The TGF-β/SMAD Signaling Pathway Prevents Follicular Atresia by Upregulating MORC2
Abstract
1. Introduction
2. Results
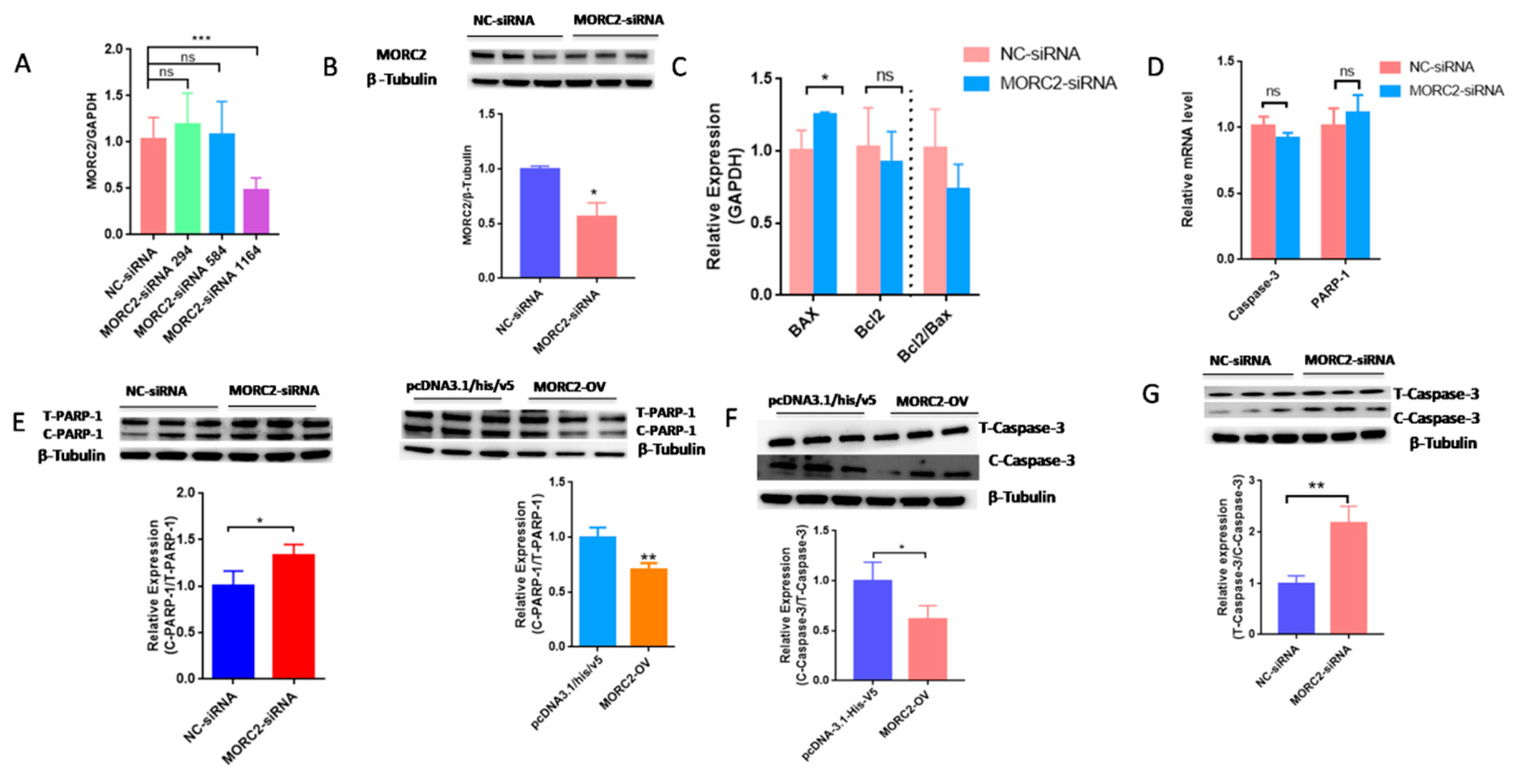
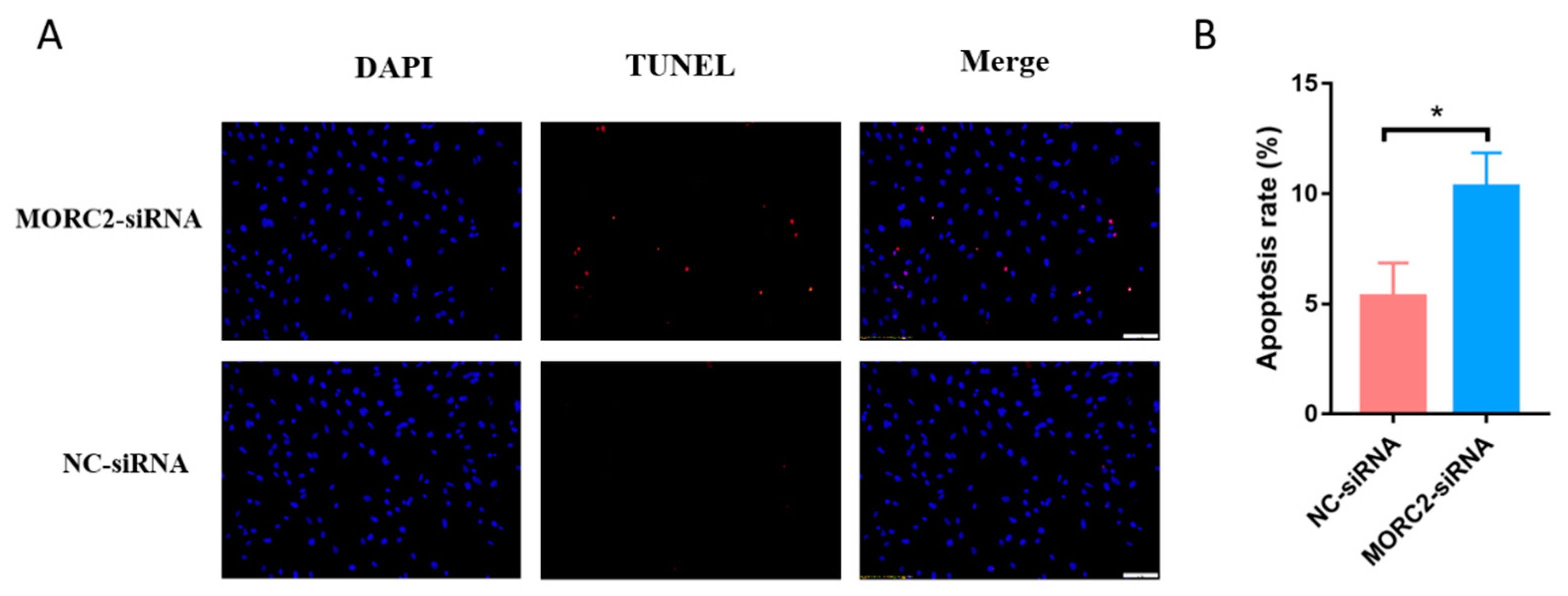
2.1. MORC2 Inhibits Apoptosis of Porcine Follicular Granulosa Cells
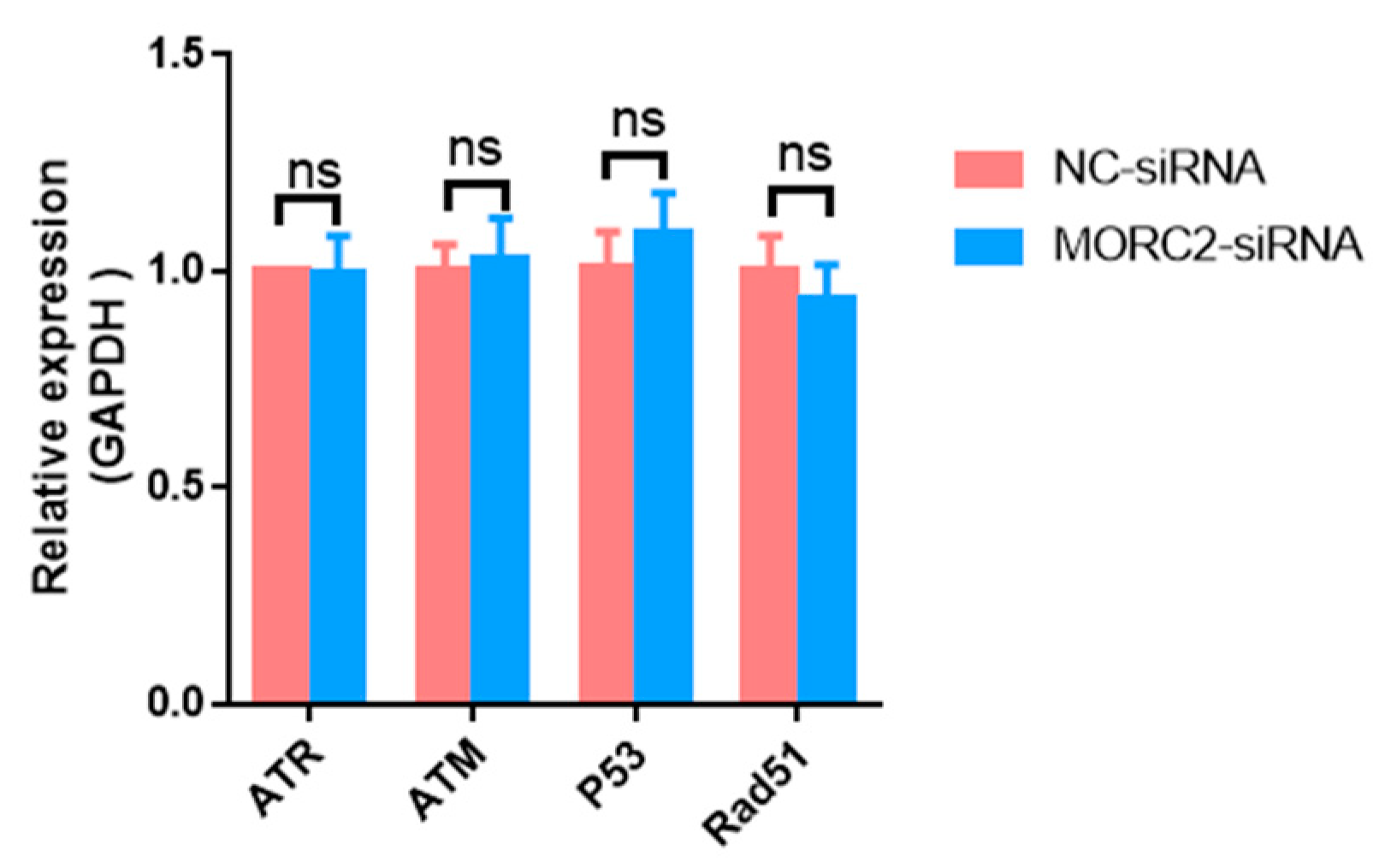
2.2. MORC2 Inhibits Apoptosis of Porcine Granulosa Cells Independently of the DNA Repair Pathway
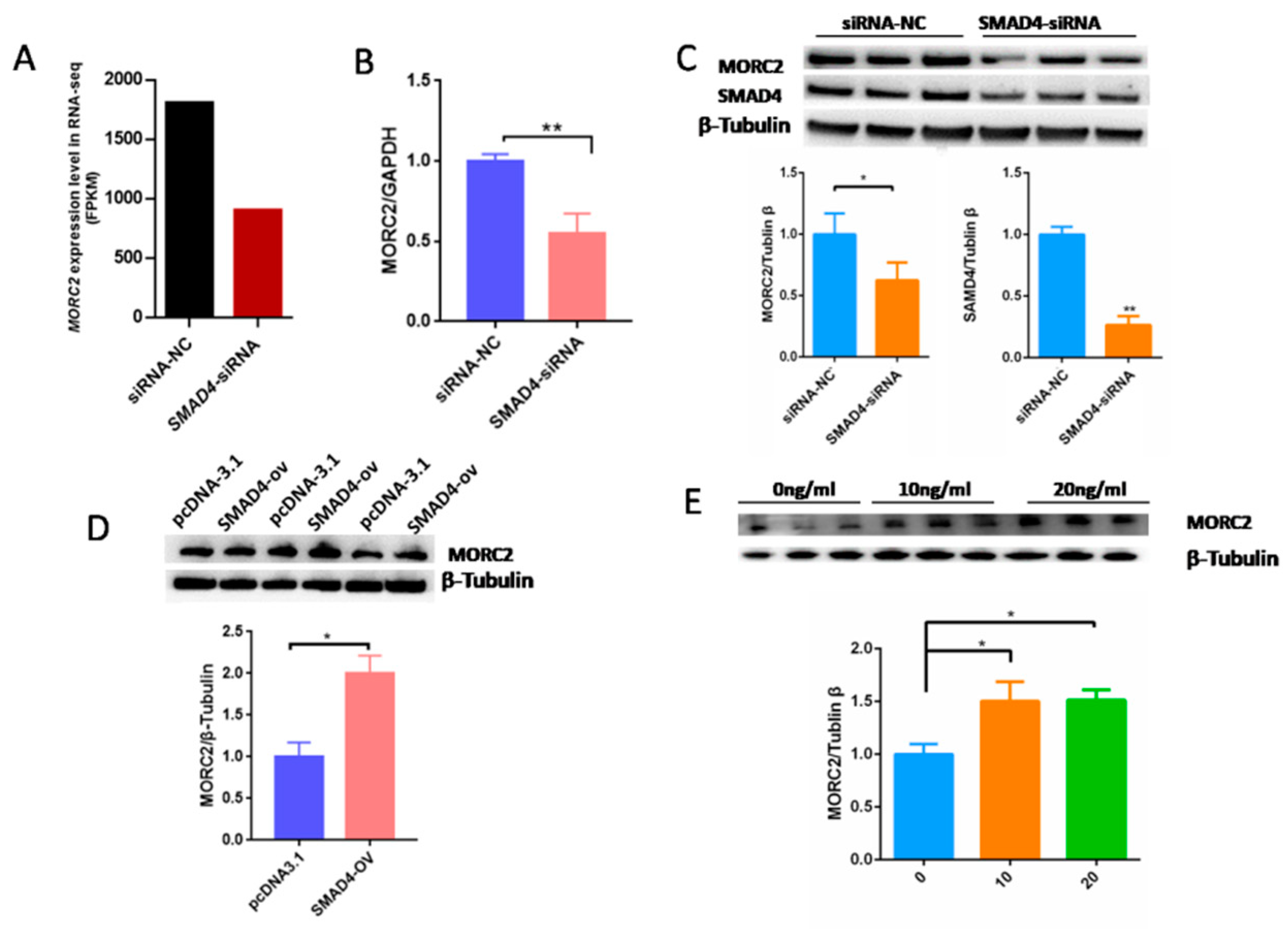
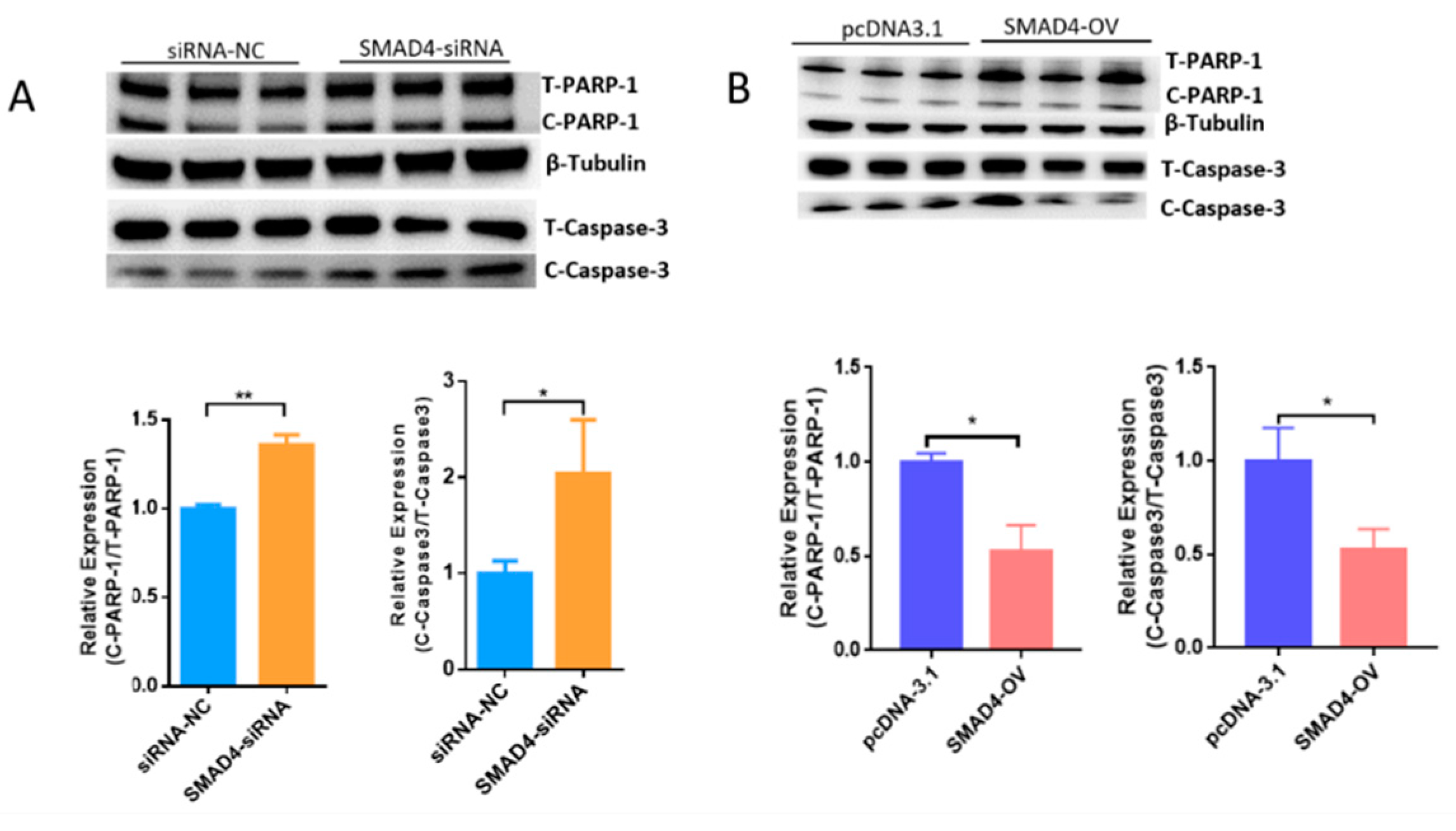
2.3. MORC2 Is a Downstream Target of the TGF-β/SMAD Signaling Pathway
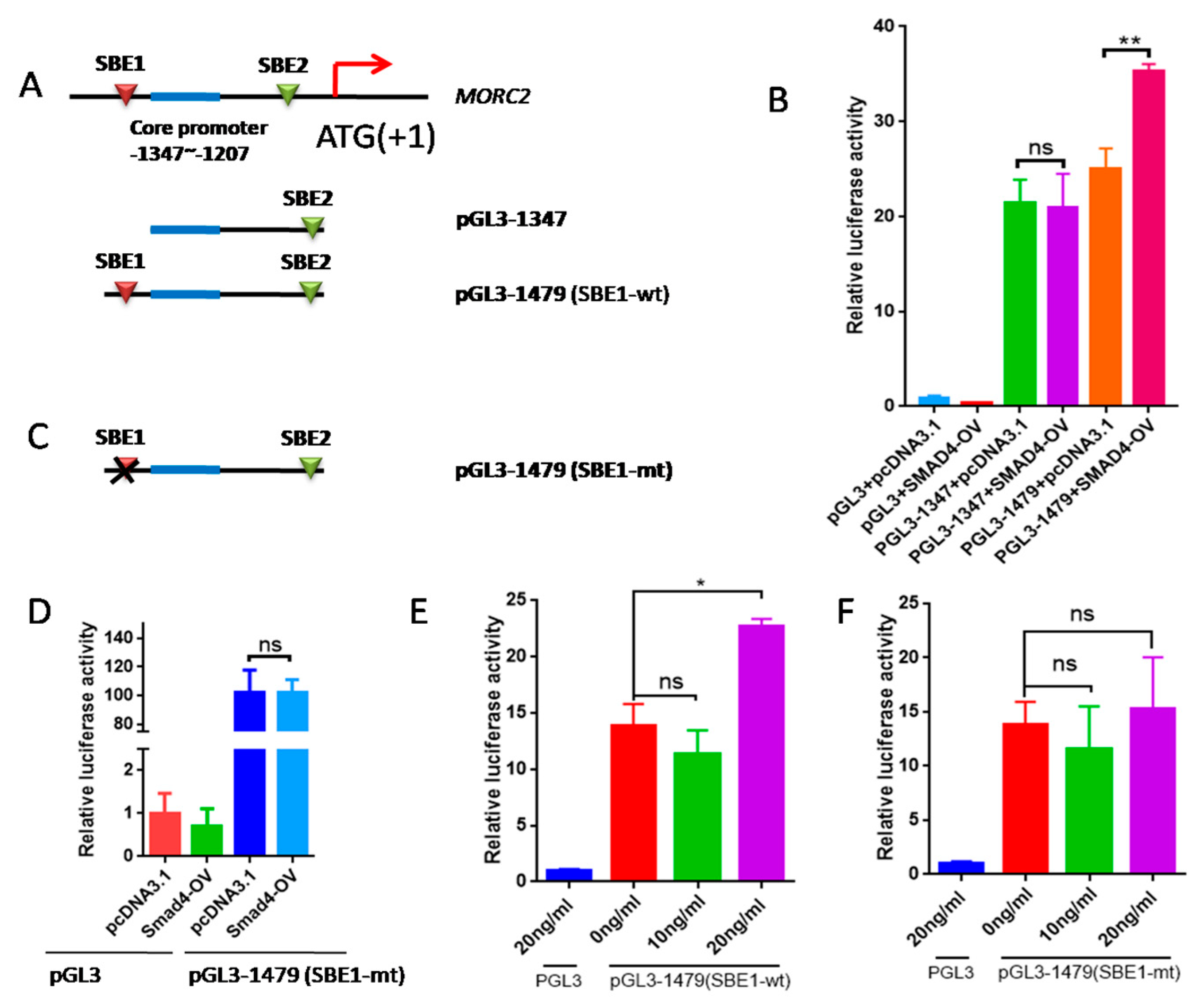
2.4. The Transcription Factor SMAD4 Directly Binds to the MORC2Promoter
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation and Culture of Porcine Granulosa Cells
4.3. Plasmid Construction
4.4. Cell Transfection
4.5. Total RNA Extraction and Quantitative PCR
4.6. WB Analysis
4.7. Tunel
4.8. Luciferase Reporter Gene Assay
4.9. Statistical Analysis
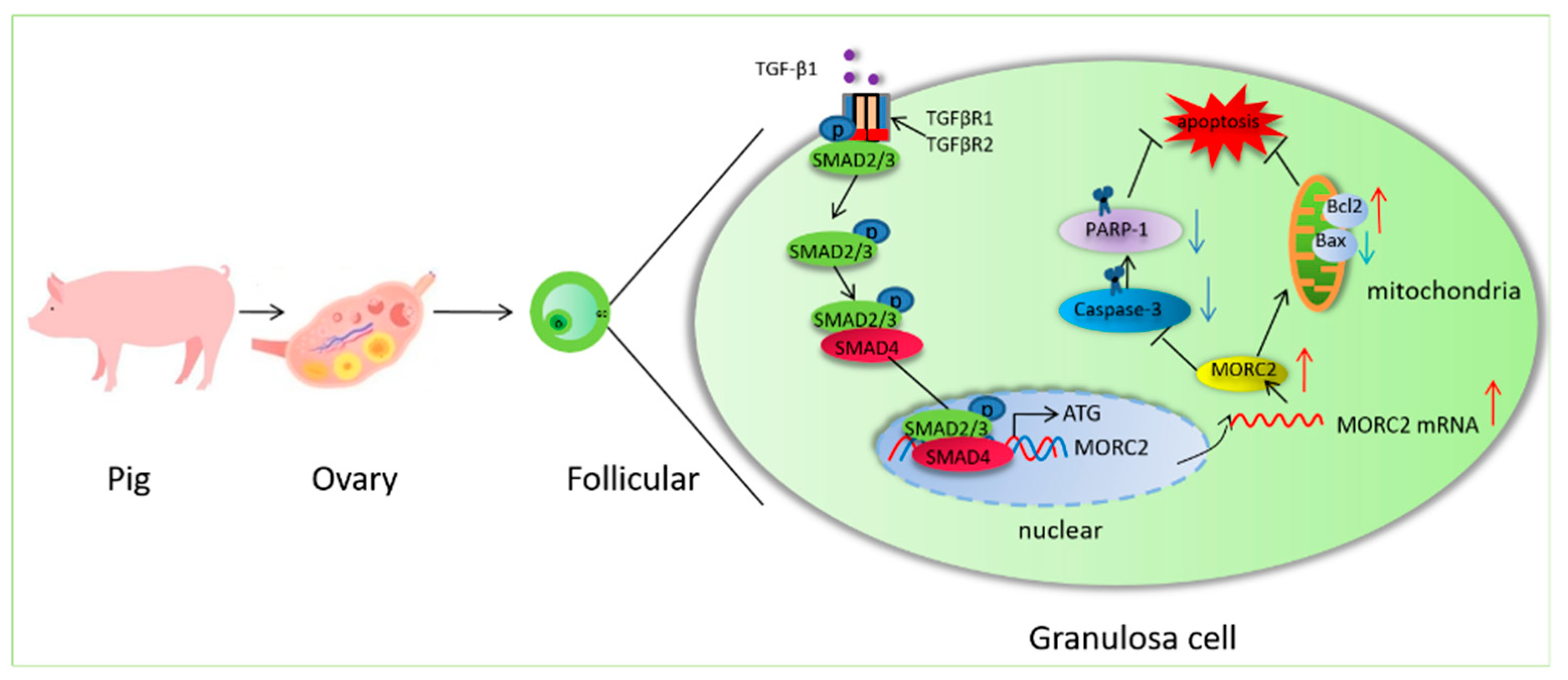
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, T.; Yao, J.; Zhou, S.; Ma, Y.; Dong, J.; Zhang, C.; Mi, Y. Naringin prevents follicular atresia by inhibiting oxidative stress in the aging chicken. Poult. Sci. 2022, 101, 101891. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Zhang, J.; Du, X.; Li, Q.; Pan, Z. circSLC41A1 Resists Porcine Granulosa Cell Apoptosis and Follicular Atresia by Promoting SRSF1 through miR-9820-5p Sponging. Int. J. Mol. Sci. 2022, 23, 1509. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cao, Y.; Jiang, Y.; Wei, Y.; Liu, H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018, 18, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Pan, Z.; Du, X.; Zhang, J.; Liu, H.; Li, Q. NORHA, a novel follicular atresia-related lncRNA, promotes porcine granulosa cell apoptosis via the miR-183-96-182 cluster and FoxO1 axis. J. Anim. Sci. Biotechnol. 