Abstract
Reactive oxygen species (ROS), which are exceptionally high in IBD lesions, are known to cause abnormal immune responses to inflammatory reactions in inflammatory bowel diseases (IBD) through damage to the intestinal mucosal linings. Moreover, they are theorized to be an agent of IBD development. Vitamin C is widely known to be an effective antioxidant for its ability to regulate inflammatory responses through its ROS scavenging effect. Therefore, we examined vitamin C’s influence on the development and progression of IBD in Gulo(−/−) mice, which cannot synthesize vitamin C like humans due to a defect in the expression of L-gulono-γ–lactone oxidase, an essential enzyme for vitamin C production. First, we found extensive oxidative stress and an inflammation increase in the colon of vitamin C-insufficient Gulo(−/−) mice. We also found decreased IL-22 production and NKp46(+) cell recruitment and the impaired activation of the p38MAPK pathway. Additionally, comparing vitamin C-insufficient Gulo(−/−) mice to vitamin C-sufficient Gulo(−/−) mice and wild-type mice, the insufficient group faced a decrease in mucin-1 expression, accompanied by an increase in IL-6 production, followed by the activation of the STAT3 and Akt pathways. The results suggest that vitamin C insufficiency induces severe colitis, meaning vitamin C could also take on a preventative role by regulating the production of cytokines and the induction of inflammation.
1. Introduction
Inflammatory bowel disease (IBD), often responsible for abdominal pain, diarrhea, and weight loss, frequently occurs in young adults. Hallmark diseases involving IBD are ulcerative colitis (UC) and Crohn’s disease (CD). Though recognized as a representative intractable inflammatory disease, effective therapeutic agents for IBD have yet to be developed. Therefore, without a definite cure, IBD patients endure a low quality of life. [1]. IBD is generally known to show a high frequency of occurrence in Western countries, but recently, it has been increasing in Asian countries, including Korea, due to changes in the environment and Westernizing diets [2,3,4,5].
Significant advances in our research on intestinal inflammation and its relationship with IBD have shown that it develops through exaggerated immune responses directed toward commensal microbiota [6,7,8]. However, the etiology of IBD ultimately remains inconclusive. Despite some uncertainties, it appears that the depletion of antioxidants coupled with oxidative stress has a substantial role in the development and progression of IBDs [9,10]. Further, it is known that the increased production of free radicals and defects in the antioxidant system needed to remove them are directly related to intestinal damage accompanied by IBD [11,12,13,14,15]. Reactive oxygen species (ROS), a byproduct of oxidative metabolism, are reactive molecules when accompanied by high electronegativity [9]. Although there are some confounding results, patients with IBD generally have more oxidized molecules in different organ systems, namely, the gastrointestinal and respiratory tracts, than healthy controls [16,17,18,19], but the total antioxidant capacity (TAC) and corrected TAC (cTAC) of plasma or serum in patients with UC and CD are, remarkably, lower. Reduced levels of cTAC indicate impaired exogenous antioxidants in IBDs [20]. Among various studies measuring the expression of antioxidants in an eclectic of organs, including colonic tissues, an imbalance in the antioxidant concentration is constant. This supports the idea that IBD patients’ vital organs withstand states of oxidative stress [9].
Vitamin C (L-ascorbic acid) not only donates electrons to enzymatic and nonenzymatic reactions as a reducing agent but is also a robust water-soluble antioxidant. It also functions as an enzymatic cofactor, such as in the synthesis of collagen. Because vitamin C is not endogenously synthesized in humans, it is considered an essential nutrient that must be supplemented through diet. The presence of vitamin C has the benefits of scavenging free radicals, such as antioxidants and anti-inflammatory effects. Conversely, vitamin C deficiency leads to scurvy in humans [21,22,23]. All animals, except humans and some primates, are known to synthesize large amounts of vitamin C in vivo because they express the gulo gene, which encodes L-gulono-γ–lactone oxidase (GULO), an essential enzyme for vitamin C biosynthesis. Hence, to determine the effect of vitamin C deficiency on the occurrence and progression of IBD, we conducted our study using animal models that cannot biosynthesize vitamin C to represent humans. Using Gulo(−/−) mice, which mirror human functions regarding vitamin C, we observed the development and progression of IBD due to vitamin C deficiency after inducing acute colitis using dextran sulfate sodium (DSS).
2. Results
2.1. Vitamin C Insufficiency Increases the Severity of DSS-Induced Colitis and Mortality
Wild-type (WT), vitamin C-insufficient Gulo(−/−) (KO), and vitamin C-sufficient Gulo(−/−) (KO + VC) mice were induced with colitis by supplementing drinking water with 3% DSS via drinking for seven days. There were no mortalities among the mice without DSS treatment. However, among the WT and KO + VC groups treated with DSS, the mortality rate was 3–6%, while it was 26% in the KO group (Figure 1A). All mice underwent a decrease in body weight (Figure 1B) and length between the colon and anus to the ileocecal valve in response to DSS; the KO group was affected more dramatically (Figure 1C, Supplementary Figure S1). The severity of colitis was evaluated using a blinded histological scoring system. The histological grade indicated that inflammation was more severe in the KO group than in the control (Figure 1D,E); it revealed that, after DSS treatment, the mice’s colons sustained destruction in the intestinal epithelium with a loss of crypts and epithelial integrity. Additionally, submucosal edema and intense infiltration of inflammatory cells occurred in all layers, which did not occur as gravely for the control group. Taken together, it suggests that vitamin C insufficiency can cause severe colitis and may increase mortality.
