Effects of Tetracycline on Scenedesmus obliquus Microalgae Photosynthetic Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Organism
2.2. Acute Toxicity Assay
2.3. Exposure Procedure of Sub-Acute Toxicity
2.4. Photosynthesis Assay
2.5. Transcriptomics Assay
2.6. Statistical Analysis
3. Results
3.1. Acute Toxicity of TC on Scenedesmus obliquus
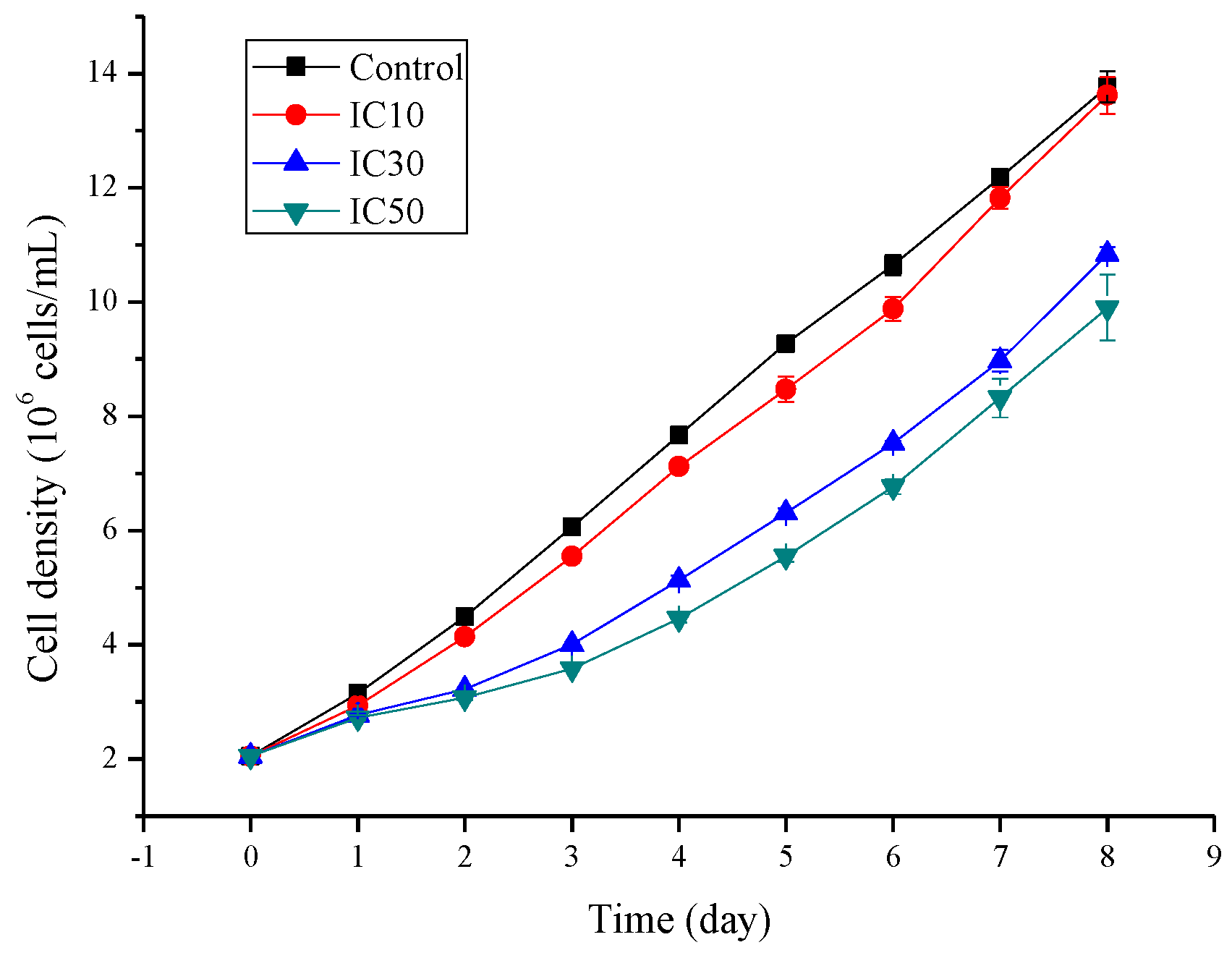
3.2. Algal Growth during the 8 d Sub-Acute Exposure
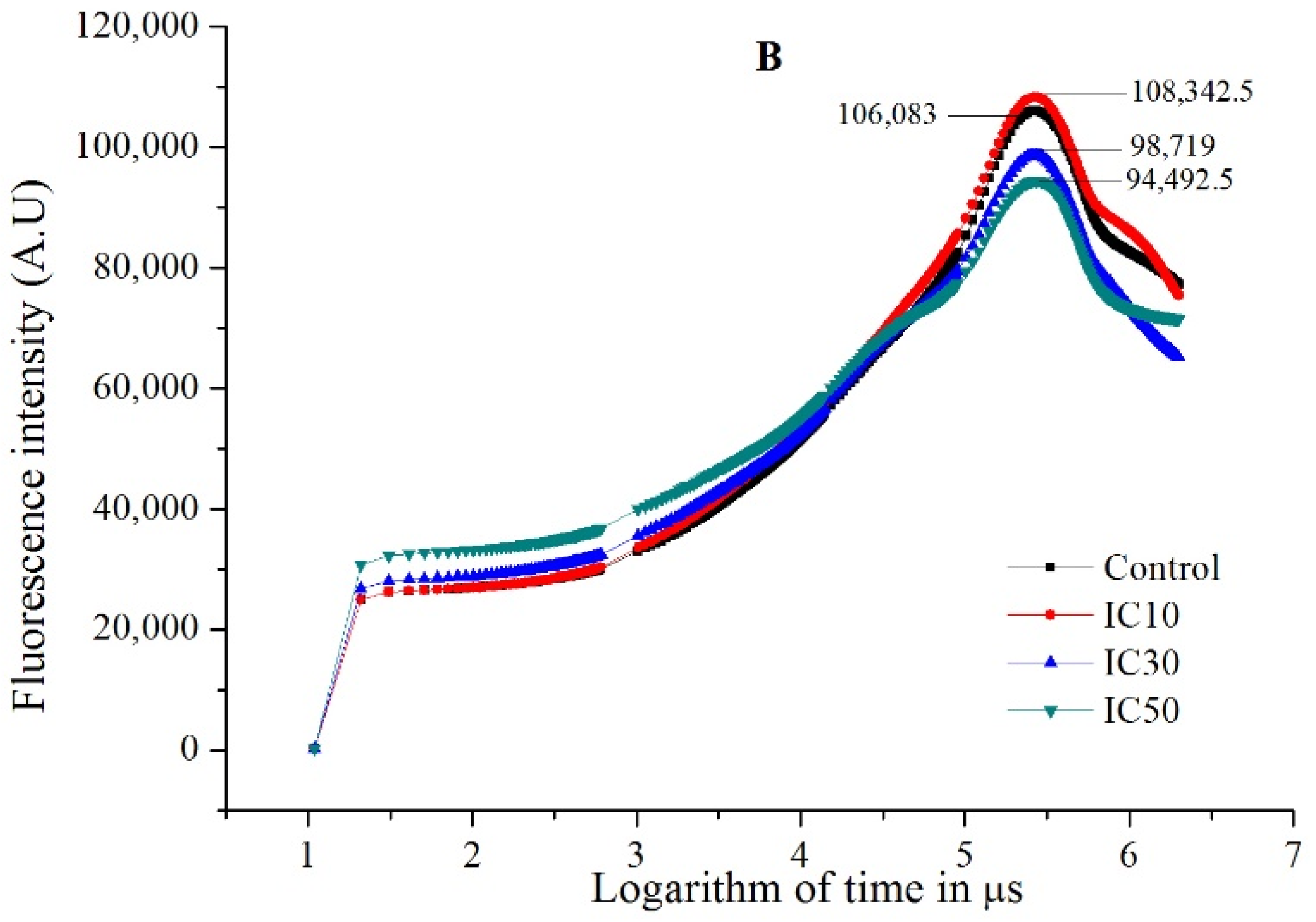
3.3. Photosynthetic Performance of Scenedesmus obliquus Exposed to TC
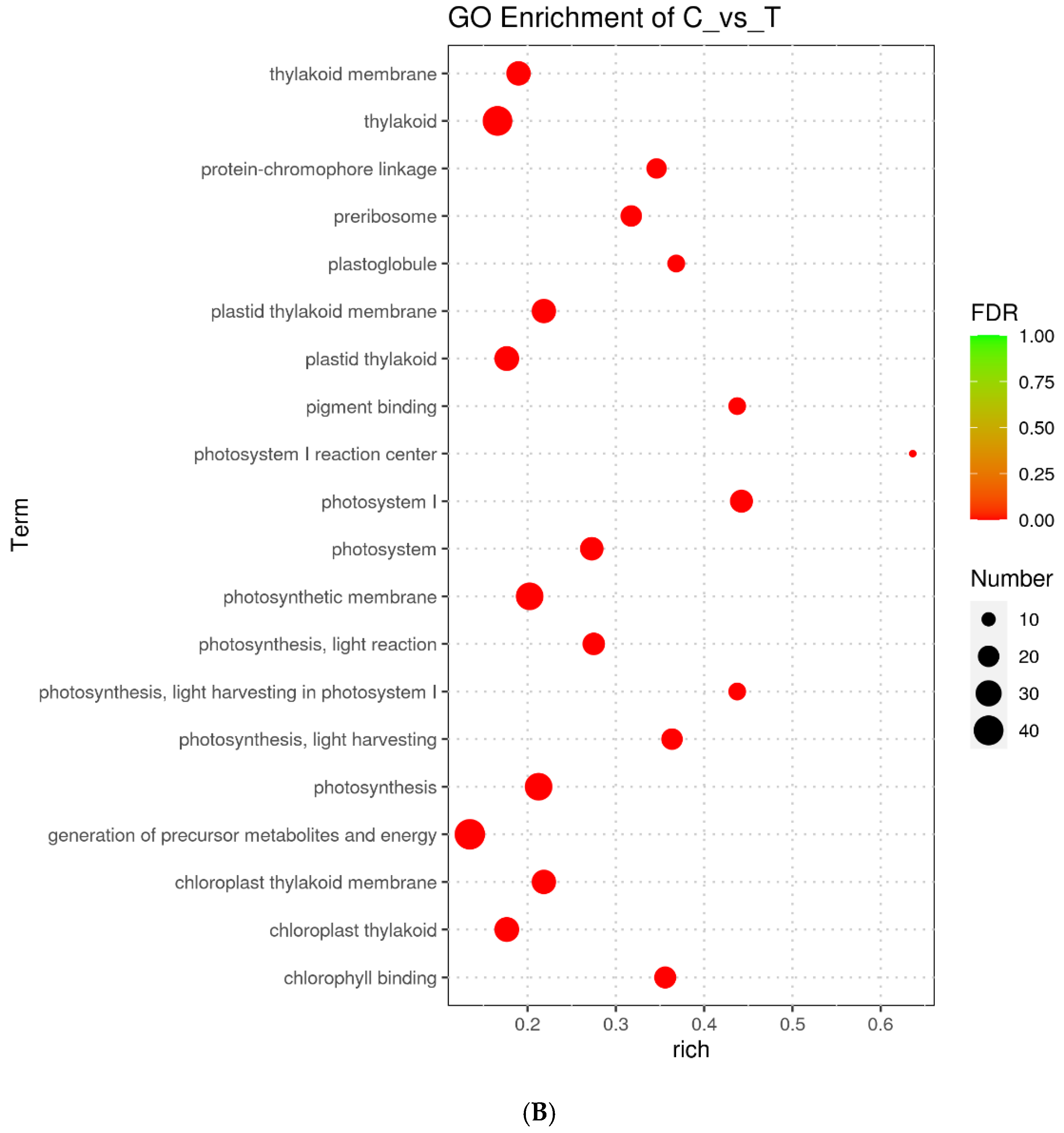
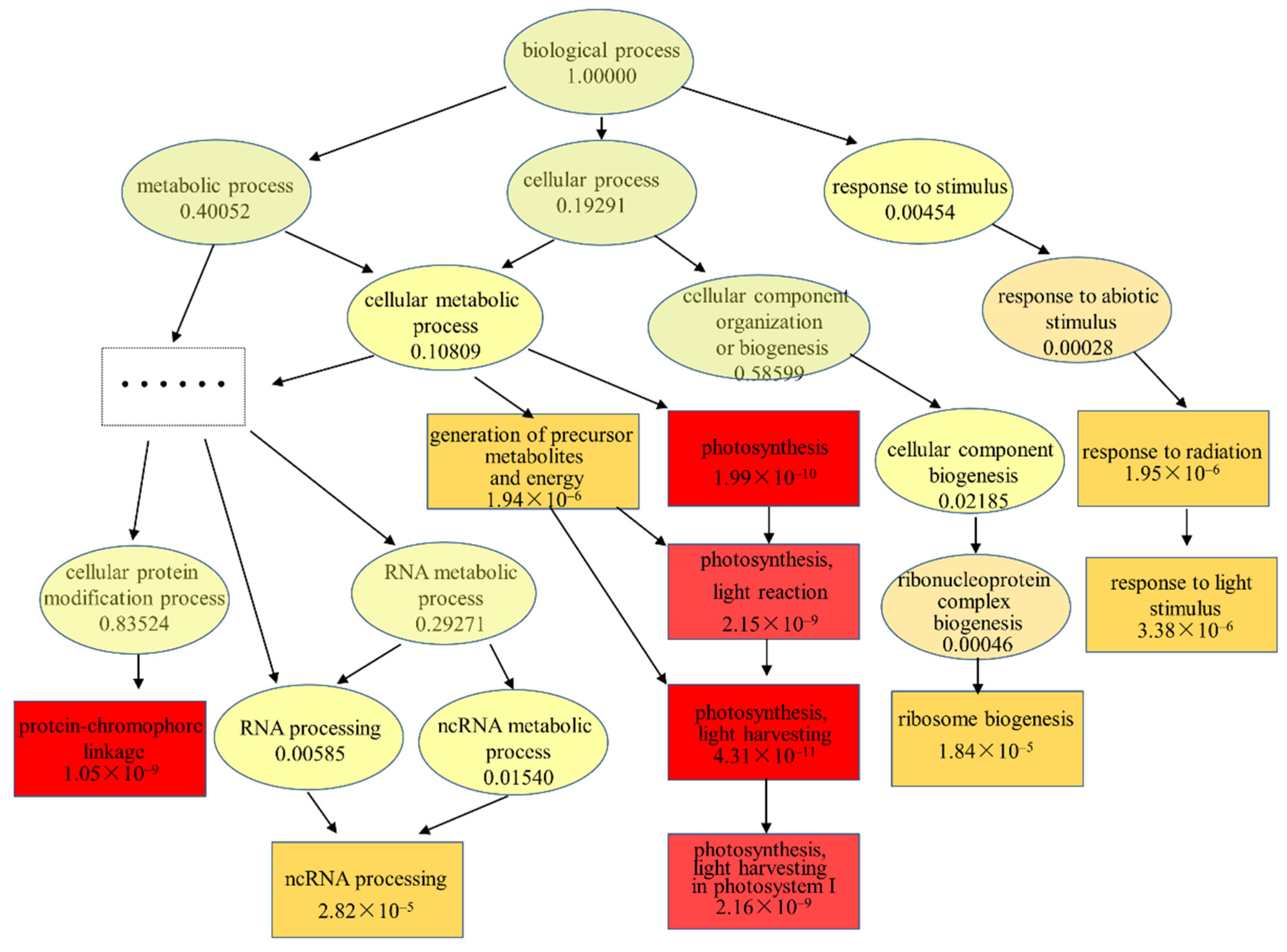
3.4. Transcriptional Responses of Scenedesmus obliquus Exposed to TC
4. Discussion
4.1. Toxicity of Tetracycline on Microalgae
4.2. Effects of Tetracycline on Algal Photosynthesis Performance
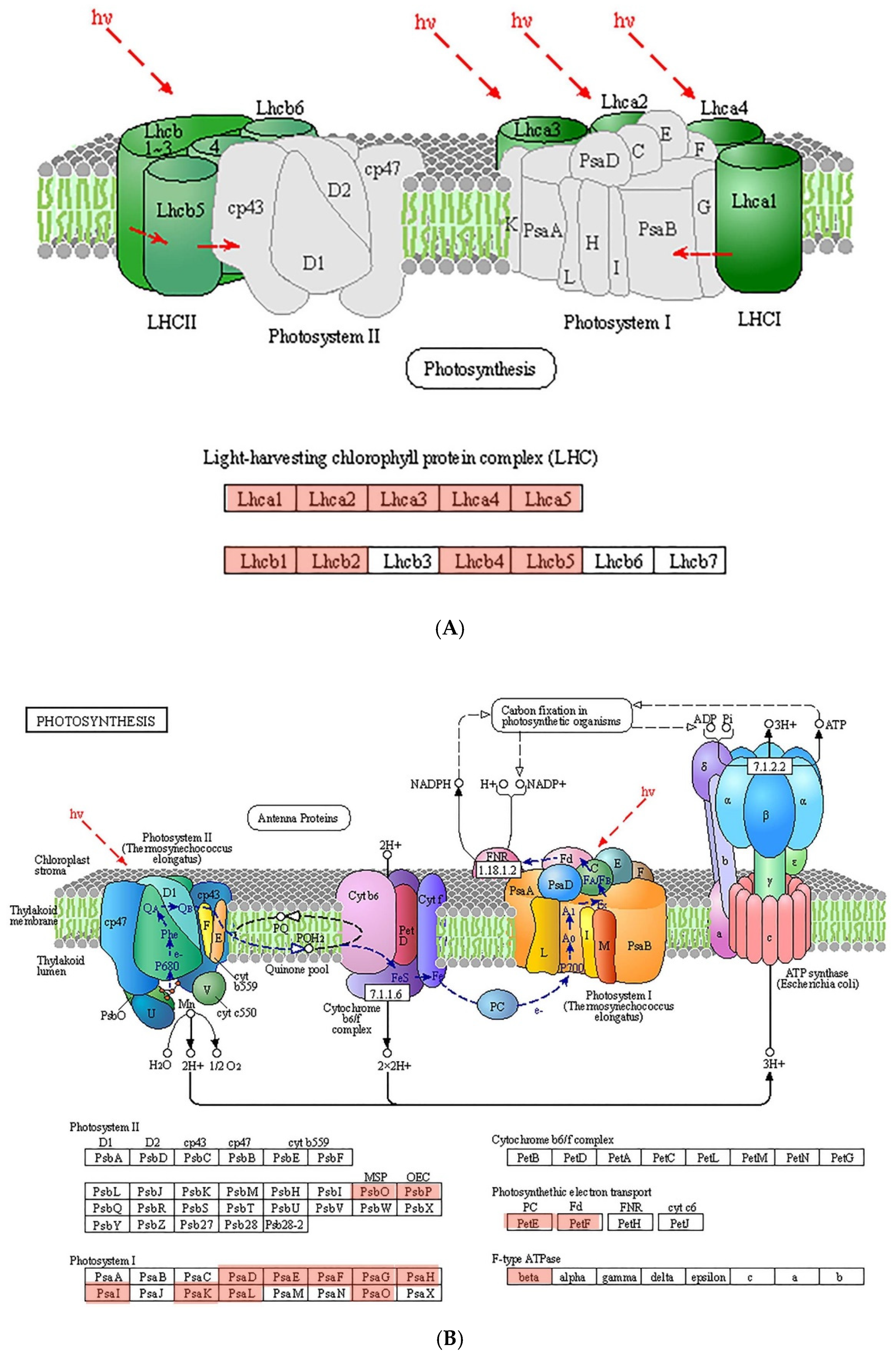
4.3. Effects of Tetracycline on Photosynthesis-Related Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS/RC | The absorption flux per reaction center |
| DEG | Differentially expressed genes |
| DIO/RC | The dissipated energy flux per reaction center |
| ETO/RC | The electron transport flux per reaction center |
| FO (O) | Basal (initial) level of chlorophyll a fluorescence at 20 μs |
| FM (P) | Maximal level of chlorophyll a fluorescence |
| Fv/FM | The maximum quantum yield of primary photochemistry |
| GO | Gene Ontology |
| IC | Inhibition concentration |
| KO | KEGG Orthology |
| LHC | The light-harvesting chlorophyll protein complex |
| MO | The slope at the origin of the relative variable fluorescence) |
| n | The number of QA reduction events between time 0 to tP) |
| OEC | The oxygen evolving center |
| PCA | Principal component analysis |
| PS | Photosystem |
| QA | The first quinone electron acceptor of Photosystem II |
