Role of the Guanidinium Groups in Ligand–Receptor Binding of Arginine-Containing Short Peptides to the Slow Sodium Channel: Quantitative Approach to Drug Design of Peptide Analgesics
Abstract
:1. Introduction
2. Results
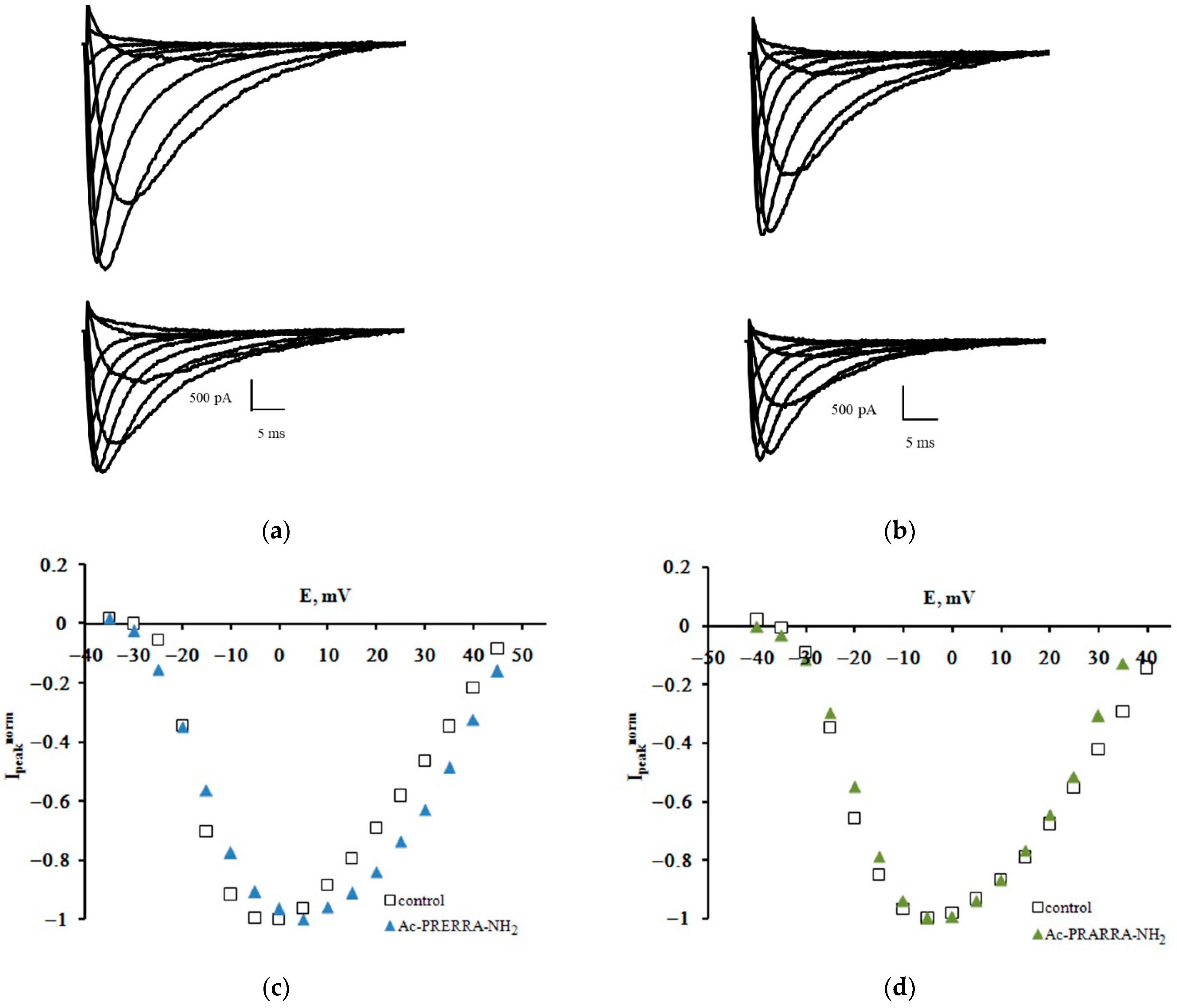
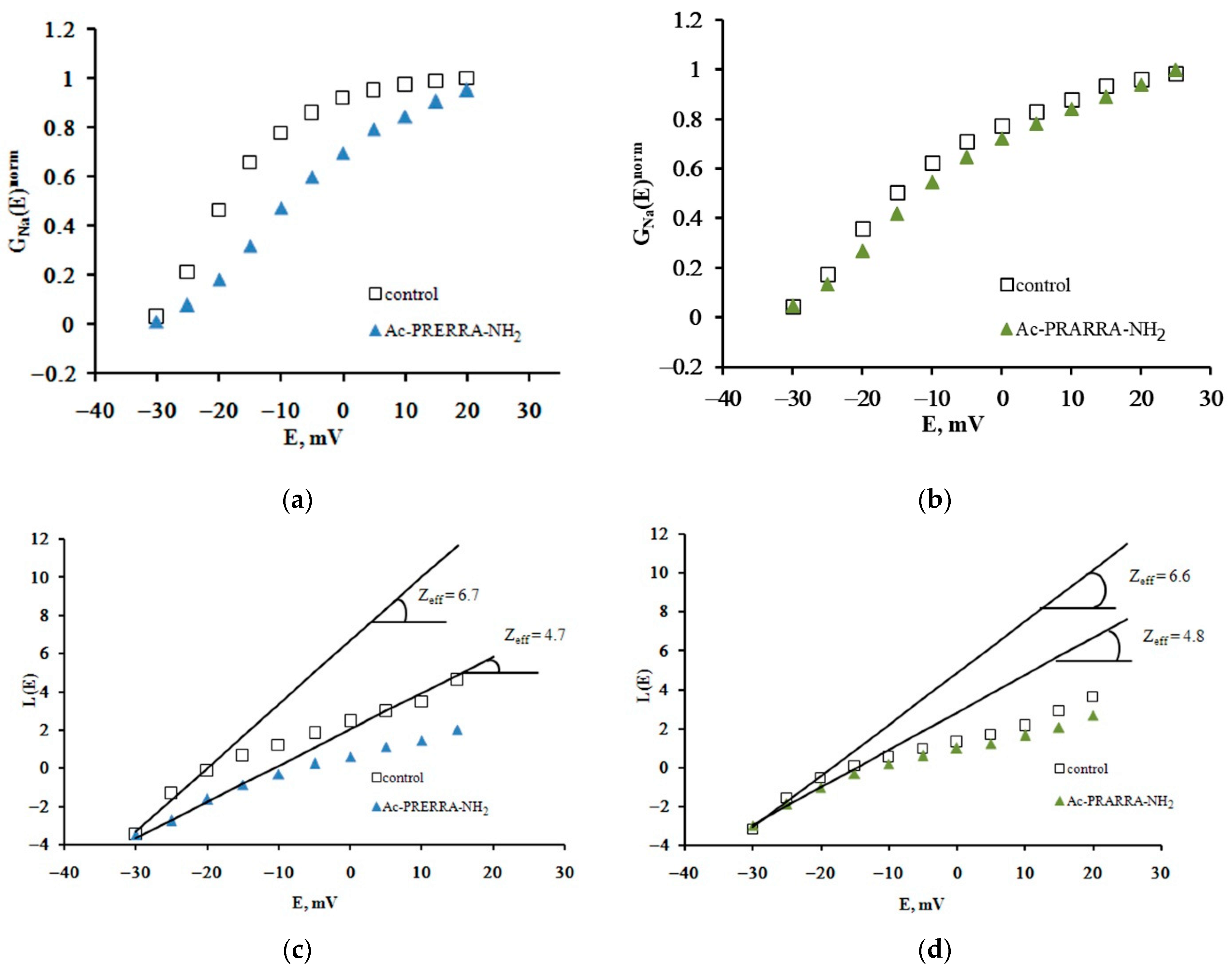
2.1. The Patch-Clamp Method
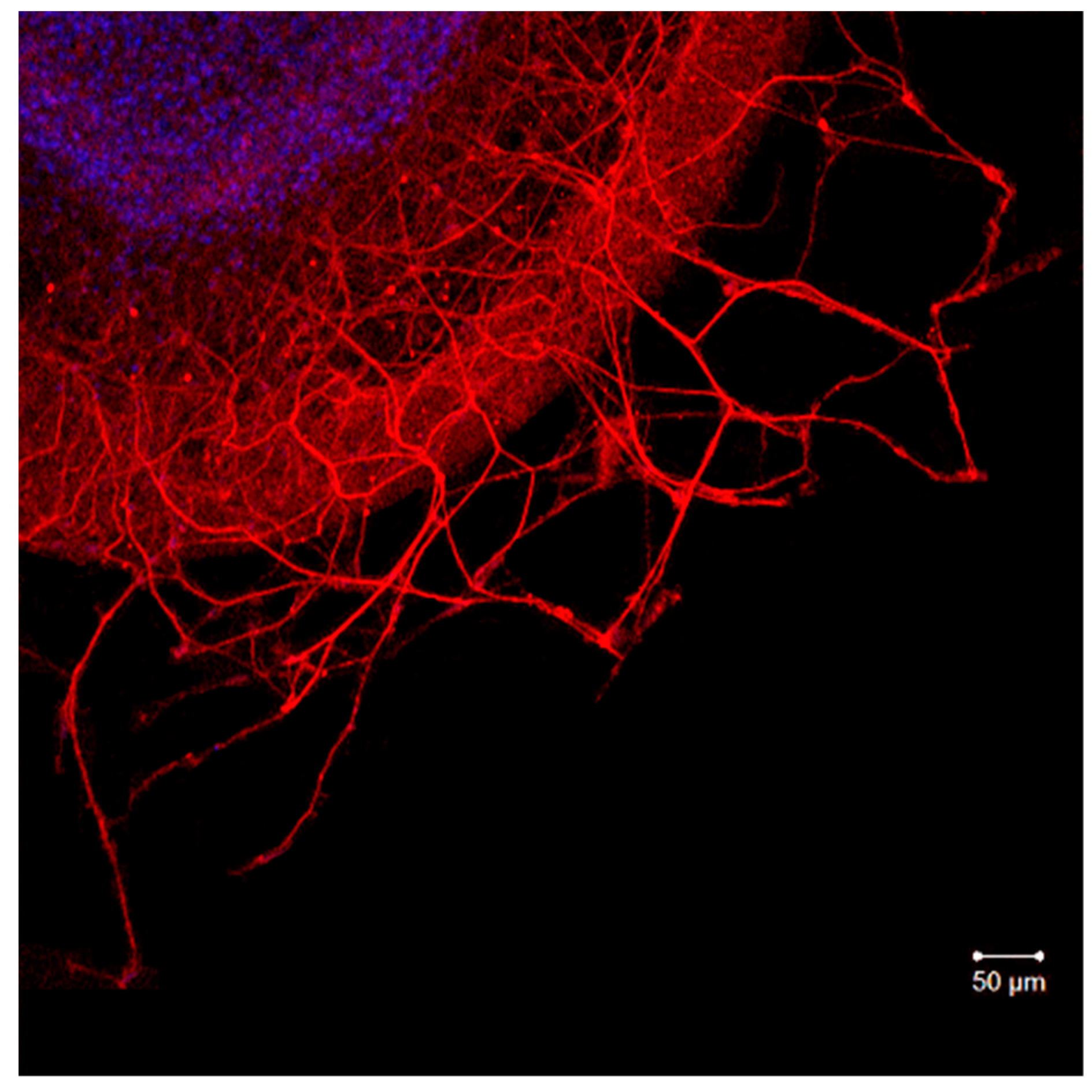
2.2. Organotypic Tissue Culture
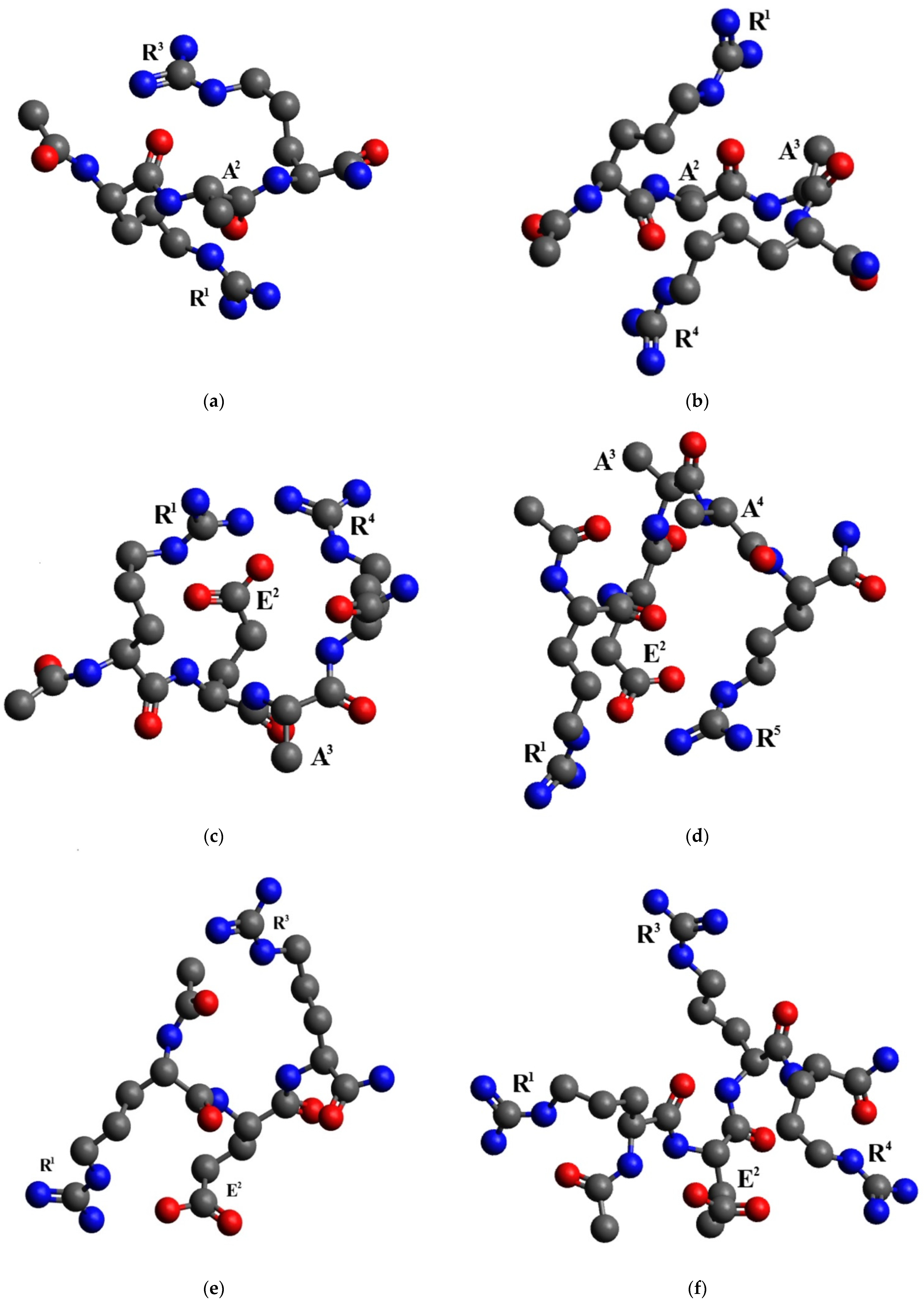
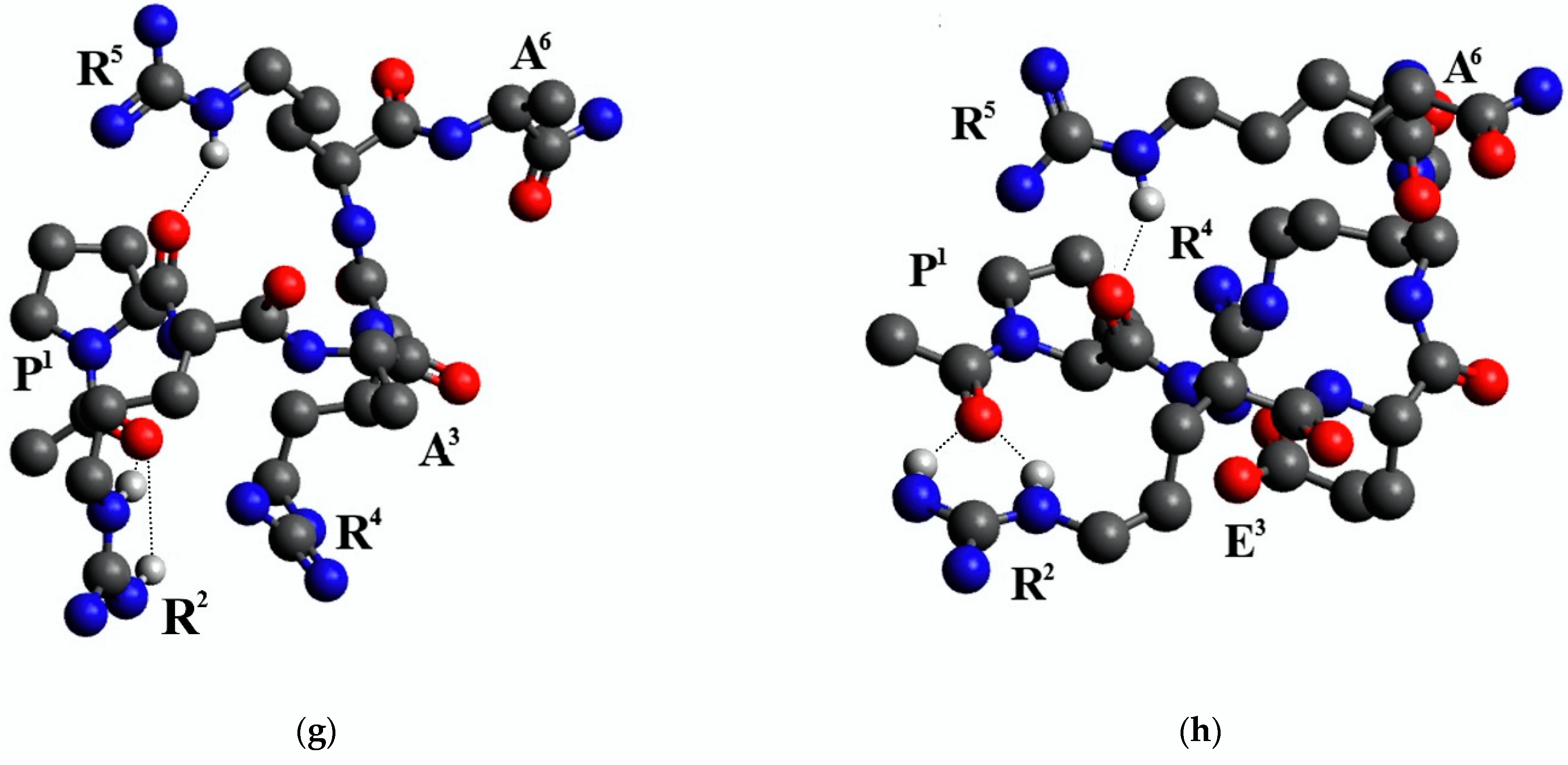
2.3. Conformational Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Patch-Clamp Method
4.2.1. Quantitative Evaluation of Zeff
4.2.2. Dissociated Cell Culture
4.2.3. Experimental Solutions
4.2.4. Hardware and Software
4.3. Organotypic Nerve Tissue Culture Method
4.4. Calculational Methods
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelykh, T.N.; Rogachevsky, I.V.; Nozdrachev, A.D.; Veselkina, O.S.; Podzorova, S.A.; Krylov, B.V.; Plakhova, V.B. Molecular Mechanism of Modulation of Nociceptive Neuron Membrane Excitability by a Tripeptide. Dokl. Biochem. Biophys. 2016, 466, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Rogachevsky, I.V.; Kalinina, A.D.; Penniyaynen, V.A.; Terekhin, S.G.; Podzorova, S.A.; Krylov, B.V.; Plakhova, V.B. A Possible Mechanism of Modulation of Slow Sodium Channels in the Sensory Neuron Membrane by Short Peptides. Biophysics 2021, 66, 579–588. [Google Scholar] [CrossRef]
- Rogachevskii, I.V.; Plakhova, V.B.; Penniyaynen, V.A.; Kalinina, A.D.; Podzorova, S.A.; Samosvat, D.M.; Zegrya, G.G.; Krylov, B.V. Arginine-Containing Tripeptides as Analgesic Substances: The Possible Mechanism of Ligand-Receptor Binding to the Slow Sodium Channel. Int. J. Mol. Sci. 2022, 23, 5993. [Google Scholar] [CrossRef] [PubMed]