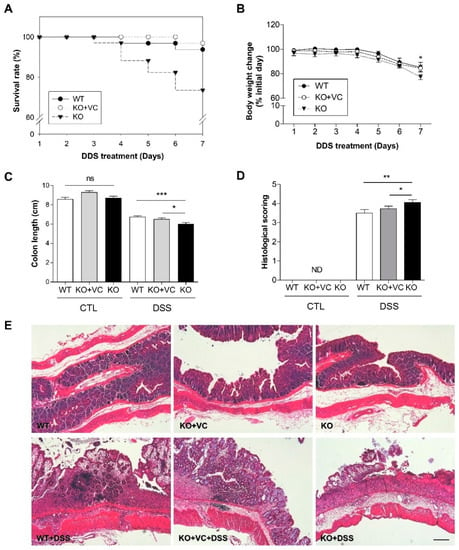
Figure 1.
Severe colitis and mortality associated with vitamin C insufficiency and DSS treatment. (A) The survival rate and (B) body weight of wild-type mice (WT, n = 32), vitamin C-sufficient KO mice (KO + VC, n = 32), and vitamin C-insufficient KO mice (KO, n = 34) were traced for 7 days with 3% DSS treatment. * p < 0.05 (WT vs. KO). (C) After DSS treatment for 7 days, colon length was measured in each group (n = 20–24). ns = not significant, * p < 0.05, *** p < 0.001. (D) Cross-sections of the colon were stained with H&E, and severity was scored according to the criteria described in Materials and Methods (n = 10). ND = not determined, * p < 0.05, ** p < 0.01. (E) Colons were longitudinally sectioned and stained with H&E. Scale bar, 200 μm. Statistical significance was determined using the Tukey multiple comparisons test after one-way ANOVA.
2.2. Vitamin C Insufficiency Increases Inflammation and Oxidative Stress after DSS Treatment
In addition to the histological assessment, we examined the infiltration of immune cells. Gr1-positive cells (mainly neutrophils) infiltrated the colonic epithelium and lamina propria of the DSS-treated groups, especially the DSS-treated KO group (Figure 2A). The activity of myeloperoxidase (MPO), which is abundantly expressed in neutrophils, was significantly increased in the colons of DSS-treated KO mice (Figure 2B). Beyond inflammation, we examined the effect of vitamin C insufficiency on tissues via oxidative stress. Nitrotyrosine (NT), known to increase in various inflammatory diseases such as arteriosclerosis and rheumatoid arthritis, is a marker that indicates the degree of cell damage caused by nitrogen oxide (NO) [24]. By immunoblotting to detect 3-NT in colonic tissues, we found that 3-NT was present in DSS-treated mice and increased in DSS-treated KO mice (Figure 2C). Although the control and DSS-treated groups had almost identical blood levels, the concentration of vitamin C in the blood was 60–80 μM in WT and KO + VC mice and 15–20 μM in KO mice (Figure 2D). DSS treatment decreased the concentration of vitamin C in colonic tissues, and vitamin C levels in colonic tissues were significantly lower in KO mice than in KO + VC mice (Figure 2E). These results suggest that DSS-induced cell infiltration and oxidative stress increased in vitamin C-insufficient KO mice, which might increase the severity of colitis and result in mortality.

Figure 2.
Vitamin C insufficiency and DSS treatment increase inflammation and oxidative stress. WT and KO mice were treated with DSS for 7 days. (A) Colons were stained with anti-Gr1. Scale bar, 100 μm. (B) The level of MPO was measured in homogenized colon lysates and normalized to tissue weight. (n = 15–17). ** p < 0.01. (C) The expression of 3-NT was evaluated with immunoblotting. The results are representative of three independent experiments. The concentration of vitamin C in (D) plasma (n = 8–12) and (E) colon lysates (n = 6–8) was measured using an ascorbate assay kit and normalized to colon weight. * p < 0.05, ** p < 0.01, *** p < 0.001. Statistical analysis was performed using the Tukey multiple comparisons test after one-way ANOVA.
