison of DNA typing success in compromised blood and touch samples based on sampling swab composition. J. Forensic Sci. 2021, 66, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Ambers, A.; Wiley, R.; Novroski, N.; Budowle, B. Direct PCR amplification of DNA from human bloodstains, saliva, and touch samples collected with microFLOQ((R)) swabs. Forensic Sci. Int. Genet. 2018, 32, 80–87. [Google Scholar] [CrossRef]
- Sherier, A.J.; Kieser, R.E.; Novroski, N.M.M.; Wendt, F.R.; King, J.L.; Woerner, A.E.; Ambers, A.; Garofano, P.; Budowle, B. Copan microFLOQ(R) Direct Swab collection of bloodstains, saliva, and semen on cotton cloth. Int. J. Leg. Med. 2020, 134, 45–54. [Google Scholar] [CrossRef]
- Dadhania, A.; Nelson, M.; Caves, G.; Santiago, R.; Podini, D. Evaluation of Copan 4N6FLOQSwabs used for crime scene evidence collection. Forensic Sci. Int. Genet. Suppl. Ser. 2013, 4, e336–e337. [Google Scholar] [CrossRef]
- Comte, J.; Baechler, S.; Gervaix, J.; Lock, E.; Milon, M.P.; Delemont, O.; Castella, V. Touch DNA collection—Performance of four different swabs. Forensic Sci. Int. Genet. 2019, 43, 102113. [Google Scholar] [CrossRef]
- Ferguson, J.; Dunn, S.; Best, A.; Mirza, J.; Percival, B.; Mayhew, M.; Megram, O.; Ashford, F.; White, T.; Moles-Garcia, E.; et al. Validation testing to determine the sensitivity of lateral flow testing for asymptomatic SARS-CoV-2 detection in low prevalence settings: Testing frequency and public health messaging is key. PLoS Biol. 2021, 19, e3001216. [Google Scholar] [CrossRef]
- Peto, T.; Team, U.C.-L.F.O. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. eClinicalMedicine 2021, 36, 100924. [Google Scholar] [CrossRef]
- Thakar, M.K.; Joshi, B.; Shrivastava, P.; Raina, A.; Lalwani, S. An assessment of preserved DNA in decomposed biological materials by using forensic DNA profiling. Egypt. J. Forensic Sci. 2019, 9, 46. [Google Scholar] [CrossRef]