- Krylov, B.V.; Rogachevskii, I.V.; Shelykh, T.N.; Plakhova, V.B. New Non-Opioid Analgesics: Understanding Molecular Mechanisms on the Basis of Patch-Clamp and Chemical Studies; Frontiers in Pain Science; Bentham Science Publishers Ltd.: Sharjah, United Arab Emirates, 2017; Volume 1, ISBN 9781608059300. [Google Scholar]
- Lopatina, E.V.; Polyakov, Y.I. Synthetic Analgesic Anoceptin: Results of Preclinical and Clinical Trials. Efferent Ther. 2011, 17, 79–81. [Google Scholar]
- Fitch, C.A.; Platzer, G.; Okon, M.; Garcia-Moreno, E.B.; McIntosh, L.P. Arginine: Its PKa Value Revisited. Protein Sci. 2015, 24, 752–761. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for High-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflügers Archiv. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Currents Carried by Sodium and Potassium Ions through the Membrane of the Giant Axon of Loligo. J. Physiol. 1952, 116, 449–472. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates Inc: Sunderland, MA, USA, 2001; ISBN 0878933220. [Google Scholar]
- Almers, W. Gating Currents and Charge Movements in Excitable Membranes. Rev. Physiol. Biochem. Pharmacol. 1978, 82, 96–190. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Zhang, Z.; Alexov, E. On the Dielectric “Constant” of Proteins: Smooth Dielectric Function for Macromolecular Modeling and Its Implementation in DelPhi. J. Chem. Theory Comput. 2013, 9, 2126–2136. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Seok, C. What Stabilizes Close Arginine Pairing in Proteins? Phys. Chem. Chem. Phys. 2013, 15, 5844. [Google Scholar] [CrossRef] [PubMed]