| QB | The second quinone electron acceptor of Photosystem II |
| RC | Reaction center |
| Sm | The working integral of the energy needed to close all reaction centers) |
| TC | Tetracycline |
| TRO/RC | The trapped energy flux per reaction center |
References
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Zhang, J. Transcriptomic mechanisms for the promotion of cyanobacterial growth against eukaryotic microalgae by a ternary antibiotic mixture. Environ. Sci. Pollut. Res. 2022, 29, 58881–58891. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Chen, W.; Xu, H.; Ding, M.; Xie, Z.; Wang, A. Removal of tetracycline hydrochloride by Z-scheme heterojunction sono-catalyst acting on ultrasound/H2O2 system. Process Saf. Environ. Prot. 2022, 165, 93–101. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut. 2017, 221, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, S.; Li, J. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Lu, S.; Yan, P.; Sui, Q. Recent advances in pharmaceuticals and personal care products in the surface water and sediments in China. Front. Environ. Sci. Eng. 2016, 10, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.S.; Zhao, J.L.; Liu, W.R.; He, L.Y.; Zhang, J.N.; Chen, J.; He, L.K.; Zhang, Q.Q.; Ying, G.G. Occurrence, fate and mass loadings of antibiotics in two swine wastewater treatment systems. Sci. Total Environ. 2018, 639, 1421–1431. [Google Scholar] [CrossRef]
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, C.; Luo, Y.; Lv, J.; Zhang, Y.; Lin, H.; Wang, L.; Xu, J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016, 213, 833–840. [Google Scholar] [CrossRef]