- Shrivastava, P.; Jain, T.; Kumawat, R.K. Direct PCR amplification from saliva sample using non-direct multiplex STR kits for forensic DNA typing. Sci. Rep. 2021, 11, 7112. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Peral, D.; Rubio, L.; Santos, I.; Gaitan, M.J.; Viguera, E.; Martin-de-Las-Heras, S. DNA degradation in human teeth exposed to thermal stress. Sci. Rep. 2021, 11, 12118. [Google Scholar] [CrossRef]
- Zapata, F.; de la Ossa, M.A.; Garcia-Ruiz, C. Differentiation of Body Fluid Stains on Fabrics Using External Reflection Fourier Transform Infrared Spectroscopy (FT-IR) and Chemometrics. Appl. Spectrosc. 2016, 70, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, R. Detection of vaginal fluid stains on common substrates via ATR FT-IR spectroscopy. Int. J. Leg. Med. 2020, 134, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Virkler, K.; Lednev, I.K. Raman spectroscopic signature of semen and its potential application to forensic body fluid identification. Forensic Sci. Int. 2009, 193, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Virkler, K.; Lednev, I.K. Raman spectroscopic signature of blood and its potential application to forensic body fluid identification. Anal. Bioanal. Chem. 2010, 396, 525–534. [Google Scholar] [CrossRef]
- McLaughlin, G.; Lednev, I.K. In Situ Identification of Semen Stains on Common Substrates via Raman Spectroscopy. J. Forensic Sci. 2015, 60, 595–604. [Google Scholar] [CrossRef]
- Niemi, M.E.K.; Daly, M.J.; Ganna, A. The human genetic epidemiology of COVID-19. Nat. Rev. Genet. 2022, 23, 533–546. [Google Scholar] [CrossRef]