- Boudon, S.; Wipff, G.; Maigret, B. Monte Carlo Simulations on the Like-Charged Guanidinium-Guanidinium Ion Pair in Water. J. Phys. Chem. 1990, 94, 6056–6061. [Google Scholar] [CrossRef]
- Volkow, N.D.; Blanco, C. The Changing Opioid Crisis: Development, Challenges and Opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef]
- Vallbo, A.B.; Hagbarth, K.-E. Micro-Electrode Recordings from Human Peripheral Nerves. In Pathological Conduction in Nerve Fibers, Electromyography of Sphincter Muscles, Automatic Analysis of Electromyogram with Computers; S. Karger AG: Basel, Switzerland; München, Germany; Paris, France; London, UK; New York, NY, USA; Sydney, Australia, 1973; Volume 2, pp. 67–84. [Google Scholar]
- Chiu, S.Y. Inactivation of Sodium Channels: Second Order Kinetics in Myelinated Nerve. J. Physiol. 1977, 273, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Akoev, G.N.; Alekseev, N.P.; Krylov, B.V. Mechanoreceptors: Their Functional Organization; Springer: London, UK; Berlin/Heidelberg, Germany; New York, NY, USA, 1998. [Google Scholar]
- Penniyaynen, V.A.; Plakhova, V.B.; Rogachevskii, I.V.; Terekhin, S.G.; Podzorova, S.A.; Krylov, B.V. Molecular Mechanisms and Signaling by Comenic Acid in Nociceptive Neurons Influence the Pathophysiology of Neuropathic Pain. Pathophysiology 2019, 26, 245–252. [Google Scholar] [CrossRef]
- Rogachevskii, I.V.; Plakhova, V.B.; Penniyaynen, V.A.; Terekhin, S.G.; Podzorova, S.A.; Krylov, B.V. New Approaches to the Design of Analgesic Medicinal Substances. Can. J. Physiol. Pharmacol. 2022, 100, 43–52. [Google Scholar] [CrossRef]