2021, 12, 103. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, H.; Chen, B.; Liu, Z.; Wu, G.; Li, C.; Li, R.; Cao, Y.; Zhou, J.; et al. Sulforaphane Acts Through NFE2L2 to Prevent Hypoxia-Induced Apoptosis in Porcine Granulosa Cells via Activating Antioxidant Defenses and Mitophagy. J. Agric. Food Chem. 2022, 70, 8097–8110. [Google Scholar] [CrossRef]
- Monsivais, D.; Matzuk, M.M.; Pangas, S.A. The TGF-beta Family in the Reproductive Tract. Cold Spring Harb. Perspect. Biol. 2017, 9, a022251. [Google Scholar] [CrossRef]
- Monsivais, D.; Nagashima, T.; Prunskaite-Hyyrylainen, R.; Nozawa, K.; Shimada, K.; Tang, S.; Hamor, C.; Agno, J.E.; Chen, F.; Masand, R.P.; et al. Endometrial receptivity and implantation require uterine BMP signaling through an ACVR2A-SMAD1/SMAD5 axis. Nat. Commun. 2021, 12, 3386. [Google Scholar] [CrossRef]
- Zhang, K.; Rajput, S.K.; Lee, K.B.; Wang, D.; Huang, J.; Folger, J.K.; Knott, J.G.; Zhang, J.; Smith, G.W. Evidence supporting a role for SMAD2/3 in bovine early embryonic development: Potential implications for embryotropic actions of follistatin. Biol. Reprod. 2015, 93, 86. [Google Scholar] [CrossRef]
- Cheng, J.C.; Fang, L.; Yan, Y.; He, J.; Guo, Y.; Jia, Q.; Gao, Y.; Han, X.; Sun, Y.P. TGF-beta1 stimulates aromatase expression and estradiol production through SMAD2 and ERK1/2 signaling pathways in human granulosa-lutein cells. J. Cell Physiol. 2021, 236, 6619–6629. [Google Scholar] [CrossRef]
- Li, Q.; Agno, J.E.; Edson, M.A.; Nagaraja, A.K.; Nagashima, T.; Matzuk, M.M. Transforming growth factor beta receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011, 7, e1002320. [Google Scholar] [CrossRef]
- Liu, J.; Du, X.; Zhou, J.; Pan, Z.; Liu, H.; Li, Q. MicroRNA-26b functions as a proapoptotic factor in porcine follicular Granulosa cells by targeting Sma-and Mad-related protein 4. Biol. Reprod. 2014, 91, 146. [Google Scholar] [CrossRef] [PubMed]