2.3. DSS-Treated KO Mice with Vitamin C Insufficiency Show Decreased IL-22 Production and p38 MAPK Activation
IL-22, belonging to the IL-10 family of cytokines, targets non-hematopoietic cells such as intestinal epithelial cells, keratinocytes, and hepatocytes because the IL-22 receptor (IL-22R) is expressed only in non-hematopoietic cells and not in hematopoietic cells [25,26]. IL-22 has both pro-inflammatory and anti-inflammatory roles in tissues depending on the inflammatory context. Previous work from our group using the mouse Th1/Th2/Th17 FlowCytomix Multiplex kit resulted in decreased plasma levels of IL-10 in DSS-treated KO mice; therefore, we examined the production of IL-22 by employing the enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry. As shown in Figure 3A, the plasma concentration of IL-22 increased upon DSS treatment, although the increase was less pronounced in the KO group. IL-22 production in colonic tissues was lower in KO mice than in WT and KO + VC mice after DSS treatment (Figure 3B,C). It is known that NKp46, an activating receptor of NK cells, expressing natural killer (NK) cells produces IL-22 in mice and may be involved in intestinal homeostasis and immune defense [27]. An immunohistochemical analysis of the population of NKp46(+) cells in the colon showed that DSS treatment induced a considerable accumulation of NKp46(+) cells. However, the colons of KO mice had a smaller NKp46(+) cell population (Figure 3D) consistent with the levels of IL-22 in Figure 3B,C. Because IL-22 activates STAT3, ERK1/2, JNK, and p38 MAPK downstream of its specific receptor, IL-22Rα, we examined p38 MAPK phosphorylation (Figure 3E) and found that p38 MAPK activation was lower in DSS-treated KO mice, which could be attributed to the lower production of IL-22 in this group. We also examined the expression of IL-22Rα in DSS-treated KO mice because heterodimeric receptors consisting of IL-22Rα and IL-10Rβ recognize IL-22 [28,29]. The results showed that IL-22Rα expression was lower in DSS-treated KO mice (Figure 3F). Based on our findings, in which IL-22 contributes to the rapid attenuation of inflammation in a mouse model of UC via the stimulation of mucus production goblet cell restitution [30], we examined mucin-1 expression with and without DSS and observed distinct colonic changes after 5 days of treatment (Supplementary Figure S2). Compared to WT and KO + VC mice, KO mice could not maintain the level of mucin-1 after being treated with DSS (Figure 4). This suggests that WT and KO + VC mice recruit a large number of NKp46(+) cells and attenuate DSS-induced inflammation and mucus depletion through the production of a large amount of IL-22, whereas KO mice lack such factors to ameliorate the colitis caused by DSS treatment.

Figure 3.
Vitamin C insufficiency and DSS treatment decrease IL-22 production and IL-22Rα expression. Mice were treated with DSS for 7 days, and the level of IL-22 in (A) plasma (n = 7–11) and (B) colon homogenates (n = 12–14) was measured with ELISA. The final tissue IL-22 concentration was normalized to proteins in colonic homogenates. * p < 0.05. (C) IL-22-producing cells and (D) the population of infiltrated NKp46(+) cells in the colon were stained using immunohistochemistry. Scale bar, 100 μm. (E) The phosphorylation of p38 MAPK in colon lysates was examined with immunoblotting. The results are representative of three independent experiments. (F) The expression of IL-22Rα was examined by immunoblotting. β-actin was used as an internal control. The results are representative of three independent experiments. Statistical analysis was performed using the Tukey multiple comparisons test after one-way ANOVA.

Figure 4.
Vitamin C insufficiency and DSS treatment decrease the expression of mucin-1. Mice were treated with DSS for 7 days, and mucin-1 expression was examined by immunohistochemistry. Scale bar, 200 μm.
2.4. Vitamin C Insufficiency and DSS Treatment Increase IL-6 Production and STAT3 and Akt Activation
Previously, we evaluated changes in various pro- and anti-inflammatory cytokines and found a notable increase in IL-6 in KO mice compared to WT or KO + VC mice with the DSS treatment. Based on this pilot result, we measured the plasma concentration of IL-6 with ELISA. Plasma IL-6 levels were high in KO mice and increased in response to the DSS treatment in the three groups of mice (Figure 5A). IL-6 levels in the colon also increased significantly due to the DSS treatment, and even more in the KO groups than in the WT and KO + VC groups (Figure 5B). The production of IL-6 in both plasma and colon tissues was highest in DSS-treated KO mice, and in addition to the IL-6 increase, a higher number of F4/80-positive macrophages accumulated in the colon of DSS-treated KO mice (Figure 5C). Furthermore, we detected that IL-6 activated three signaling pathways, Shp2-Ras-ERK, JAK1/2-STAT3, and PI3K-Akt-mTOR [31,32,33]. Thus, we found it appropriate to evaluate the activation of Akt and STAT3 in DSS-treated colonic tissue through immunohistochemistry and immunoblotting. The results demonstrated that DSS amplified the phosphorylation of Akt and STAT3, especially in KO mice (Figure 5D). STAT3 was also activated with the DSS treatment in both the intestinal epithelium and infiltration immune cells in the lamina propria, and the number of cells with phosphorylated STAT3 was higher in the KO mice than the WT and KO + VC mice (Figure 5E). These results indicate that vitamin C-insufficient KO mice produce a higher amount of colonic IL-6 in response to DSS treatment, activating the Akt and STAT3 signaling pathways.