- Dalmaso, G.; Bini, M.; Paroni, R.; Ferrari, M. Qualification of high-recovery, flocked swabs as compared to traditional rayon swabs for microbiological environmental monitoring of surfaces. PDA J. Pharm. Sci. Technol. 2008, 62, 191–199. [Google Scholar]
- Hedman, J.; Akel, Y.; Jansson, L.; Hedell, R.; Wallmark, N.; Forsberg, C.; Ansell, R. Enhanced forensic DNA recovery with appropriate swabs and optimized swabbing technique. Forensic Sci. Int. Genet. 2021, 53, 102491. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, M.S.; Stasulli, D.M.; Sobestanovich, E.M.; Bille, T.W. Evaluation of methods to improve the extraction and recovery of DNA from cotton swabs for forensic analysis. PLoS ONE 2014, 9, e116351. [Google Scholar] [CrossRef] [PubMed]
- Seiberle, I.; Wahrer, J.; Kron, S.; Flury, K.; Girardin, M.; Schocker, A.; Schulz, I. Collaborative swab performance comparison and the impact of sampling solution volumes on DNA recovery. Forensic Sci. Int. Genet. 2022, 59, 102716. [Google Scholar] [CrossRef]
- Garriga, J.A.; Ubelaker, D.H.; Zapico, S.C. Evaluation of macroscopic changes and the efficiency of DNA profiling from burnt teeth. Sci. Justice 2016, 56, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]


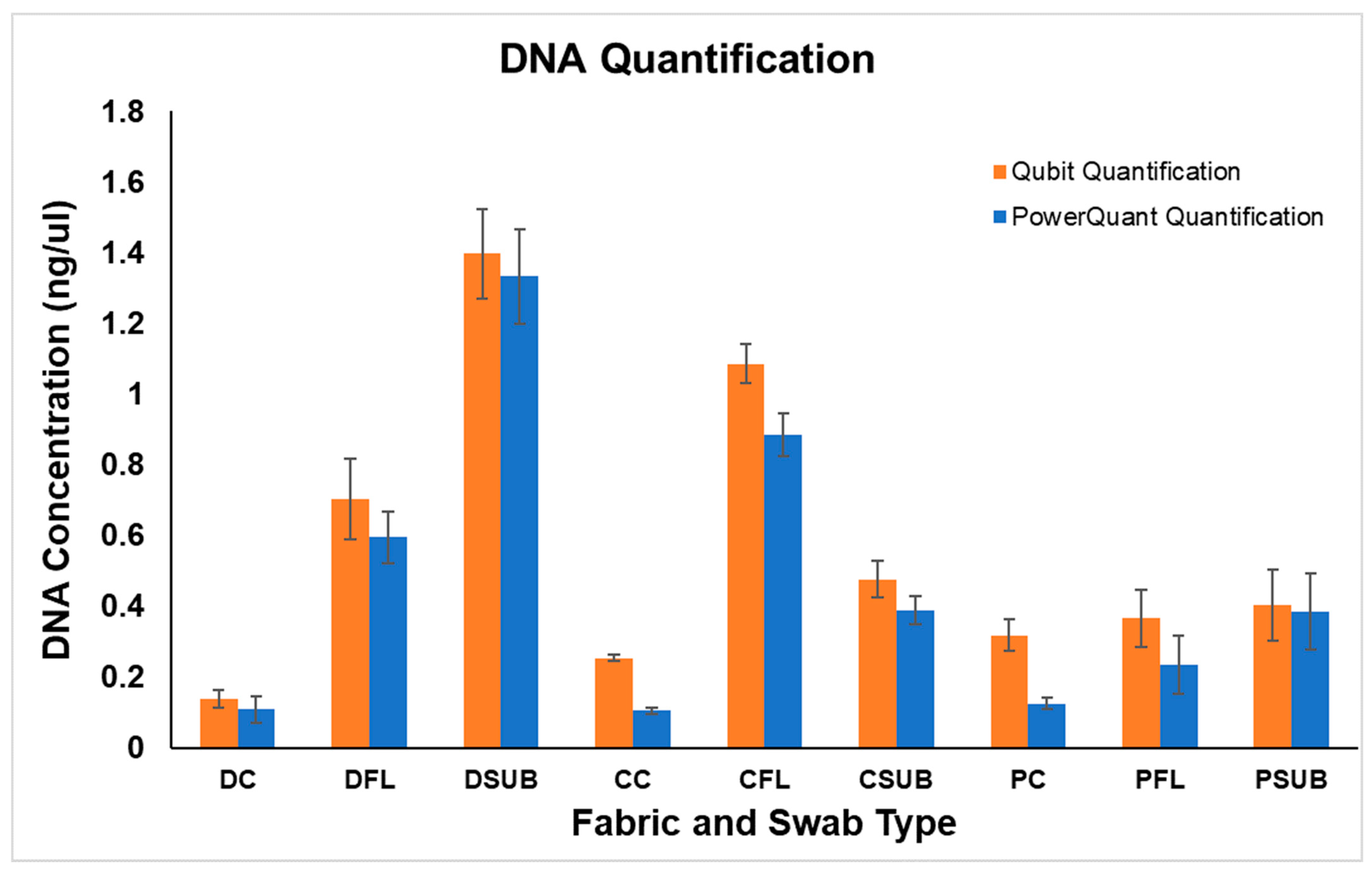
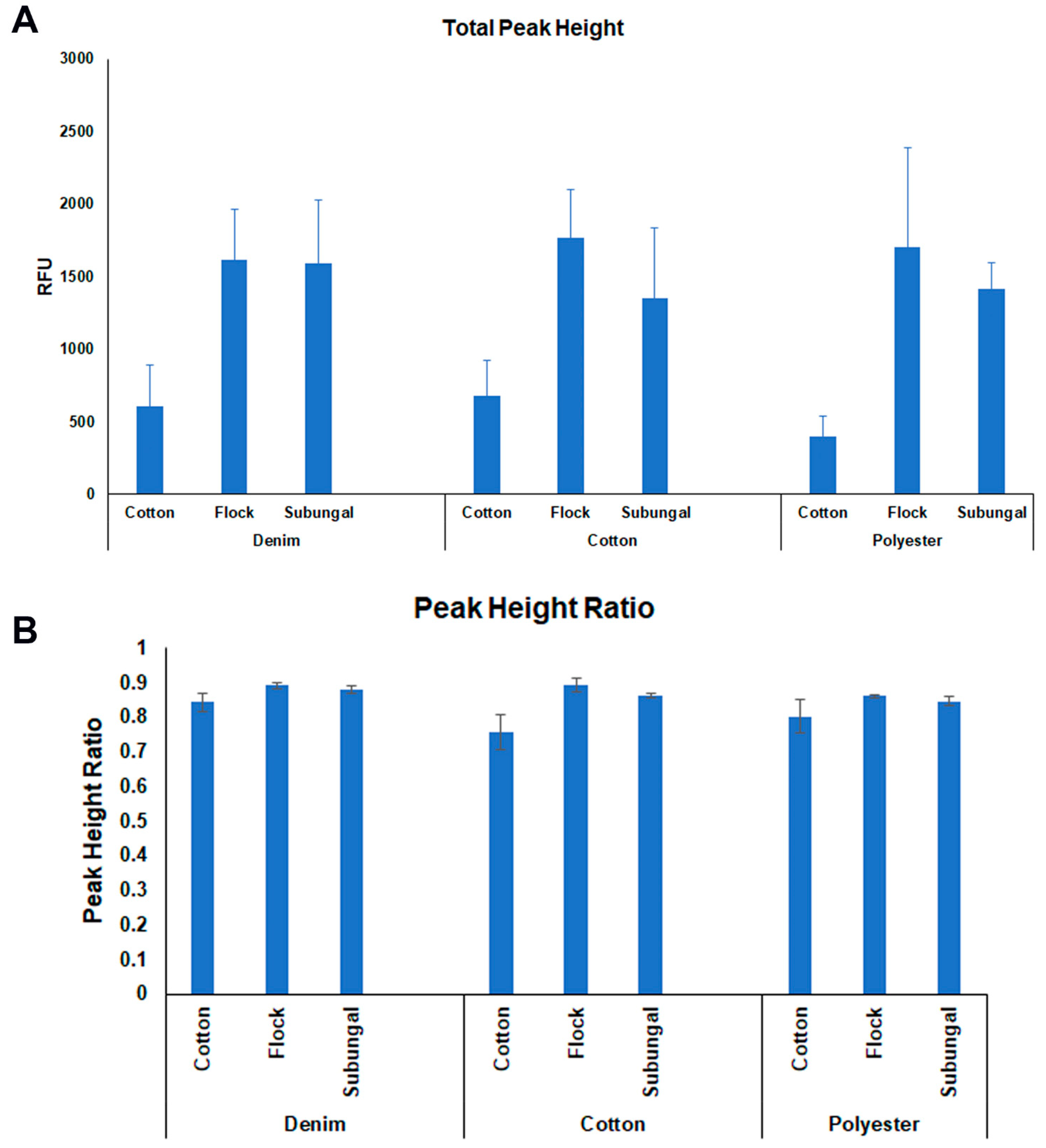

| Fabric | Swab Type | Replicate | Rank | Qubit Quantification ng/μl | Human Quantification ng/μl |
|---|---|---|---|---|---|
| 1 | G3.5 | 0.204 | 0.2319 | ||
| Cotton | 2 | G3 | 0.159 | 0.0832 | |
| 3 | G3.5 | 0.058 | 0.015 | ||
| 1 | G7 | 0.334 | 0.3666 | ||
| Denim | 4N6FLOQ Swabs Regular | 2 | G8 | 0.769 | 0.6067 |
| 3 | G8 | 1.010 | 0.8106 | ||
| 1 | G5 | 1.660 | 1.5858 | ||
| 4N6FLOQ Swabs Subungual | 2 | G7 | 0.962 | 0.8649 | |
| 3 | G8 | 1.570 | 1.5468 | ||
| 1 | G5 | 0.268 | 0.0785 | ||
| Cotton | 2 | G6 | 0.223 | 0.1285 | |
| 3 | G4 | 0.273 | 0.1098 | ||