- Terenina, E.; Fabre, S.; Bonnet, A.; Monniaux, D.; Robert-Granie, C.; SanCristobal, M.; Sarry, J.; Vignoles, F.; Gondret, F.; Monget, P.; et al. Differentially expressed genes and gene networks involved in pig ovarian follicular atresia. Physiol. Genom. 2017, 49, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Du, X.; Zhang, J.; Wang, Y.; Wang, M.; Pan, Z.; Li, Q. SMAD4-induced knockdown of the antisense long noncoding RNA BRE-AS contributes to granulosa cell apoptosis. Mol. Ther. Nucleic Acids 2021, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, J.; Pan, Z.; Du, X.; Li, X.; Ma, B.; Yao, W.; Li, Q.; Liu, H. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-beta type 1 receptor. Mol. Cell. Endocrinol. 2015, 409, 103–112. [Google Scholar] [CrossRef]
- Granados-Aparici, S.; Hardy, K.; Franks, S.; Sharum, I.B.; Waite, S.L.; Fenwick, M.A. SMAD3 directly regulates cell cycle genes to maintain arrest in granulosa cells of mouse primordial follicles. Sci. Rep. 2019, 9, 6513. [Google Scholar] [CrossRef]
- Lee, G.S.; Kwak, G.; Bae, J.H.; Han, J.P.; Nam, S.H.; Lee, J.H.; Song, S.; Kim, G.D.; Park, T.S.; Choi, Y.K.; et al. Morc2a p.S87L mutant mice develop peripheral and central neuropathies associated with neuronal DNA damage and apoptosis. Dis. Model. Mech. 2021, 14, dmm049123. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Morrow, A.; Fairgrieve, M.R.; Karr, J.P.; Yosef, N.; Vance, R.E. Self-guarding of MORC3 enables virulence factor-triggered immunity. Nature 2021, 600, 138–142. [Google Scholar] [CrossRef]
- Wang, T.; Qin, Z.Y.; Wen, L.Z.; Guo, Y.; Liu, Q.; Lei, Z.J.; Pan, W.; Liu, K.J.; Wang, X.W.; Lai, S.J.; et al. Epigenetic restriction of Hippo signaling by MORC2 underlies stemness of hepatocellular carcinoma cells. Cell Death Differ. 2018, 25, 2086–2100. [Google Scholar] [CrossRef]
