Precision Killing of Sinoporphyrin Sodium-Mediated Photodynamic Therapy against Malignant Tumor Cells
Abstract
:1. Introduction
2. Results
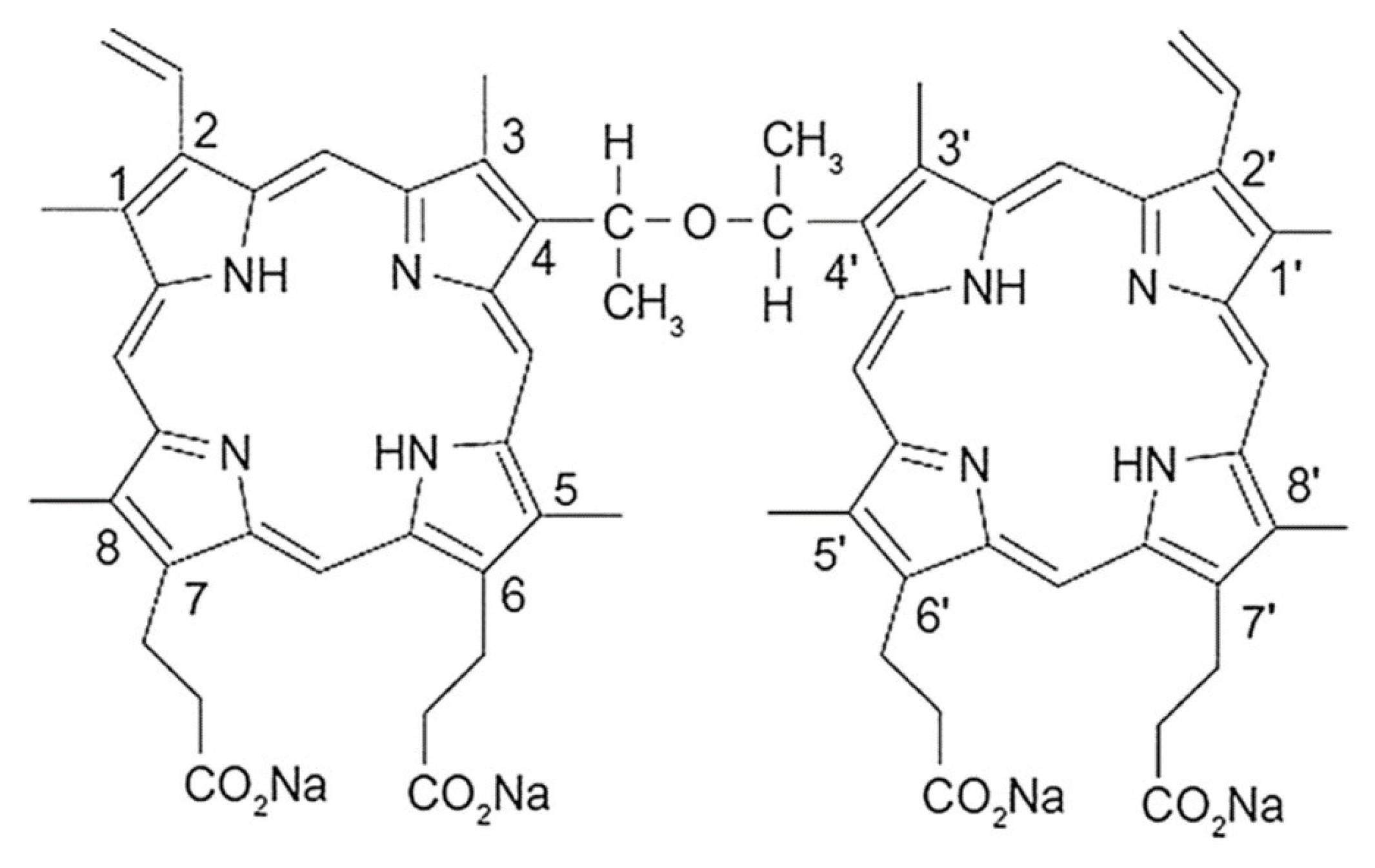
2.1. Spectral Characteristics of DVDMS
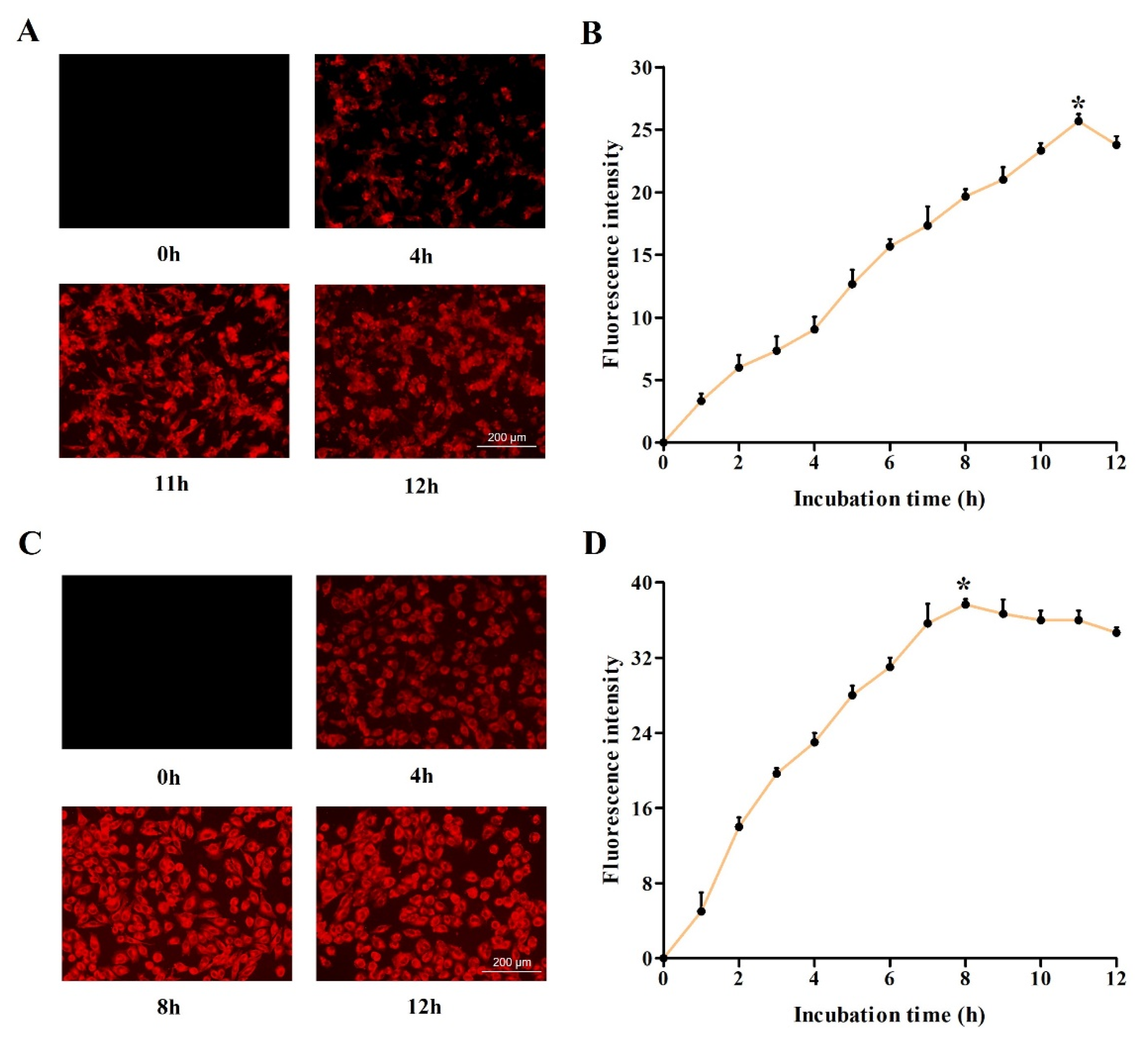
2.2. Investigation of Optimum Cellular Uptake of DVDMS
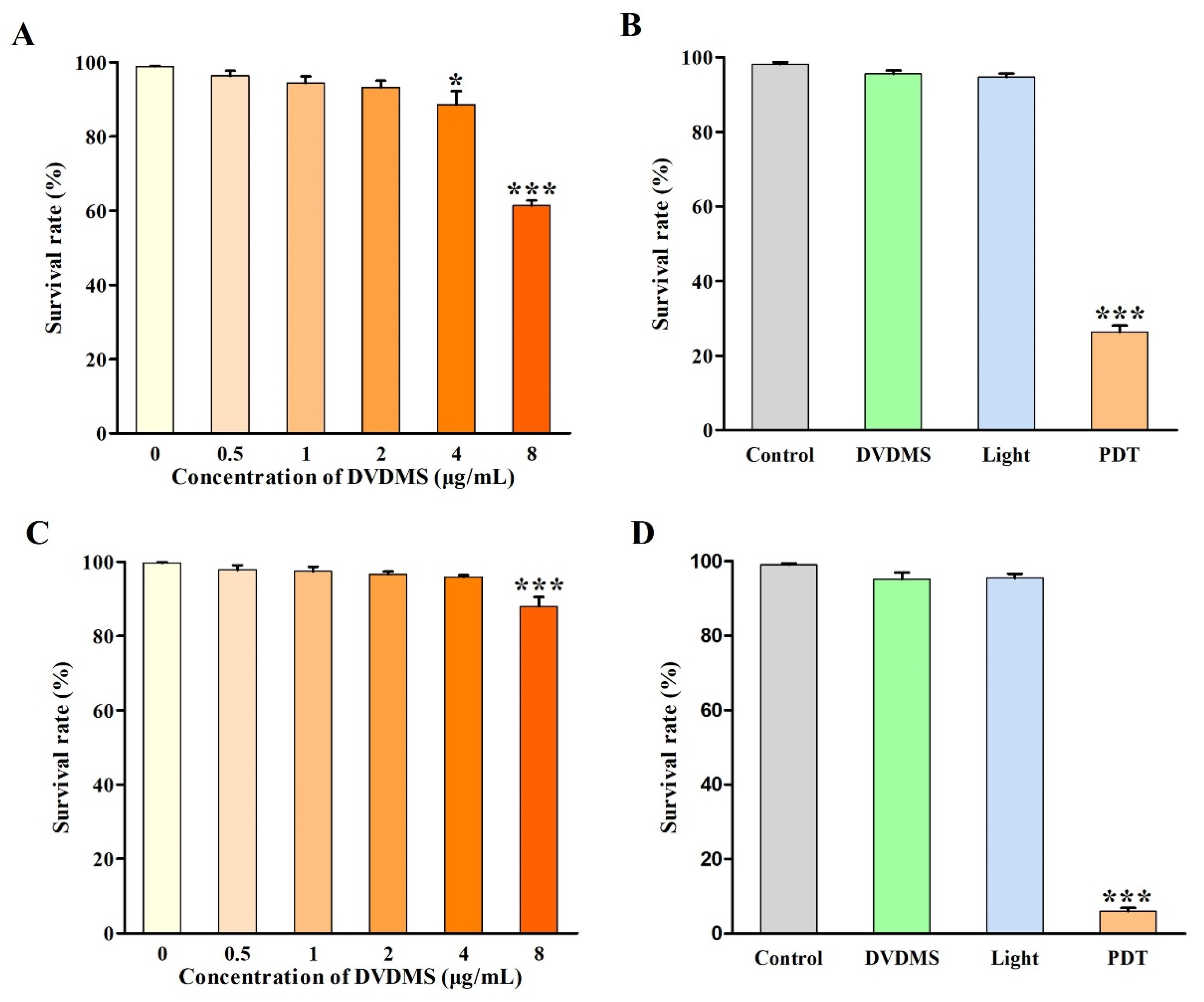
2.3. Identification of DVDMS-PDT Efficacy
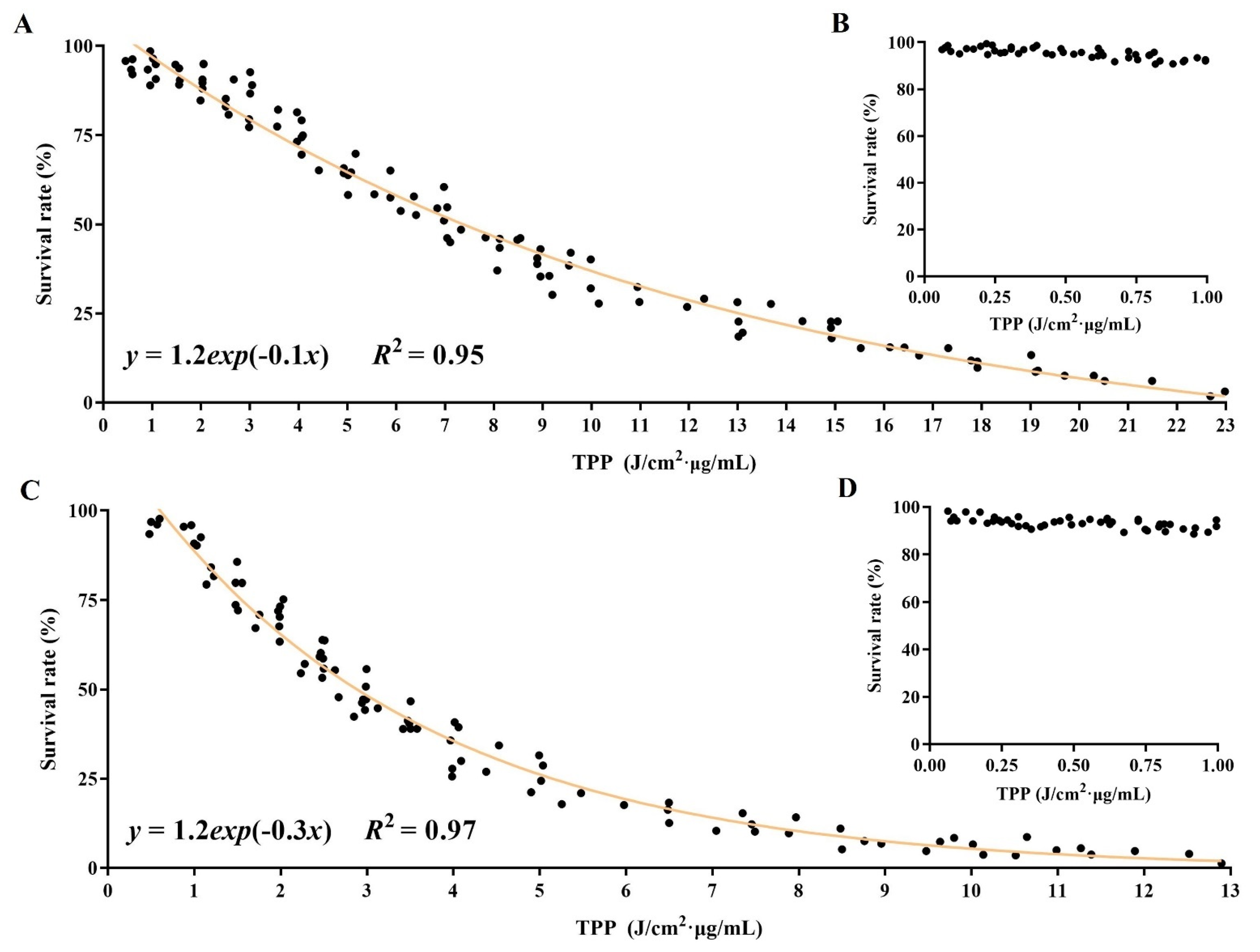
2.4. In-Depth Analysis Associated Parameters with Outcomes in DVDMS-PDT
2.5. Establishment of Relationship among Parameters for PDT Efficacy
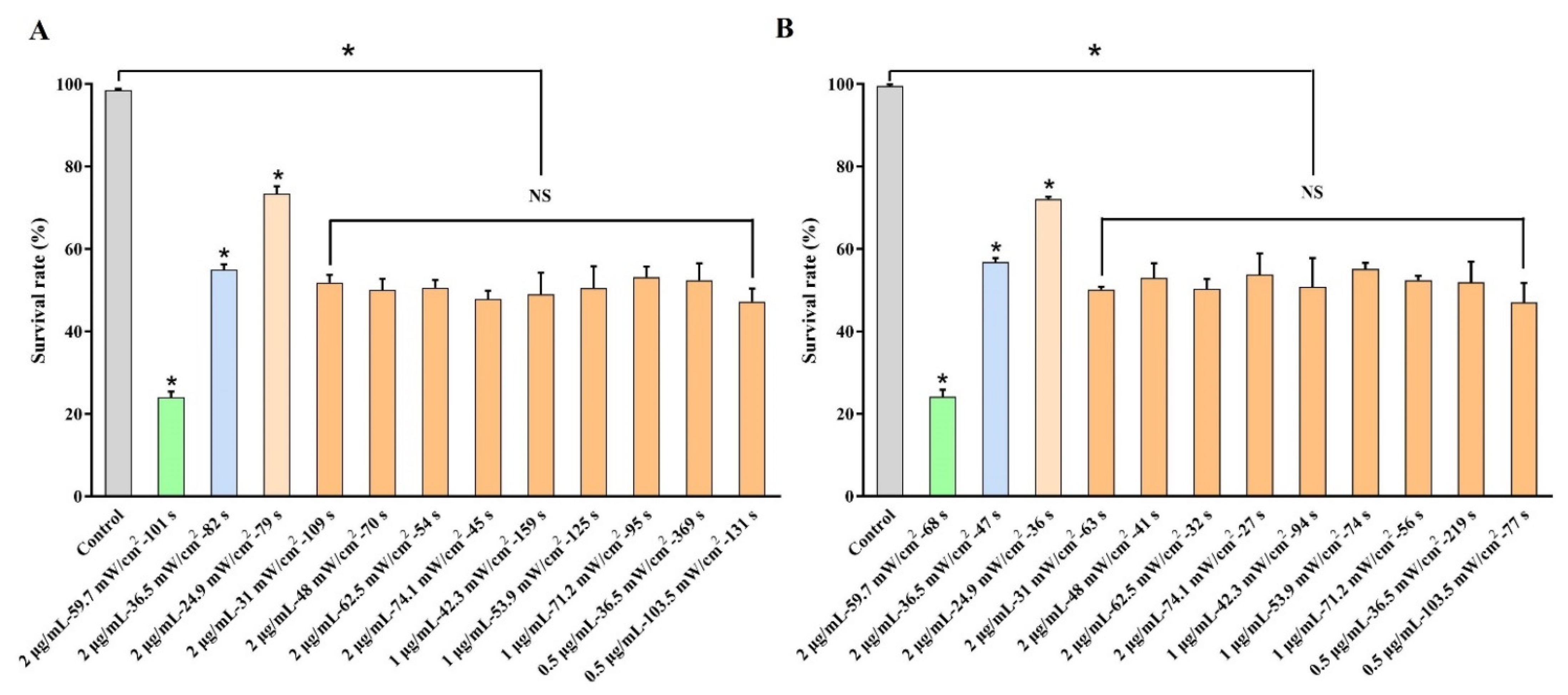
2.6. Realization of Controllable Outcomes by Regulating Parameters of DVDMS-PDT
3. Discussion
4. Materials and Methods
4.1. Reagents
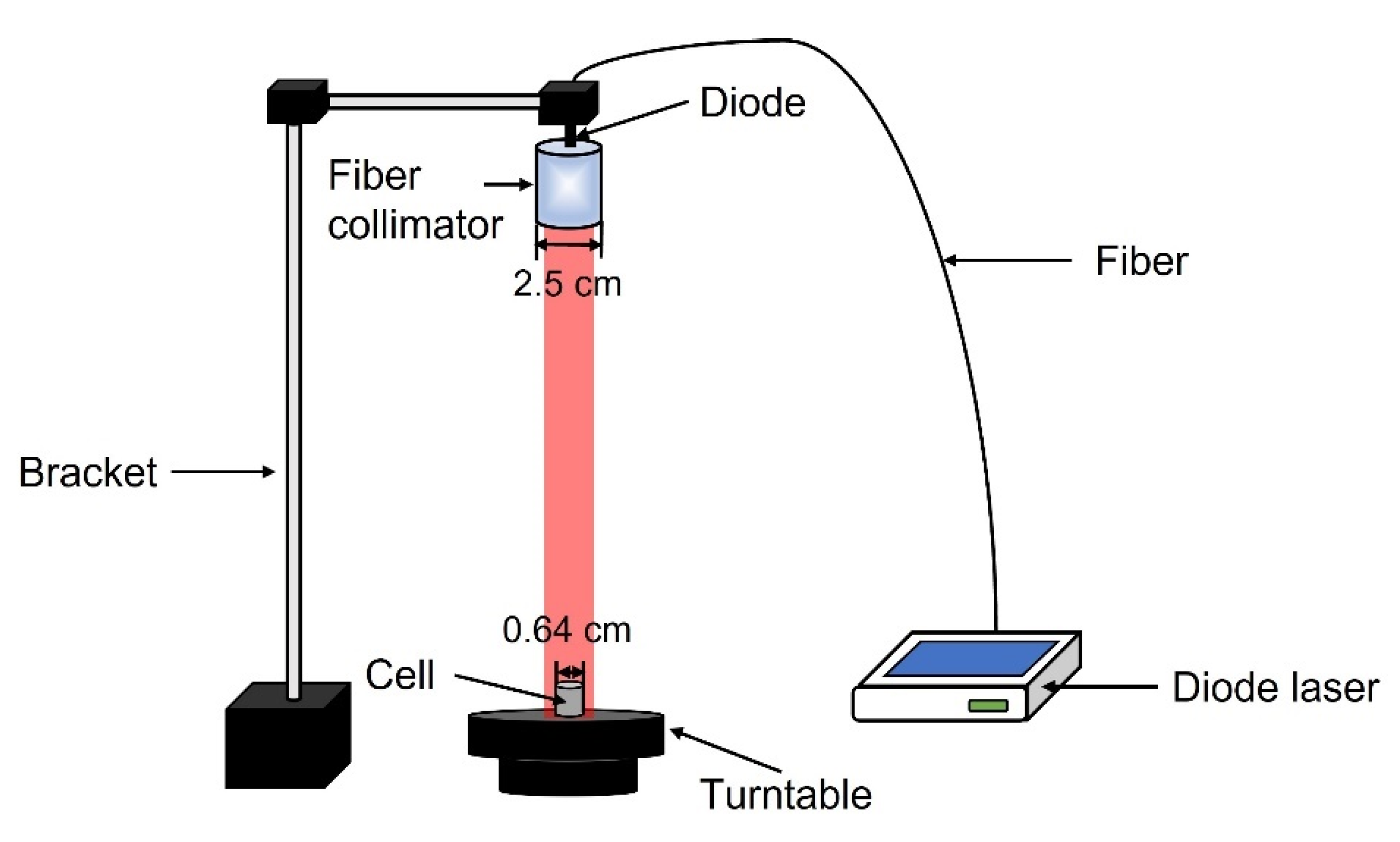
4.2. Construction of PDT Device
4.3. Cell Line and Cell Culture
4.4. Spectral Characteristics Analysis
4.5. Cellular-Uptake Assay
4.6. Cytotoxicity Assay
4.7. PDT Treatment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Yang, M.; Yang, T.; Mao, C. Enhancement of Photodynamic Cancer Therapy by Physical and Chemical Factors. Angew. Chem. Int. Ed. 2019, 58, 14066–14080. [Google Scholar] [CrossRef]