- Osipchuk, Y.V.; Timin, E.N. Electrical Measurements on Professed Cells. In Intracellular Perfusion of Excitable Cells; Kostyuk, P.G., Krishtal, O.A., Eds.; JohnWiley and Sons: London, UK, 1984; pp. 103–129. [Google Scholar]
- Sakmann, B.; Neher, E. (Eds.) Single-Channel Recording; Springer: Boston, MA, USA, 1995; ISBN 978-1-4419-1230-5. [Google Scholar]
- Kostyuk, P.G.; Veselovsky, N.S.; Tsyndrenko, A.Y. Ionic Currents in the Somatic Membrane of Rat Dorsal Root Ganglion Neurons-I. Sodium Currents. Neuroscience 1981, 6, 2423–2430. [Google Scholar] [CrossRef]
- Dib-Hajj, S.; Black, J.A.; Cummins, T.R.; Waxman, S.G. NaN/Nav1.9: A sodium channel with unique properties. Trends Neurosci. 2002, 25, 253–259. [Google Scholar] [CrossRef]
- Rackers, J.A.; Wang, Z.; Lu, C.; Laury, M.L.; Lagardère, L.; Schnieders, M.J.; Piquemal, J.-P.; Ren, P.; Ponder, J.W. Tinker 8: Software Tools for Molecular Design. J. Chem. Theory Comput. 2018, 14, 5273–5289. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck Molecular Force Field. I. Basis, Form, Scope, Parameterization, and Performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Kolossváry, I.; Guida, W. Low Mode Conformational Search Elucidated: Application to C39H80 and Flexible Docking of 9-Deazaguanine Inhibitors into PNP. J. Comput. Chem. 1999, 20, 1671–1684. [Google Scholar] [CrossRef]
- Mongan, J.; Simmerling, C.; McCammon, J.A.; Case, D.A.; Onufriev, A. Generalized Born Model with a Simple, Robust Molecular Volume Correction. J. Chem. Theory Comput. 2007, 3, 156–169. [Google Scholar] [CrossRef] [PubMed]


| Cutoff, kcal/mol | Ac-RAR-NH2 | Ac-RAAR-NH2 | Ac-REAR-NH2 ch * | Ac-REAR-NH2 unch * | ||||
|---|---|---|---|---|---|---|---|---|
| ε = 10 | ε = 10 | ε = 10 | ε = 10 | |||||
| Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | |
| None | 102,328 | R1–R3 10.4 ± 3.6 | 102,412 | R1–R4 10.7 ± 4.5 | 103,238 | R1–R4 10.8 ± 4.2 | 103,022 | R1–R4 11.3 ± 4.4 |
| 7 | 8407 | R1–R3 8.1 ± 2.8 | 5903 | R1–R4 7.8 ± 2.3 | 2706 | R1–R4 8.1 ± 2.7 | 10,442 | R1–R4 8.9 ± 3.0 |
| 6 | 4327 | R1–R3 7.8 ± 2.5 | 2915 | R1–R4 7.7 ± 2.2 | 1073 | R1–R4 8.0 ± 2.6 | 6222 | R1–R4 8.5 ± 2.7 |
| 5 | 1882 | R1–R3 7.5 ± 2.3 | 1208 | R1–R4 7.8 ± 2.0 | 410 | R1–R4 8.1 ± 2.6 | 3280 | R1–R4 8.0 ± 2.4 |
| 4.5 | 1145 | R1–R3 7.3 ± 2.3 | 729 | R1–R4 7.8 ± 1.8 | 232 | R1–R4 8.1 ± 2.6 | 2233 | R1–R4 7.9 ± 2.3 |
| 4 | 698 | R1–R3 7.2 ± 2.2 | 449 | R1–R4 7.7 ± 1.8 | 149 | R1–R4 7.9 ± 2.4 | 1440 | R1–R4 7.8 ± 2.2 |
| 3 | 201 | R1–R3 6.6 ± 2.1 | 150 | R1–R4 7.8 ± 1.6 | 48 | R1–R4 7.6 ± 2.4 | 460 | R1–R4 7.8 ± 2.1 |
| 2 | 26 | R1–R3 6.5 ± 1.5 | 33 | R1–R4 7.7 ± 1.8 | 11 | R1–R4 7.1 ± 1.7 | 106 | R1–R4 7.9 ± 2.1 |
| Cutoff, kcal/mol | Ac-RER-NH2 ch * | Ac-RER-NH2 ch * | Ac-RER-NH2 unch * | Ac-RER-NH2 unch * | ||||
| ε = 10 | ε = 80 | ε = 10 | ε = 80 | |||||
| Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | |
| None | 102,410 | R1–R3 10.8 ± 3.6 | 102,660 | R1–R3 10.9 ± 3.7 | 102,042 | R1–R3 11.0 ± 3.6 | 102,538 | R1–R3 11.0 ± 3.6 |
| 7 | 221 | R1–R3 7.7 ± 3.0 | 1534 | R1–R3 8.0 ± 2.9 | 6870 | R1–R3 9.1 ± 3.0 | 7601 | R1–R3 9.2 ± 3.0 |
| 6 | 81 | R1–R3 7.0 ± 2.6 | 645 | R1–R3 7.8 ± 2.9 | 3262 | R1–R3 8.8 ± 2.8 | 3739 | R1–R3 8.8 ± 2.8 |
| 5 | 25 | R1–R3 6.9 ± 2.4 | 241 | R1–R3 7.3 ± 2.7 | 1298 | R1–R3 8.3 ± 2.6 | 1565 | R1–R3 8.5 ± 2.6 |
| 4.5 | 16 | R1–R3 6.7 ± 2.2 | 158 | R1–R3 7.3 ± 2.7 | 796 | R1–R3 8.2 ± 2.6 | 955 | R1–R3 8.4 ± 2.6 |
| 4 | 9 | R1–R3 7.1 ± 2.5 | 95 | R1–R3 7.1 ± 2.6 | 474 | R1–R3 8.2 ± 2.7 | 576 | R1–R3 8.3 ± 2.6 |