- Groh, S.; Milton, A.V.; Marinelli, L.K.; Sickinger, C.V.; Russo, A.; Bollig, H.; de Almeida, G.P.; Schmidt, A.; Forne, I.; Imhof, A.; et al. Morc3 silences endogenous retroviruses by enabling Daxx-mediated histone H3.3 incorporation. Nat. Commun. 2021, 12, 5996. [Google Scholar] [CrossRef]
- Ding, Q.S.; Zhang, L.; Wang, B.C.; Zeng, Z.; Zou, X.Q.; Cao, P.B.; Zhou, G.M.; Tang, M.; Wu, L.; Wu, L.L.; et al. Aberrant high expression level of MORC2 is a common character in multiple cancers. Hum. Pathol. 2018, 76, 58–67. [Google Scholar] [CrossRef]
- Douse, C.H.; Bloor, S.; Liu, Y.; Shamin, M.; Tchasovnikarova, I.A.; Timms, R.T.; Lehner, P.J.; Modis, Y. Neuropathic MORC2 mutations perturb GHKL ATPase dimerization dynamics and epigenetic silencing by multiple structural mechanisms. Nat. Commun. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.Q. MORC2 regulates DNA damage response through a PARP1-dependent pathway. Nucleic Acids Res. 2019, 47, 8502–8520. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xie, H.Y.; Yang, L.F.; Zhang, L.; Zhang, F.L.; Liu, H.Y.; Li, D.Q.; Shao, Z.M. Stabilization of MORC2 by estrogen and antiestrogens through GPER1- PRKACA-CMA pathway contributes to estrogen-induced proliferation and endocrine resistance of breast cancer cells. Autophagy 2020, 16, 1061–1076. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, H.Y.; Yu, T.J.; Lu, Q.; Zhang, F.L.; Liu, G.Y.; Shao, Z.M.; Li, D.Q. O-GlcNAcylation of MORC2 at threonine 556 by OGT couples TGF-beta signaling to breast cancer progression. Cell Death Differ. 2022, 29, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Wang, C.Y.; Cai, X.Z.; Chen, W.; Wang, X.H.; Li, F. Identification and expression analysis of a novel CW-type zinc finger protein MORC2 in cancer cells. Anat. Rec. 2010, 293, 1002–1009. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Ruan, B.; Chen, W.; Zheng, J.; Xu, B.; Jiang, P.; Miao, Z.; Li, F.; Guo, J.Y.; et al. MORC2 regulates C/EBPalpha-mediated cell differentiation via sumoylation. Cell Death Differ. 2019, 26, 1905–1917. [Google Scholar] [CrossRef]