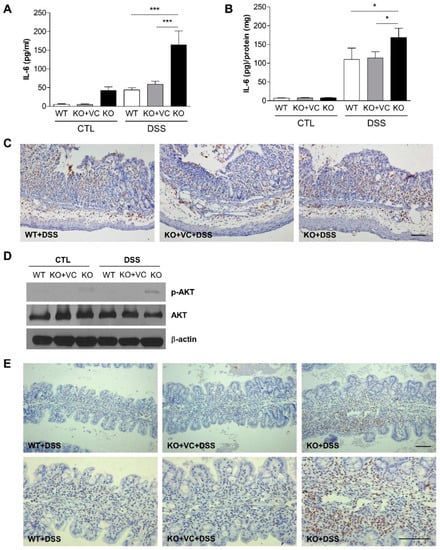
Figure 5.
Vitamin C insufficiency and DSS treatment increase IL-6 production. Mice were treated with DSS for 7 days, and the level of IL-6 was measured in (A) plasma (n = 15–18) and (B) colon homogenates (n = 9–13) by ELISA. The final tissue IL-6 concentration was normalized to proteins in colonic homogenates. * p < 0.05, *** p < 0.001. (C) Infiltrated macrophages were stained with F4/80 antibodies. Scale bar, 100 μm. (D) Akt phosphorylation was examined in colon lysates with immunoblotting. The results are representative of three independent experiments. (E) Phosphorylated STAT3 in the colon was examined by immunohistochemistry. Scale bar, 100 μm. Statistical analysis was performed using the Tukey multiple comparisons test after one-way ANOVA.
3. Discussion
The present study showed that vitamin C insufficiency increased the severity of DSS-induced colitis. This effect is associated with decreased IL-22 production, leading to mucin loss in the colon epithelium as well as increased IL-6 production.
The exact mechanism related to IBD’s development is still unclear, but recent studies have shown that inflammatory reactions caused by an imbalance in the intestinal microbiome of susceptible hosts mark the beginning of IBD development [34]. ROS production, as in many immunoregulatory factors, occurs at abnormally high levels when met with IBD [9]. Additionally, irrespective of disease activity, TAC and cTAC are reduced in IBD patients [20]. Amplified oxidative stress, coupled with a reduction in antioxidant defenses, leads to an increase in damage to DNA in IBD patients [18,35,36]. Adult patients with IBD often become underweight and lack nutrients due to appetite loss, decreased intake due to abdominal pain, absorption defects, and chronic gastrointestinal defects [37,38]. Restrictive diets in the inactive stage of IBD further cases of nutritional deficiency, correlating to the depletion of vital micronutrients such as vitamin C [16,37,39,40].
After being synthesized in the kidneys or liver, vitamin C circulates into tissues. In some species, including humans, the terminal rate-limiting enzyme gluconolactone oxidase is lost. Vitamin C scavenges ROS as an antioxidant, a crucial dietary function [21,41]. Our previous reports established that an increased uptake of vitamin C by keratinocytes exposed to UVB inhibits the production of IL-8 and monocyte chemotactic protein (MCP)-1; this suggests that vitamin C acts as an anti-inflammatory agent by effectively inhibiting the inflammatory response [42]. Therefore, as an essential micronutrient with antioxidative and anti-inflammatory functions, vitamin C may be supplementary in managing IBD. Concurrent with its role as a ROS scavenger and a reducing agent, vitamin C also acts as a cofactor for the hydrolysis of lysine and proline in collagen synthesis and cross-linkage pathways. Supplementing vitamin C in some animals revealed that it is valuable: inducing collagen accumulation, promoting inflammatory rejoinders, and increasing anastomotic strength [43,44,45]. Along with clinical remission and steroid independence, mucosal healing is considered a pivotal prognostic aspect of IBD management. As vitamin C can enhance mucosal healing, it can improve outcomes for select IBD patients. A recent study examined the effect of vitamin C deficiency on the pathogenesis of UC and CD in humans via the antioxidants in vegetables containing vitamin C and miscellaneous micronutrients [46,47].
During chronic inflammation, IL-22 is induced and often protects against damaging tissues similar to IL-10, specifically exerting its effects during acute inflammation in mouse colitis models [25,30]. We found increased production of IL-22 plasma and tissue in the WT and KO+VC groups treated with DSS (Figure 3A,B). However, DSS-induced IL-22 production was significantly lower in the KO group than in the other groups. The natural killer cell expressing NKp46 on its surface plays an important role in IL-22-mediated innate intestinal immune defense [27,48]. As shown in Figure 3D, a smaller number of NKp46(+) cells infiltrated the colonic tissues of KO mice after the DSS treatment, which correlated to the decreased production of IL-22 in KO mice. Moreover, the expression of mucin-1 was decreased in the colons of DSS-treated mice (Figure 4), consistent with a report that IL-22 is dispensable for mucin production to attenuate inflammation [30]. This suggests that the impaired production of colonic IL-22 and mucin, which increases the severity of colitis, is caused by vitamin C insufficiency.