| 1 | G10 | 0.896 | 0.682 | ||
| Cotton | 4N6FLOQ Swabs Regular | 2 | G9 | 1.170 | 0.9544 |
| 3 | G10 | 1.190 | 1.0185 | ||
| 1 | G7 | 0.654 | 0.5249 | ||
| 4N6FLOQ Swabs Subungual | 2 | G8 | 0.378 | 0.3397 | |
| 3 | G5 | 0.397 | 0.3071 | ||
| 1 | G6 | 0.233 | 0.1148 | ||
| Cotton | 2 | G7 | 0.253 | 0.0856 | |
| 3 | G8 | 0.470 | 0.1788 | ||
| 1 | G9 | 0.218 | 0.0022 | ||
| Polyester | 4N6FLOQ Swabs Regular | 2 | G7 | 0.232 | 0.2105 |
| 3 | G7 | 0.648 | 0.4905 | ||
| 1 | G5 | 0.270 | 0.2401 | ||
| 4N6FLOQ Swabs Subungual | 2 | G6 | 0.192 | 0.1582 | |
| 3 | G7 | 0.748 | 0.7569 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
C. Zapico, S.; Dytso, A.; Rubio, L.; Roca, G. The Perfect Match: Assessment of Sample Collection Efficiency for Immunological and Molecular Findings in Different Types of Fabrics. Int. J. Mol. Sci. 2022, 23, 10686. https://doi.org/10.3390/ijms231810686
C. Zapico S, Dytso A, Rubio L, Roca G. The Perfect Match: Assessment of Sample Collection Efficiency for Immunological and Molecular Findings in Different Types of Fabrics. International Journal of Molecular Sciences. 2022; 23(18):10686. https://doi.org/10.3390/ijms231810686
Chicago/Turabian StyleC. Zapico, Sara, Alex Dytso, Leticia Rubio, and Gabriela Roca. 2022. "The Perfect Match: Assessment of Sample Collection Efficiency for Immunological and Molecular Findings in Different Types of Fabrics" International Journal of Molecular Sciences 23, no. 18: 10686. https://doi.org/10.3390/ijms231810686
APA StyleC. Zapico, S., Dytso, A., Rubio, L., & Roca, G. (2022). The Perfect Match: Assessment of Sample Collection Efficiency for Immunological and Molecular Findings in Different Types of Fabrics. International Journal of Molecular Sciences, 23(18), 10686. https://doi.org/10.3390/ijms231810686