- Shi, B.; Xue, J.; Zhou, J.; Kasowitz, S.D.; Zhang, Y.; Liang, G.; Guan, Y.; Shi, Q.; Liu, M.; Sha, J.; et al. MORC2B is essential for meiotic progression and fertility. PLoS Genet. 2018, 14, e1007175. [Google Scholar] [CrossRef]
- Zhang, L.; Du, X.; Wei, S.; Li, D.; Li, Q. A comprehensive transcriptomic view on the role of SMAD4 gene by RNAi-mediated knockdown in porcine follicular granulosa cells. Reproduction 2016, 152, 81–89. [Google Scholar] [CrossRef]
- Liu, L.; Luo, G.; Situ, X.; Li, M. Cloning and Identification of Promoter Region of Porcine MORC2 Gene. Chin. J. Anim. Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Kojima-Kita, K.; Kuramochi-Miyagawa, S.; Nakayama, M.; Miyata, H.; Jacobsen, S.E.; Ikawa, M.; Koseki, H.; Nakano, T. MORC3, a novel MIWI2 association partner, as an epigenetic regulator of piRNA dependent transposon silencing in male germ cells. Sci. Rep. 2021, 11, 20472. [Google Scholar] [CrossRef]
- Inoue, N.; Hess, K.D.; Moreadith, R.W.; Richardson, L.L.; Handel, M.A.; Watson, M.L.; Zinn, A.R. New gene family defined by MORC, a nuclear protein required for mouse spermatogenesis. Hum. Mol. Genet. 1999, 8, 1201–1207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pastor, W.A.; Stroud, H.; Nee, K.; Liu, W.; Pezic, D.; Manakov, S.; Lee, S.A.; Moissiard, G.; Zamudio, N.; Bourc’His, D.; et al. MORC1 represses transposable elements in the mouse male germline. Nat. Commun. 2014, 5, 5795. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Parrella, A.; Rosenwaks, Z.; Palermo, G.D. Genetic and epigenetic profiling of the infertile male. PLoS ONE 2019, 14, e214275. [Google Scholar] [CrossRef]