- Borghi, A.A.; Palma, M.S.A. Tetracycline: Production, waste treatment and environmental impact assessment. Braz. J. Pharm. Sci. 2014, 50, 25–40. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Yu, Y.; Ruan, Y.; Kong, D.; Lv, X.; Xu, P.; Awasthi, M.K.; Dong, M. Interrelationships between tetracyclines and nitrogen cycling processes mediated by microorganisms: A review. Bioresour. Technol. 2021, 319, 124036. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, S.; Fan, J.; Long, S.; Wang, L.; Tang, C.; Tam, N.F.; Yang, Y. Predicting distribution coefficients for antibiotics in a river water–sediment using quantitative models based on their spatiotemporal variations. Sci. Total Environ. 2019, 655, 1301–1310. [Google Scholar] [CrossRef]
- Yang, L.H.; Ying, G.G.; Su, H.C.; Stauber, J.L.; Adams, M.S.; MT, B. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 2000, 40, 731–739. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef]
- Pomati, F.; Netting, A.G.; Calamari, D.; Neilan, B.A. Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat. Toxicol. 2004, 67, 387–396. [Google Scholar] [CrossRef]
- Wollenberger, L.; Halling-Sørensen, B.; Kusk, K.O. Acute and chronic toxicity of veterinary antibiotics to Daphnia magna. Chemosphere 2000, 40, 723–730. [Google Scholar] [CrossRef]
- Nunes, B.; Antunes, S.C.; Gomes, R.; Campos, J.C.; Braga, M.R.; Ramos, A.S.; Correia, A.T. Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef]
- Kim, S.C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, K.; Sun, S.; Li, X. Effect of tetracycline on the growth and nutrient removal capacity of Chlamydomonas reinhardtii in simulated effluent from wastewater treatment plants. Bioresour. Technol. 2016, 218, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Li, X.; Luo, Q.; Yang, C.; Lou, W.; Liu, H.; Du, C.; Nie, L.; Zhong, Y. Effects of humic acids on biotoxicity of tetracycline to microalgae Coelastrella sp. Algal Res. 2020, 50, 101962. [Google Scholar] [CrossRef]