- Leroy, H.; Baert, G.; Guerin, L.; Delhem, N.; Mordon, S.; Reyns, N.; Vignion-Dewalle, A. Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use. Cancers 2021, 13, 5754. [Google Scholar] [CrossRef]
- Berroeta, L.; Clark, C.; Dawe, R.S.; Ibbotson, S.H.; Fleming, C.J. A randomized study of minimal curettage followed by topical photodynamic therapy compared with surgical excision for low-risk nodular basal cell carcinoma. Br. J. Dermatol. 2007, 157, 401–403. [Google Scholar] [CrossRef]
- Overholt, B.F.; Wang, K.K.; Burdick, J.S.; Lightdale, C.J.; Kimmey, M.; Nava, H.R.; Sivak, M.V., Jr.; Nishioka, N.; Barr, H.; Marcon, N.; et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest. Endosc. 2007, 66, 460–468. [Google Scholar] [CrossRef]
- Furuse, K.; Fukuoka, M.; Kato, H.; Horai, T.; Kubota, K.; Kodama, N.; Kusunoki, Y.; Takifuji, N.; Okunaka, T.; Konaka, C.; et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J. Clin. Oncol. 1993, 11, 1852–1857. [Google Scholar] [CrossRef]
- Hopper, C.; Kubler, A.; Lewis, H.; Tan, I.B.; Putnam, G. mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int. J. Cancer 2004, 111, 138–146. [Google Scholar] [CrossRef]