- Desai, V.P.; Chouaref, J.; Wu, H.; Pastor, W.A.; Kan, R.L.; Oey, H.M.; Li, Z.; Ho, J.; Vonk, K.; San, L.G.D.; et al. The role of MORC3 in silencing transposable elements in mouse embryonic stem cells. Epigenet. Chromatin 2021, 14, 49. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, Y.Y.; Yang, F.; Zhang, L.; Zhang, F.L.; Hu, X.; Shao, Z.M.; Li, D.Q. Acetylation of MORC2 by NAT10 regulates cell-cycle checkpoint control and resistance to DNA-damaging chemotherapy and radiotherapy in breast cancer. Nucleic Acids Res. 2020, 48, 3638–3656. [Google Scholar] [CrossRef]
- Li, Q.; Du, X.; Wang, L.; Shi, K.; Li, Q. TGF-beta1 controls porcine granulosa cell states: A miRNA-mRNA network view. Theriogenology 2021, 160, 50–60. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Li, Q.; Wu, W.; Shang, P.; Chamba, Y.; Pan, Z.; Li, Q. miR-130a/TGF-beta1 axis is involved in sow fertility by controlling granulosa cell apoptosis. Theriogenology 2020, 157, 407–417. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y. Activation of TGF-beta1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells. J. Cell. Physiol. 2019, 234, 11976–11985. [Google Scholar] [CrossRef]
- Zheng, X.; Boerboom, D.; Carriere, P.D. Transforming growth factor-beta1 inhibits luteinization and promotes apoptosis in bovine granulosa cells. Reproduction 2009, 137, 969–977. [Google Scholar] [CrossRef]
- Du, X.; Li, Q.; Yang, L.; Liu, L.; Cao, Q.; Li, Q. SMAD4 activates Wnt signaling pathway to inhibit granulosa cell apoptosis. Cell Death Dis. 2020, 11, 373. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, L.; Li, Q.; Du, X. SMAD4 Feedback Activates the Canonical TGF-beta Family Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 24. [Google Scholar] [CrossRef]