- Nie, X.-P.; Liu, B.-Y.; Yu, H.-J.; Liu, W.-Q.; Yang, Y.-F. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environ. Pollut. 2013, 172, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Cho, M.-G. The effect of kanamycin and tetracycline on growth and photosynthetic activity of two chlorophyte algae. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tang, Z.; Zhou, F.; Zhang, W.; Song, L. Toxicity studies of tetracycline on Microcystis aeruginosa and Selenastrum capricornutum. Environ. Toxicol. Pharmacol. 2013, 35, 320–324. [Google Scholar] [CrossRef] [PubMed]
- McLean, T.I. “Eco-omics”: A review of the application of genomics, transcriptomics, and proteomics for the study of the ecology of harmful algae. Microb. Ecol. 2013, 65, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L. Insights into the Regulation of Algal Proteins and Bioactive Peptides Using Proteomic and Transcriptomic Approaches. Molecules 2019, 24, 1708. [Google Scholar] [CrossRef]
- Wan, J.; Guo, P.; Peng, X.; Wen, K. Effect of erythromycin exposure on the growth, antioxidant system and photosynthesis of Microcystis flos-aquae. J. Hazard. Mater. 2015, 283, 778–786. [Google Scholar] [CrossRef]
- OECD. Freshwater Alga and Cyanobacteria, Growth Inhibition Test; Publications: Paris, France, 2006. [Google Scholar]
- Sun, C.; Xu, Y.; Hu, N.; Ma, J.; Sun, S.; Cao, W.; Klobucar, G.; Hu, C.; Zhao, Y. To evaluate the toxicity of atrazine on the freshwater microalgae Chlorella sp. using sensitive indices indicated by photosynthetic parameters. Chemosphere 2020, 244, 125514. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Chen, S.; Xu, X.; Dai, X.; Yang, C.; Qiang, S. Identification of tenuazonic acid as a novel type of natural photosystem II inhibitor binding in QB-site of Chlamydomonas reinhardtii. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 306–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise OJIP. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Du, C.; Zhang, B.; He, Y.; Hu, C.; Ng, Q.X.; Zhang, H.; Ong, C.N. Biological effect of aqueous C60 aggregates on Scenedesmus obliquus revealed by transcriptomics and non-targeted metabolomics. J. Hazard. Mater. 2017, 324, 221–229. [Google Scholar] [CrossRef]
- Teixeira, J.R.; Granek, E.F. Effects of environmentally-relevant antibiotic mixtures on marine microalgal growth. Sci. Total Environ. 2017, 580, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Cervera, B.; Cordoba-Diaz, M.; García-Balboa, C.; Costas, E.; López-Rodas, V.; Cordoba-Diaz, D. Assessing the effect of high doses of ampicillin on five marine and freshwater phytoplankton species: A biodegradation perspective. J. Appl. Phycol. 2019, 31, 2999–3010. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Gao, B.; Yue, Q. Antioxidant responses and degradation of two antibiotic contaminants in Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2012, 86, 23–30. [Google Scholar] [CrossRef]
- Guo, J.; Selby, K.; Boxall, A. Effects of Antibiotics on the Growth and Physiology of Chlorophytes, Cyanobacteria, and a Diatom. Arch. Environ. Contam. Toxicol. 2016, 71, 589–602. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Bernier, S.P.; Surette, M.G. Concentration-dependent activity of antibiotics in natural environments. Front. Microbiol. 2013, 4, 20. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Yang, Z.; Yang, W.; Zhou, J.; Cen, K. Microstructure and antioxidative capacity of the microalgae mutant Chlorella PY-ZU1 during tilmicosin removal from wastewater under 15% CO2. J. Hazard. Mater. 2017, 324, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wan, L.; Zhang, W.; Ding, H.; Yang, W. Resistance of cyanobacteria Microcystis aeruginosa to erythromycin with multiple exposure. Chemosphere 2020, 249, 126147. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Du, Y.; Wang, L.; Qian, J.; Chen, J.; Wu, Q.; Hu, X. Toxin release of cyanobacterium Microcystis aeruginosa after exposure to typical tetracycline antibiotic contaminants. Toxins 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Liu, Y.; Zhang, J. Sulfamethoxazole and tetracycline induced alterations in biomass, photosynthesis, lipid productivity, and proteomic expression of Synechocystis sp. PCC 6803. Environ. Sci. Pollut. Res. 2020, 27, 30437–30447. [Google Scholar] [CrossRef]
- Le, V.V.; Tran, Q.-G.; Ko, S.-R.; Lee, S.-A.; Oh, H.-M.; Kim, H.-S.; Ahn, C.-Y. How do freshwater microalgae and cyanobacteria respond to antibiotics? Crit. Rev. Biotechnol. 2022, 21, 1–21. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Polyphasic chlorophyll alpha fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef]
- Ohad, N.; Hirschberg, J. Mutations in the D1 subunit of photosystem II distinguish between quinone and herbicide binding sites. Plant Cell 1992, 4, 273–282. [Google Scholar]
- Wilhelm, C.; Selmar, D. Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Nie, X.-P.; Liu, W.-Q.; Snoeijs, P.; Guan, C.; Tsui, M.T. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2011, 74, 1027–1035. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Pan, X.; Sun, Z.; Ye, C.; Jin, G.; Fu, Z. Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ. Toxicol. 2012, 27, 229–237. [Google Scholar] [CrossRef]
- Moro, I.; La Rocca, N.; Rascio, N. Photosynthetic apparatus in cyanobacteria and microalgae. In Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018; pp. 349–367. [Google Scholar]
- Jensen, P.E.; Bassi, R.; Boekema, E.J.; Dekker, J.P.; Jansson, S.; Leister, D.; Robinson, C.; Scheller, H.V. Structure, function and regulation of plant photosystem I. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 335–352. [Google Scholar] [CrossRef] [Green Version]