- Chen, Q.; Li, K.T.; Tian, S.; Yu, T.H.; Yu, L.H.; Lin, H.D.; Bai, D.Q. Photodynamic Therapy Mediated by Aloe-Emodin Inhibited Angiogenesis and Cell Metastasis Through Activating MAPK Signaling Pathway on HUVECs. Technol. Cancer Res. Treat. 2018, 17, 1533033818785512. [Google Scholar] [CrossRef]
- Sun, Y.; Xing, D.; Shen, L.; Sun, M.; Fang, M.; Bi, L.; Sui, Y.; Zhang, Z.; Cao, W. Bactericidal effects of hematoporphyrin monomethyl ether-mediated photosensitization against pathogenic communities from supragingival plaque. Appl. Microbiol. Biotechnol. 2013, 97, 5079–5087. [Google Scholar] [CrossRef]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting photodynamic therapy dosimetry: Reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar]
- Li, Q.; Cheng, J.; Peng, C.; Li, Z.; Shi, S.; Liang, H.; Tian, Y.; Zhang, Z.; Cao, W. Apoptosis of vascular smooth muscle cells induced by photodynamic therapy with protoporphyrin IX. Biochem. Biophys. Res. Commun. 2010, 391, 69–72. [Google Scholar] [CrossRef]
- Ma, W.; Wang, T.; Zang, L.; Jiang, Z.; Zhang, Z.; Bi, L.; Cao, W. Bactericidal effects of hematoporphyrin monomethyl ether-mediated blue-light photodynamic therapy against Staphylococcus aureus. Photochem. Photobiol. Sci. 2019, 18, 92–97. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic therapy for treatment of solid tumors--potential and technical challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef]