- Sivera, R.; Lupo, V.; Frasquet, M.; Argente-Escrig, H.; Alonso-Perez, J.; Diaz-Manera, J.; Querol, L.; Del, M.G.M.; Ignacio, P.S.; Garcia-Sobrino, T.; et al. Charcot-Marie-Tooth disease due to MORC2 mutations in Spain. Eur. J. Neurol. 2021, 28, 3001–3011. [Google Scholar] [CrossRef]
- Tong, Y.; Li, Y.; Gu, H.; Wang, C.; Liu, F.; Shao, Y.; Li, F. HSF1, in association with MORC2, downregulates ArgBP2 via the PRC2 family in gastric cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1104–1114. [Google Scholar] [CrossRef]
- Guddeti, R.K.; Thomas, L.; Kannan, A.; Karyala, P.; Pakala, S.B. The chromatin modifier MORC2 affects glucose metabolism by regulating the expression of lactate dehydrogenase A through a feed forward loop with c-Myc. FEBS Lett. 2021, 595, 1289–1302. [Google Scholar] [CrossRef]
| siRNA | Sequence(5′ to 3′) |
|---|---|
| NC-siRNA | F: UUCUCCGAACGUGUCACGUTT R: UUCUCCGAACGUGUCACGUTT |
| SMAD4-siRNA | F: CACCAGGAAUUGAUCUCUCAGGAUU R: CACCAGGAAUUGAUCUCUCAGGAUU |
| MORC2-294 | F: GCAAUACGGGAAUGGGUUATT R: UAACCCAUUCCCGUAUUGCTT |
| MORC2-584 | F: GCGGAACGCUGGUCAUCAUTT R: AUGAUGACCAGCGUUCCGCTT |
| MORC2-1164 | F: CCGCCUGAUCAAGAUGUAUTT R: AUACAUCUUGAUCAGGCGGTT |
| Name | Primer Sequence | Anneal T (°C) | Product Size (bp) | Usage |
|---|---|---|---|---|
| MORC2 | F: TCACCAAGAAGGAAGACACTA R: AGATGATGACCAGCGTTCC | 58.9 | 252 | qRT-PCR |
| Bax | F: GCCGAAATGTTTGCTGAC R: GCCGATCTCGAAGGAAGT | 58 | 154 | qRT-PCR |
| Bcl2 | F: TCAGGGATGGGGTGAACT R: TCAGAGACAGCCAGGAGAAAT | 60 | 240 | qRT-PCR |
| GAPDH | F: GGACTCATGACCACGGTCCAT R: TCAGATCCACAACCGACACGT | 60 | 220 | qRT-PCR |
| SMAD4 | F: ATTGGTGTTCCATTGCCTAC R: TGGTCACTAAGGCACCTGAC | 58 | 250 | qRT-PCR |
| ATR | F: TATCTGGGCTCCCTCCTC R: CTCCTTTCTGTATTCCTGTAGAACT | 58.6 | 204 | qRT-PCR |
| ATM | F: CTGTGAGATTGGCGTTAGT R: AACATGCAAACTTGGTGAT | 59 | 154 | qRT-PCR |
| P53 | F: TGACTGTACCACCATCCACTAC R: AGGCACAAACACGCACCT | 60 | 149 | qRT-PCR |
| Rad51 | F: GGTGGAGGTGAAGGAAAG R: CTGGGTCTGGTGGTCTGT | 60 | 156 | qRT-PCR |