Lymphoid lineage cells, including T cells, NK cells, and ILCs—especially NKp46+ ILC3—are known as the main producers of IL-22 [49,50,51]. On this basis, we focused the experiment on the protective role of IL-22 from NKp46+ cells regulated by vitamin C on DSS-induced colitis. However, there is a report regarding the production of IL-22 from activated macrophages and mast cells [52,53], particularly regarding incidents of increased tryptase-positive mast cells in the colons of IBD patients [54,55]. In the case of mast cells in a patient’s colon, it is known that the anti-inflammatory response in the mucous membrane caused by IL-22 decreases due to the reduction in IL-22 production because of a defect in IL-33/ST2 signaling [56]. Therefore, more extensive studies about the effect of vitamin C on mast cells in the gut epithelium are required to clarify whether the IL-22-dependent anti-inflammatory effects of vitamin C on colitis are through mast cell regulation.
Both IL-22 and IL-6 activate STAT3 signaling, but epithelial STAT3 activation in DSS-induced colitis depends more on IL-22 than on IL-6 [31]. However, in this study, STAT3 phosphorylation was increased in the colons of DSS-treated KO mice, concomitant with increased IL-6 production and decreased IL-22 production. IL-22 can activate STAT3 in epithelial cells because of the limited expression of receptors. Figure 5E shows that phosphorylated STAT3 expression was higher in nonepithelial cells in the lamina propria than in the epithelium. Because DSS caused the accumulation of nonepithelial cells in the colonic tissues of KO mice, phosphorylated STAT3 expression induced by increased IL-6 may be enhanced in DSS-treated KO mice. An analysis of signal transduction pathways revealed that Akt and STAT3 were activated, whereas p38 MAPK was not activated, in DSS-treated KO mice (Figure 3E and Figure 5D,E). IL-6 activates ERK/STAT3/Akt [32], and IL-22 activates ERK/STAT3/p38 MAPK [28]. Considering the increase in IL-6 and the more minor increase in IL-22 in DSS-treated KO mice, the different activation of Akt, STAT3, and p38 MAPK is consistent with the different levels of IL-6 and IL-22. Moreover, the lower level of p38 MAPK activation might be related not only to the lower production of IL-22 in KO mice but also to decreased IL-22Rα expression in KO mice after DSS treatment (Figure 3F).
4. Materials and Methods
4.1. Animals
C57BL/6 WT mice and KO mice were maintained under specific pathogen-free conditions at the animal facility at the Seoul National University College of Medicine. Male mice (8–10 weeks old) were used for experiments. The KO group was maintained with the supplementation of vitamin C (3.3 g/L) in their drinking water to prevent death caused by vitamin C deficiency. For colitis experiments, vitamin C supplementation was discontinued for 3 weeks (KO group) or continued (KO + VC group). KO mice were not in a vitamin C-depleted state, but in a vitamin C-insufficient state, and the plasma level of vitamin C in the KO group was 1/3–1/4 of that in WT and KO + VC mice [57,58]. All experiments using animals were reviewed and approved by the Institutional Animal Care and Use Committee of Seoul National University.
4.2. Induction of Acute Colitis
Mice were provided 3% DSS (MP Biochemicals, Irvine, CA, USA) dissolved in drinking water for 5 or 7 days to induce acute colitis. The mice were checked daily for behavior, water and food consumption, body weight, stool consistency, and the presence of gross blood in the stool or the anus. Weight change was calculated as the percentage change in weight compared with body weight on day 0. The three groups of mice were peritoneally injected with azoxymethane (AOM, 12 mg/kg). One week after AOM injection, 2% DSS was supplemented in drinking water for 5 days. Two weeks after DSS treatment, 2% DSS has supplemented in the drinking water for 5 days once more. Lastly, after 2 weeks, 2% DSS supplementation was given to mice for 5 days. After staining with indigo carmine solution (Sigma, St. Louis, MO, USA), the number of tumors was counted.
4.3. Histological Evaluation of Colonic Damage
The colon samples were fixed in 4% paraformaldehyde (PFA), paraffin-embedded, and sectioned and stained with hematoxylin and eosin (H&E). A histological assessment was performed by a trained pathologist who was blind to the treatment. Histological quantification was performed in a blind method using a scoring system. Briefly, cellularity, edema, erosion (or ulceration of the mucosa), and loss of mucosal architecture, were evaluated as follows: 0, normal; 1, a slight increase in cellularity (primarily lymphocytes in the lamina propria); 2, increase in cellularity, neutrophils present, mild edema; 3, diffuse increase in cellularity, focal erosions, or ulcerations of mucosa; 4, increased cellularity, large and/or multifocal mucosal ulcerations; 5, diffuse ulceration, loss of mucosal architecture [59].
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
Blood was collected from the intraorbital plexuses of mice with a capillary tube and readily centrifuged at 14,000 rpm for 30 min at 4 °C. Sera of the upper layer were collected into new tubes and stored at −70 °C until use. Colonic tissues were homogenized with lysis buffer and quantified with the BCA method. The final concentrations of IL-6 (R&D system, Minneapolis, MN, USA) and IL-22 (BioLegend, San Diego, CA, USA) in the colon were normalized to the amounts of total protein in the colonic tissue lysates. ELISA was performed according to the manufacturer’s instructions.