- van Solinge, T.; Nieland, L.; Chiocca, E.; Broekman, M. Advances in local therapy for glioblastoma—Taking the fight to the tumour. Nat. Rev. Neurol. 2022, 18, 221–236. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Q.; Wang, Y.; Hua, S.; Sauvé, C.; Ong, D.; Lan, Z.; Chang, Q.; Ho, Y.; Monasterio, M.; et al. Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth. Cell 2016, 167, 1281–1295.e18. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Sugiyama, K.; Takeshima, Y.; Amatya, V.; Yamasaki, F.; Takayasu, T.; Nosaka, R.; Muragaki, Y.; Kawamata, T.; Kurisu, K. Prognostic implications of the subcellular localization of survivin in glioblastomas treated with radiotherapy plus concomitant and adjuvant temozolomide. J. Neurosurg. 2018, 128, 679–684. [Google Scholar] [CrossRef]
- Abd-El-Barr, M.; Chiocca, E. How much is enough? The question of extent of resection in glioblastoma multiforme. World Neurosurg. 2014, 82, e109–e110. [Google Scholar] [CrossRef]
- Li, X.; Feng, H.; Li, F. Progression of basic research, clinical application of photodynamic therapy and fluorescence-guided surgery in glioma treatment. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2018, 43, 360–367. [Google Scholar] [PubMed]
- Luby, B.; Walsh, C.; Zheng, G. Advanced Photosensitizer Activation Strategies for Smarter Photodynamic Therapy Beacons. Angew. Chem.-Int. Ed. 2019, 58, 2558–2569. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kajimoto, Y.; Furuse, M.; Miyatake, S. A surgical loupe system for observing protoporphyrin IX fluorescence in high-grade gliomas after administering 5-aminolevulinic acid. Photodiagnosis Photodyn. Ther. 2013, 10, 379–381. [Google Scholar] [CrossRef]
- Fang, Q.; Yang, D. A Porphyrin Dimer Combined with an Ether Bond and Its Manufacturing Method. China Patent ZL200910179116.5 29 August 2012. [Google Scholar]
- Mai, B.; Wang, X.; Liu, Q.; Zhang, K.; Wang, P. The Application of DVDMS as a Sensitizing Agent for Sono-/Photo-Therapy. Front. Pharmacol. 2020, 11, 19. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Liu, Q.; Zhang, K.; Xiong, W.; Xu, C.; Wang, P.; Leung, A.W. Antitumor Effect of Sinoporphyrin Sodium-Mediated Photodynamic Therapy on Human Esophageal Cancer Eca-109 Cells. Photochem. Photobiol. 2014, 90, 1404–1412. [Google Scholar] [CrossRef]