| PARP-1 | F: AGCACCTGTGACGGGCTAC R: CTTGCTGATGTGCGAAGC | 61 | 171 | qRT-PCR |
| Caspase-3 | F: TTGGACTGTGGGATTGAGACG R: CGCTGCACAAAGTGACTGGA | 59 | 165 | qRT-PCR |
| pcDNA3.1-his-v5-MORC2 | F: CCCAAGCTTGCCACCATGGCTTTCACAAATTACAGCAGTCTGAATC R: CCGCTCGAGGTCCCCCTTGGTGATCAGGTCC | 68 | 3102 | Over-expression |
| pcDNA3.1-SMAD4 | F: CCCAAGCTTGCCACCATGGACAATATGTCTATTACAAATACACCAACAAG R: CCGCTCGAGTCAGTCTAAAGGCTGTGGGTCGGC | 68 | 1659 | Over-expression |
| pGL3-MORC2-1347 | F: CGGGTACCGGTCAAGAAAGCAGGAAGAGAG | 68 | 1347 | promoter |
| pGL3-MORC2-1479 | F: CGGGTACCGCTTCTTCGAGTTAGGGGCCTGACT | 68 | 1479 | promoter |
| pGL3-MORC2-R | R: CCGCTCGAGTGCAGTGAGGTCTCCAGTTCTTT | - | promoter | |
| pGL3-SBE1-mut | F: TGACTCCCCGGCGGATGGCTCCTCCGGTCCGC R: CATCTCGCCGGGGATCAGGCCCCTAACTCGAA | SEB mutation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qi, N.; Xing, W.; Li, M.; Qian, Y.; Luo, G.; Yu, S. The TGF-β/SMAD Signaling Pathway Prevents Follicular Atresia by Upregulating MORC2. Int. J. Mol. Sci. 2022, 23, 10657. https://doi.org/10.3390/ijms231810657
Liu J, Qi N, Xing W, Li M, Qian Y, Luo G, Yu S. The TGF-β/SMAD Signaling Pathway Prevents Follicular Atresia by Upregulating MORC2. International Journal of Molecular Sciences. 2022; 23(18):10657. https://doi.org/10.3390/ijms231810657
Chicago/Turabian StyleLiu, Jiying, Nannan Qi, Wenwen Xing, Mengxuan Li, Yonghang Qian, Gang Luo, and Shali Yu. 2022. "The TGF-β/SMAD Signaling Pathway Prevents Follicular Atresia by Upregulating MORC2" International Journal of Molecular Sciences 23, no. 18: 10657. https://doi.org/10.3390/ijms231810657
APA StyleLiu, J., Qi, N., Xing, W., Li, M., Qian, Y., Luo, G., & Yu, S. (2022). The TGF-β/SMAD Signaling Pathway Prevents Follicular Atresia by Upregulating MORC2. International Journal of Molecular Sciences, 23(18), 10657. https://doi.org/10.3390/ijms231810657