4.5. Myeloperoxidase (MPO) Assay
Weighted colons were homogenized in 0.5 mL of ice-cold 0.5% hexadecyltrimethylammonium bromide (HTAB, Sigma) in 50 mM of phosphate buffer (pH 6.0). HTAB was used to negate the pseudoperoxidase activity of hemoglobin and to solubilize the membrane-bound MPO. The homogenate was centrifuged at 18,000× g for 20 min at 4 °C. The supernatant reacted with a mixture containing 0.167 mg/mL O-dianisidine dihydrochloride (Sigma) and 0.005% H2O2 and an amount of MPO for 10 min at room temperature. The reaction was terminated by adding 0.02% sodium azide. The absorbance was measured at 460 nm, and the amount of MPO was normalized to the weight of the colon.
4.6. Immunoblotting
Colonic tissues were homogenized with lysis buffer and quantified with the BCA method. Protein was mixed with 5× SDS sample buffer and loaded onto each lane of a 10% SDS-PAGE gel. Proteins were separated by electrophoresis and transferred to a nitrocellulose membrane with an electroblotting apparatus. Nonspecific sites were blocked with 5% skim milk for 1 h, and the membranes were then incubated with primary antibody against 3-nitrotyrosine (NT), p38 MAPK (Santa Cruz, Palo Alto, CA, USA), IL-22R (R&D Systems), p-Akt, Akt, p-p38 MAPK (Cell Signaling, Danvers, MA, USA), or β-actin (Sigma) at 4 °C overnight. After washing with PBS-T (0.05% Tween-20 in PBS), membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cell signaling) and detected with the ECL detection kit (Amersham, Piscataway, NJ, USA).
4.7. Immunohistochemistry
Colon tissues were freshly isolated and fixed in 4% PFA at 4 °C. Paraffin-embedded tissues were sectioned at a thickness of 4 μm. After deparaffinization and hydration, the antigen epitope was retrieved by heating it in 0.1 M citrate buffer (pH 6.0) in a microwave. After blocking endogenous peroxidase with H2O2 and inhibiting nonspecific signals with 5% goat serum, sections were incubated with primary antibodies against p-STAT3 (Santa Cruz), Gr1 (MACS, Bergisch Gladbach, Germany), NKp46 (BD Bioscience, San Jose, CA, USA), mucin-1 (Abcam, Cambridge, MA, USA), or IL-22 (R&D Systems) at 4 °C overnight in a humidified chamber. Then, sections were incubated with the corresponding biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. ABC solution (Vector Laboratories) was loaded on sections for 30 min, and a DAB kit (Vector Laboratories) was used for chromogenic detection. Subsequent to dehydration and clearing, the sections were mounted with DPX mounting medium (Fluka, St. Louis, MO, USA) and observed under a light microscope (Olympus, Center Valley, PA, USA).
4.8. Statics
Data from independent experiments are expressed as the mean ± SEM of each group. For comparisons of three or more groups, data were analyzed using the Tukey multiple comparisons test after one-way ANOVA. p < 0.05 was considered statistically significant. Statistical tests were performed using GraphPad InStat (GraphPad Software, San Diego, CA, USA).
5. Conclusions
Vitamin C insufficiency increased inflammatory cell infiltration and oxidative stress. Vitamin C insufficiency resulted in decreased IL-22 and mucin production and increased the production of IL-6 in the DSS-induced colons of vitamin C-insufficient KO mice, which seems to cause severe colitis. These results suggest that vitamin C is a potential treatment for IBD patients due to its antioxidant and anti-inflammatory effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810612/s1.
Author Contributions
Conceptualization, J.S.K.; methodology, J.P.I., Y.K. and J.S.K.; formal analysis, H.J. and Y.K.; investigation, H.J., D.L., C.G., Y.J., N.C. and S.B.; resources, Y.K. and J.S.K.; data curation, J.P.I., Y.K. and J.S.K.; writing—original draft preparation, H.J., Y.K. and J.S.K.; writing—review and editing, H.J., N.C., D.K., Y.K. and J.S.K.; visualization, H.J.; project administration, J.S.K.; funding acquisition, Y.K. and J.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the NRF of Korea (No.: 2017R1A2B2010948, 2017R1A6A3A11032576, 2020R1C1C1009334).