- Zang, L.; Zhao, H.; Fang, Q.; Fan, M.; Chen, T.; Tian, Y.; Yao, J.; Zheng, Y.; Zhang, Z.; Cao, W. Photophysical properties of sinoporphyrin sodium and explanation of its high photo-activity. J. Porphyr. Phthalocyanines 2017, 21, 59–66. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neuro-Oncol. 2008, 87, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Wilson, B. Photodynamic therapy of brain tumors—A work in progress. Lasers Surg. Med. 2006, 38, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Fujimoto, S.; Yamaguchi, H.; Yamauchi, T.; Yoshimoto, T.; Tokuda, K. Photodynamic Therapy of Malignant Gliomas. Prog. Neurol. Surg. 2018, 32, 1–13. [Google Scholar]
- Li, L.; Li, W.; Xiang, L.; Qing, Z.; Wang, L.; Lin, J.; Ma, Y.; Luo, R.; Huang, Z. Photodynamic therapy: Clinical research and application in China. Chin. J. Laser Med. Surg. 2012, 21, 278–307. [Google Scholar]
- Rosenthal, M.; Kavar, B.; Hill, J.; Morgan, D.; Nation, R.; Stylli, S.; Basser, R.; Uren, S.; Geldard, H.; Green, M.; et al. Phase I and pharmacokinetic study of photodynamic therapy for high-grade gliomas using a novel boronated porphyrin. J. Clin. Oncol. 2001, 19, 519–524. [Google Scholar] [CrossRef]