- Nickelsen, J.; Rengstl, B. Photosystem II assembly: From cyanobacteria to plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, G.; Singh, V.P.; Srivastava, P.K.; Kumar, S.; Prasad, S.M. High light intensity augments mercury toxicity in cyanobacterium Nostoc muscorum. Biol. Trace Elem. Res. 2012, 149, 262–272. [Google Scholar] [CrossRef]
- Deng, C.-N.; Zhang, D.-Y.; Pan, X.-L. Toxic effects of erythromycin on photosystem I and II in Microcystis aeruginosa. Photosynthetica 2014, 52, 574–580. [Google Scholar] [CrossRef]
- Williamson, A.K. Structural and functional aspects of the MSP (PsbO) and study of its differences in thermophilic versus mesophilic organisms. Photosynth. Res. 2008, 98, 365–389. [Google Scholar] [CrossRef]
- Montané, M.-H.; Kloppstech, K. The family of light-harvesting-related proteins (LHCs, ELIPs, HLIPs): Was the harvesting of light their primary function? Gene 2000, 258, 1–8. [Google Scholar] [CrossRef]
- Shimakawa, G.; Roach, T.; Krieger-Liszkay, A. Changes in photosynthetic electron transport during leaf senescence in two barley varieties grown in contrasting growth regimes. Plant Cell Physiol. 2020, 61, 1986–1994. [Google Scholar] [CrossRef]



| Probability | 95% Confidence Limits for TC Concentration (mg/L) | ||
|---|---|---|---|
| Estimate | Lower Bound | Upper Bound | |
| IC10 | 1.8 | 0.5 | 3.1 |
| IC30 | 4.1 | 2.1 | 6.2 |
| IC50 | 6.9 | 4.3 | 11.1 |
| Time (day) | Parameters | Treatment | |||
|---|---|---|---|---|---|
| Control | IC10 | IC30 | IC50 | ||
| 4 | Fv/Fm | 0.76 ± 0.00 a | 0.72 ± 0.00 b | 0.32 ± 0.02 c | 0.23 ± 0.00 d |
| Mo | 0.13 ± 0.01 a | 0.16 ± 0.00 b | 0.44 ± 0.01 c | 0.56 ± 0.04 d | |
| Sm | 307.0 ± 7.3 a | 332.0 ± 1.0 b | 947.7 ± 68.0 c | 1501.1 ± 18.6 d | |