Institutional Review Board Statement
All experiments using animals were reviewed and approved by the Institutional Animal Care and Use Committee of Seoul National University (No. SNU-120903-1-3).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to give our special thanks to Amelie Pak (Gunderson High School) for her hard work in correcting and revising our manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Blumberg, R.; Cho, J.; Lewis, J.; Wu, G. Inflammatory bowel disease: An update on the fundamental biology and clinical man-agement. Gastroenterology 2011, 140, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Loftus, E.V., Jr.; Sandborn, W.J.; Yang, S.-K. An update on the epidemiology of inflammatory bowel disease in Asia. Off. J. Am. Coll. Gastroenterol. ACG 2008, 103, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Manne, S.; Treem, W.R.; Bennett, D. Prevalence of inflammatory bowel disease in pediatric and adult populations: Recent estimates from large national databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020, 26, 619–625. [Google Scholar] [CrossRef]
- Im, J.P. Evaluation of Vitamin C Effect on Murine Colitis in Gulo Knock-Out Mouse. Ph.D. Thesis, Seoul National University College of Medicine, Seoul, Korea, 2012. [Google Scholar]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 1991, 325, 928–937. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- de Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- La, K.; Verspaget, H. oxidative stress as a pathogenic factor in inflammatory bowel disease—Radicals or ridiculous? Aliment. Pharmacol. Ther. 2002, 16, 1997–2015. [Google Scholar]
- Piechota-Polanczyk, A.; Fichna, J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, K.; Sano, R.; Goto, K.; Hiramoto, K.; Ooi, K. Administration of high-dose vitamin C and irinotecan ameliorates colorectal cancer induced by azoxymethane and dextran sodium sulfate in mice. Biol. Pharm. Bull. 2018, 41, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Hiramoto, K.; Yamate, Y.; Goto, K.; Sekijima, H.; Ooi, K. Ameliorative effect of high-dose vitamin C administration on dextran sulfate sodium-induced colitis mouse model. Biol. Pharm. Bull. 2019, 42, 954–959. [Google Scholar] [CrossRef]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Hengstermann, S.; Valentini, L.; Schaper, L.; Buning, C.; Koernicke, T.; Maritschnegg, M.; Buhner, S.; Tillinger, W.; Regano, N.; Guglielmi, F.; et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin. Nutr. 2008, 27, 571–578. [Google Scholar] [CrossRef]
- Gatti, S.; Cianfruglia, L.; Galeazzi, T.; Palpacelli, A.; Quattrini, S.; Quatraccioni, C.; Catassi, G.; Monachesi, C.; Di Sario, A.; Armeni, T.; et al. P378 Oxidative status and antioxidant capacity in children and adults with IBD: Preliminary results of the OxIBDiet trial. J. Crohn’s Colitis 2021, 15 (Suppl. S1), S392–S393. [Google Scholar] [CrossRef]
- D’Odorico, S.B.R.; Cardin, R.; D’Inca’, D.; Martines, A.; Ferronato, G.C.; Sturniolo, A. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand. J. Gastroenterol. 2001, 36, 1289–1294. [Google Scholar]
- Guan, G.; Lan, S. Implications of antioxidant systems in inflammatory bowel disease. BioMed Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Malliaraki, N.; Dimoulios, P.D.; Karmiris, K.; Castanas, E.; Kouroumalis, E.A. Decreased total and corrected an-tioxidant capacity in patients with inflammatory bowel disease. Dig. Dis. Sci. 2004, 49, 1433–1437. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Park, S.W.; Ghim, W.; Oh, S.; Kim, Y.; Park, U.C.; Kang, J.; Yu, H.G. Association of vitreous vitamin C depletion with diabetic macular ischemia in proliferative diabetic retinopathy. PLoS ONE 2019, 14, e0218433. [Google Scholar] [CrossRef] [PubMed]
- Hampl, J.S.; Taylor, C.A.; Johnston, C.S. Vitamin C deficiency and depletion in the United States: The third national health and nutrition examination survey, 1988 to 1994. Am. J. Public Health 2004, 94, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kawanishi, S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res. Commun. 2004, 316, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008, 29, 947–957. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Dumoutier, L.; Lesjean-Pottier, S.; Ribeiro, V.S.; Mandelboim, O.; Renauld, J.-C.; Vosshenrich, C.A.; Di Santo, J.P. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J. Immunol. 2009, 183, 6579–6587. [Google Scholar] [CrossRef]
- Mizoguchi, A. Healing of intestinal inflammation by IL-22. Inflamm. Bowel Dis. 2012, 18, 1777–1784. [Google Scholar] [CrossRef]