- Stebbins, W.G.; Hanke, C.W. MAL-PDT for difficult to treat nonmelanoma skin cancer. Dermatol. Ther. 2011, 24, 82–93. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Ibbotson, S.; Murrell, D.F.; Rubel, D.; Frambach, Y.; de Berker, D.; Dummer, R.; Kerrouche, N.; Villemagne, H.; Excilight Study Group. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1302–1311. [Google Scholar] [CrossRef]
- Vinciullo, C.; Elliott, T.; Francis, D.; Gebauer, K.; Spelman, L.; Nguyen, R.; Weightman, W.; Sheridan, A.; Reid, C.; Czarnecki, D.; et al. Photodynamic therapy with topical methyl aminolaevulinate for ‘difficult-to-treat’ basal cell carcinoma. Br. J. Dermatol. 2005, 152, 765–772. [Google Scholar] [CrossRef]
- Kim, M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Ibbotson, S.; Wong, T.; Morton, C.; Collier, N.; Haylett, A.; McKenna, K.; Mallipeddi, R.; Moseley, H.; Rhodes, L.; Seukeran, D.; et al. Adverse effects of topical photodynamic therapy: A consensus review and approach to management. Br. J. Dermatol. 2019, 180, 715–729. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Lemée, J.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro-Oncol. 