| 3 | 2 | R1–R3 5.2 ± 0.1 | 41 | R1–R3 6.6 ± 2.5 | 116 | R1–R3 8.3 ± 2.7 | 129 | R1–R3 8.0 ± 2.8 |
| 2 | 1 | R1–R3 5.3 ± 0.0 | 19 | R1–R3 6.5 ± 2.5 | 17 | R1–R3 9.0 ± 2.7 | 24 | R1–R3 7.3 ± 2.6 |
| Cutoff, kcal/mol | Ac-REAAR-NH2 ch * | Ac-REAAR-NH2 ch * | Ac-REAAR-NH2 unch * | |||||
| ε = 10 | ε = 80 | ε = 10 | ||||||
| Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | |||
| None | 101,946 | R1–R5 10.7 ± 4.4 | 101,848 | R1–R5 10.9 ± 4.5 | 101,968 | R1–R5 11.1 ± 4.7 | ||
| 7 | 1180 | R1–R5 7.7 ± 2.4 | 2526 | R1–R5 8.2 ± 2.6 | 5106 | R1–R5 8.2 ± 2.6 | ||
| 6 | 490 | R1–R5 7.3 ± 2.2 | 1119 | R1–R5 8.0 ± 2.4 | 2310 | R1–R5 8.0 ± 2.4 | ||
| 5 | 185 | R1–R5 6.9 ± 2.0 | 484 | R1–R5 7.8 ± 2.3 | 877 | R1–R5 7.9 ± 2.3 | ||
| 4.5 | 126 | R1–R5 6.7 ± 2.0 | 289 | R1–R5 7.7 ± 2.2 | 510 | R1–R5 7.8 ± 2.2 | ||
| 4 | 70 | R1–R5 6.5 ± 2.1 | 186 | R1–R5 7.6 ± 2.2 | 287 | R1–R5 7.7 ± 2.1 | ||
| 3 | 23 | R1–R5 6.2 ± 1.4 | 68 | R1–R5 7.0 ± 2.4 | 81 | R1–R5 8.0 ± 1.9 | ||
| 2 | 4 | R1–R5 5.8 ± 0.7 | 25 | R1–R5 6.8 ± 2.4 | 18 | R1–R5 7.8 ± 1.2 | ||
| Cutoff, kcal/mol | Ac-RERR-NH2 ch * | Ac-PRERRA-NH2 ch * | Ac-PRERRA-NH2 unch * | Ac-PRARRA-NH2 | ||||
|---|---|---|---|---|---|---|---|---|
| ε = 10 | ε = 10 | ε = 10 | ε = 10 | |||||
| Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | Nconf | Distances, Å | |
| None | 101,546 | R1–R3 9.8 ± 3.4 R1–R4 9.2 ± 3.7 R3–R4 9.4 ± 2.7 | 102,238 | R2–R4 12.9 ± 3.4 R2–R5 10.7 ± 3.7 R4–R5 10.3 ± 2.7 | 101,716 | R2–R4 12.6 ± 3.6 R2–R5 10.6 ± 3.7 R4–R5 10.1 ± 2.8 | 101,517 | R2–R4 10.0 ± 3.5 R2–R5 10.0 ± 4.1 R4–R5 10.7 ± 2.3 |