- Li, L.-J.; Gong, C.; Zhao, M.-H.; Feng, B.-S. Role of interleukin-22 in inflammatory bowel disease. World J. Gastroenterol. WJG 2014, 20, 18177. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Pereira, C.; Coelho, R.; Grácio, D.; Dias, C.; Silva, M.; Peixoto, A.; Lopes, P.; Costa, C.; Teixeira, J.P.; Macedo, G.; et al. DNA damage and oxidative DNA damage in inflammatory bowel disease. J. Crohn’s Colitis 2016, 10, 1316–1323. [Google Scholar] [CrossRef]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahigashi, M.; Saniabadi, A. Diet and inflammatory bowel disease–epidemiology and treatment. Aliment. Pharmacol. Ther. 2009, 30, 99–112. [Google Scholar] [CrossRef]
- Hartman, C.; Eliakim, R.; Shamir, R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J. Gastroenterol. WJG 2009, 15, 2570. [Google Scholar] [CrossRef]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.-B.; Hébuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 85–91. [Google Scholar] [CrossRef]
- Guerreiro, C.S.; Cravo, M.; Costa, A.R.; Miranda, A.; Tavares, L.; Moura-Santos, P.; MarquesVidal, P.; Leitão, C.N. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: A case-control study. Off. J. Am. Coll. Gastroenterol. ACG 2007, 102, 2551–2556. [Google Scholar] [CrossRef]
- Wheeler, G.; Ishikawa, T.; Pornsaksit, V.; Smirnoff, N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 2015, 4, e06369. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, H.N.; Kim, J.E.; Mun, G.H.; Kim, Y.S.; Cho, D.; Shin, D.H.; Hwang, Y.I.; Lee, W.J. Regulation of UVB-induced IL-8 and MCP-1 production in skin keratinocytes by increasing vitamin C uptake via the redistribution of SVCT-1 from the cytosol to the membrane. J. Investig. Dermatol. 2007, 127, 698–706. [Google Scholar] [CrossRef]
- Ellinger, S.; Stehle, P. Efficacy of vitamin supplementation in situations with wound healing disorders: Results from clinical intervention studies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 588–595. [Google Scholar] [CrossRef]
- Cevikel, M.; Tuncyurek, P.; Ceylan, F.; Meteoglu, I.; Kozaci, D.; Boylu, S. Supplementation with high-dose ascorbic acid improves intestinal anastomotic healing. Eur. Surg. Res. 2008, 40, 29–33. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Nagata, C.; Furukawa, S.; Andoh, A.; Yokoyama, T.; Yoshimura, N.; Mori, K.; Ninomiya, T.; Yamamoto, Y.; et al. Dietary intake of vegetables, fruit, and antioxidants and risk of ulcerative colitis: A case-control study in Japan. Nutrition 2021, 91, 111378. [Google Scholar] [CrossRef]
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A prospective analysis of mi-cronutrient status in quiescent inflammatory bowel disease. Clin. Nutr. 2021, 40, 327–331. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef]
- Rankin, L.C.; Girard-Madoux, M.J.H.; Seillet, C.; Mielke, L.A.; Kerdiles, Y.; Fenis, A.; Wieduwild, E.; Putoczki, T.; Mondot, S.; Lantz, O.; et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat. Immunol. 2016, 17, 179–186. [Google Scholar] [CrossRef]
- Yeste, A.; Mascanfroni, I.D.; Nadeau, M.; Burns, E.J.; Tukpah, A.-M.; Santiago, A.; Wu, C.; Patel, B.; Kumar, D.; Quintana, F.J. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 2014, 5, 3753. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Vosshenrich, C.A.J.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef]
- Hansson, M.; Silverpil, E.; Lindén, A.; Glader, P. Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm. Res. 2013, 62, 561–569. [Google Scholar] [CrossRef]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.-Q.; Yu, T.-Y.; Yin, Y.-Y.; Liu, Y.; Wang, X.-D.; He, Z.G.; Yin, L.; Chen, C.Q.; Li, J.Y. Mast Cell Tryptase Promotes Inflammatory Bowel Disease–Induced Intestinal Fibrosis. Inflamm. Bowel Dis. 2021, 27, 242–255. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Sinnamon, M.J.; Lyng, G.D.; Glickman, J.N.; Wang, X.; Xing, W.; Krilis, S.A.; Blumberg, R.S.; Adachi, R.; Lee, D.M.; et al. Essential role for mast cell tryptase in acute experimental colitis. Proc. Natl. Acad. Sci. USA 2011, 108, 290–295. [Google Scholar] [CrossRef]
- He, Z.; Song, J.; Hua, J.; Yang, M.; Ma, Y.; Yu, T.; Feng, J.; Liu, B.; Wang, X.; Li, Y.; et al. Mast cells are essential intermediaries in regulating IL-33/ST2 signaling for an immune network favorable to mucosal healing in experimentally inflamed colons. Cell Death Dis. 2018, 9, 1173. [Google Scholar] [CrossRef]
- Bae, S.; Cho, C.-H.; Kim, H.; Kim, Y.; Kim, H.-R.; Hwang, Y.-I.; Yoon, J.H.; Kang, J.S.; Lee, W.J. In vivo consequence of vitamin C insufficiency in liver injury: Vitamin C ameliorates T-cell-mediated acute liver injury in Gulo(−/−) mice. Antioxid. Redox Signal. 2013, 19, 2040–2053. [Google Scholar] [CrossRef]
- Kim, H.; Bae, S.; Yu, Y.; Kim, Y.; Kim, H.-R.; Hwang, Y.-I.; Kang, J.S.; Lee, W.J. The analysis of vitamin C concentration in organs of gulo-/-mice upon vitamin C withdrawal. Immune Netw. 2012, 12, 18–26. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Brown, W.A.; Smith, C.L.; Byrne, F.R.; Viney, J.L. Methods of inducing inflammatory bowel disease in mice. Curr. Protoc. Pharmacol. 2009, 72, 5–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).