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Dong, Z.; Zhao, Y.; Ma, N.; Jiang, X.; Li, J.; Wang, J.; Wang, J.; Zhang, W.; Lin, X.; et al. Precision Killing of Sinoporphyrin Sodium-Mediated Photodynamic Therapy against Malignant Tumor Cells. Int. J. Mol. Sci. 2022, 23, 10561. https://doi.org/10.3390/ijms231810561
Lv G, Dong Z, Zhao Y, Ma N, Jiang X, Li J, Wang J, Wang J, Zhang W, Lin X, et al. Precision Killing of Sinoporphyrin Sodium-Mediated Photodynamic Therapy against Malignant Tumor Cells. International Journal of Molecular Sciences. 2022; 23(18):10561. https://doi.org/10.3390/ijms231810561
Chicago/Turabian StyleLv, Guixiang, Zhihui Dong, Yunhan Zhao, Ning Ma, Xiaochen Jiang, Jia Li, Jinyue Wang, Jiaxin Wang, Wenxiu Zhang, Xin Lin, and et al. 2022. "Precision Killing of Sinoporphyrin Sodium-Mediated Photodynamic Therapy against Malignant Tumor Cells" International Journal of Molecular Sciences 23, no. 18: 10561. https://doi.org/10.3390/ijms231810561
APA StyleLv, G., Dong, Z., Zhao, Y., Ma, N., Jiang, X., Li, J., Wang, J., Wang, J., Zhang, W., Lin, X., & Hu, Z. (2022). Precision Killing of Sinoporphyrin Sodium-Mediated Photodynamic Therapy against Malignant Tumor Cells. International Journal of Molecular Sciences, 23(18), 10561. https://doi.org/10.3390/ijms231810561