| 7 | 2354 | R1–R3 9.7 ± 3.1 R1–R4 8.0 ± 2.8 R3–R4 9.1 ± 2.4 | 3389 | R2–R4 12.4 ± 2.8 R2–R5 9.3 ± 3.2 R4–R5 10.1 ± 2.4 | 5661 | R2–R4 12.3 ± 2.9 R2–R5 8.8 ± 3.3 R4–R5 10.0 ± 2.4 | 4756 | R2–R4 10.2 ± 3.1 R2–R5 9.0 ± 4.1 R4–R5 10.7 ± 2.2 |
| 6 | 1023 | R1–R3 9.7 ± 3.1 R1–R4 8.2 ± 2.7 R3–R4 9.2 ± 2.3 | 1600 | R2–R4 12.1 ± 2.8 R2–R5 9.3 ± 3.3 R4–R5 10.1 ± 2.4 | 2885 | R2–R4 12.2 ± 2.8 R2–R5 8.2 ± 3.3 R4–R5 9.9 ± 2.2 | 2353 | R2–R4 10.0 ± 3.0 R2–R5 8.9 ± 4.2 R4–R5 10.6 ± 2.1 |
| 5 | 408 | R1–R3 9.3 ± 3.2 R1–R4 8.2 ± 2.4 R3–R4 9.3 ± 2.3 | 663 | R2–R4 11.8 ± 2.7 R2–R5 9.2 ± 3.3 R4–R5 10.1 ± 2.3 | 1242 | R2–R4 12.1 ± 2.4 R2–R5 8.1 ± 3.4 R4–R5 9.9 ± 2.2 | 1030 | R2–R4 10.1 ± 2.9 R2–R5 8.7 ± 4.1 R4–R5 10.6 ± 2.0 |
| 4.5 | 258 | R1–R3 9.1 ± 3.3 R1–R4 8.1 ± 2.4 R3–R4 9.4 ± 2.2 | 411 | R2–R4 11.6 ± 2.7 R2–R5 9.3 ± 3.3 R4–R5 10.1 ± 2.2 | 728 | R2–R4 12.1 ± 2.3 R2–R5 8.1 ± 3.4 R4–R5 9.9 ± 2.2 | 629 | R2–R4 10.0 ± 2.8 R2–R5 8.6 ± 4.0 R4–R5 10.5 ± 2.0 |
| 4 | 157 | R1–R3 8.6 ± 3.2 R1–R4 8.2 ± 2.4 R3–R4 9.5 ± 2.2 | 235 | R2–R4 11.4 ± 2.8 R2–R5 9.5 ± 3.4 R4–R5 10.1 ± 2.2 | 417 | R2–R4 12.1 ± 2.2 R2–R5 8.0 ± 3.1 R4–R5 10.0 ± 2.1 | 364 | R2–R4 10.0 ± 2.8 R2–R5 8.3 ± 3.9 R4–R5 10.4 ± 1.9 |
| 3 | 55 | R1–R3 7.6 ± 2.9 R1–R4 8.7 ± 2.1 R3–R4 9.9 ± 2.0 | 82 | R2–R4 10.7 ± 2.9 R2–R5 10.1 ± 3.3 R4–R5 10.1 ± 2.1 | 91 | R2–R4 11.7 ± 2.3 R2–R5 7.2 ± 2.6 R4–R5 9.7 ± 2.0 | 105 | R2–R4 9.7 ± 2.8 R2–R5 8.2 ± 3.4 R4–R5 10.3 ± 1.9 |
| 2 | 21 | R1–R3 6.3 ± 2.3 R1–R4 8.3 ± 1.6 R3–R4 9.8 ± 1.5 | 25 | R2–R4 9.8 ± 3.0 R2–R5 10.3 ± 3.1 R4–R5 9.8 ± 1.8 | 24 | R2–R4 12.3 ± 2.3 R2–R5 7.9 ± 3.1 R4–R5 9.2 ± 2.1 | 31 | R2–R4 8.5 ± 2.9 R2–R5 9.2 ± 3.7 R4–R5 10.3 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plakhova, V.B.; Samosvat, D.M.; Zegrya, G.G.; Penniyaynen, V.A.; Kalinina, A.D.; Ke, M.; Podzorova, S.A.; Krylov, B.V.; Rogachevskii, I.V. Role of the Guanidinium Groups in Ligand–Receptor Binding of Arginine-Containing Short Peptides to the Slow Sodium Channel: Quantitative Approach to Drug Design of Peptide Analgesics. Int. J. Mol. Sci. 2022, 23, 10640. https://doi.org/10.3390/ijms231810640
Plakhova VB, Samosvat DM, Zegrya GG, Penniyaynen VA, Kalinina AD, Ke M, Podzorova SA, Krylov BV, Rogachevskii IV. Role of the Guanidinium Groups in Ligand–Receptor Binding of Arginine-Containing Short Peptides to the Slow Sodium Channel: Quantitative Approach to Drug Design of Peptide Analgesics. International Journal of Molecular Sciences. 2022; 23(18):10640. https://doi.org/10.3390/ijms231810640
Chicago/Turabian StylePlakhova, Vera B., Dmitriy M. Samosvat, Georgy G. Zegrya, Valentina A. Penniyaynen, Arina D. Kalinina, Ma Ke, Svetlana A. Podzorova, Boris V. Krylov, and Ilya V. Rogachevskii. 2022. "Role of the Guanidinium Groups in Ligand–Receptor Binding of Arginine-Containing Short Peptides to the Slow Sodium Channel: Quantitative Approach to Drug Design of Peptide Analgesics" International Journal of Molecular Sciences 23, no. 18: 10640. https://doi.org/10.3390/ijms231810640
APA StylePlakhova, V. B., Samosvat, D. M., Zegrya, G. G., Penniyaynen, V. A., Kalinina, A. D., Ke, M., Podzorova, S. A., Krylov, B. V., & Rogachevskii, I. V. (2022). Role of the Guanidinium Groups in Ligand–Receptor Binding of Arginine-Containing Short Peptides to the Slow Sodium Channel: Quantitative Approach to Drug Design of Peptide Analgesics. International Journal of Molecular Sciences, 23(18), 10640. https://doi.org/10.3390/ijms231810640