| n | 224.9 ± 0.6 a | 265.8 ± 11.5 b | 1172.4 ± 100.1 c | 2005.2 ± 108.6 d | |
| ABS/RC | 0.97 ± 0.03 a | 1.11 ± 0.06 b | 3.82 ± 0.23 c | 5.72 ± 0.21 d | |
| TRO/RC | 0.73 ± 0.02 a | 0.80 ± 0.04 b | 1.24 ± 0.02 c | 1.34 ± 0.06 d | |
| ETO/RC | 0.60 ± 0.01 a | 0.64 ± 0.04 a | 0.80 ± 0.01 b | 0.77 ± 0.05 b | |
| DIO/RC | 0.24 ± 0.01 a | 0.31 ± 0.02 b | 2.58 ± 0.22 c | 4.38 ± 0.15 d | |
| 8 | Fv/Fm | 0.75 ± 0.00 a | 0.72 ± 0.00 b | 0.73 ± 0.01 b | 0.65 ± 0.03 c |
| Mo | 0.14 ± 0.01 a | 0.15 ± 0.00 a | 0.15 ± 0.01 a | 0.19 ± 0.02 b | |
| Sm | 121.9 ± 8.0 a | 118.4 ± 0.6 a | 363.1 ± 30.8 b | 304.8 ± 107.7 b | |
| n | 82.1 ± 5.4 a | 79.2 ± 0.14 a | 264.1 ± 34.0 b | 255.6 ± 119.9 b | |
| ABS/RC | 0.93 ± 0.00 a | 0.93 ± 0.00 a | 1.00 ± 0.05 b | 1.31 ± 0.13 c | |
| TRO/RC | 0.67 ± 0.00 a | 0.67 ± 0.00 a | 0.73 ± 0.03 a | 0.85 ± 0.05 b | |
| ETO/RC | 0.53 ± 0.00 a | 0.52 ± 0.00 a | 0.58 ± 0.03 b | 0.66 ± 0.04 c | |
| DIO/RC | 0.25 ± 0.00 a | 0.26 ± 0.00 a | 0.27 ± 0.02 a | 0.46 ± 0.08 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Gu, G.; Wang, Z.; Ou, D.; Liang, X.; Hu, C.; Li, X. Effects of Tetracycline on Scenedesmus obliquus Microalgae Photosynthetic Processes. Int. J. Mol. Sci. 2022, 23, 10544. https://doi.org/10.3390/ijms231810544
Chen Z, Gu G, Wang Z, Ou D, Liang X, Hu C, Li X. Effects of Tetracycline on Scenedesmus obliquus Microalgae Photosynthetic Processes. International Journal of Molecular Sciences. 2022; 23(18):10544. https://doi.org/10.3390/ijms231810544
Chicago/Turabian StyleChen, Zhehua, Gan Gu, Ziyun Wang, Dong Ou, Xianrui Liang, Changwei Hu, and Xi Li. 2022. "Effects of Tetracycline on Scenedesmus obliquus Microalgae Photosynthetic Processes" International Journal of Molecular Sciences 23, no. 18: 10544. https://doi.org/10.3390/ijms231810544
APA StyleChen, Z., Gu, G., Wang, Z., Ou, D., Liang, X., Hu, C., & Li, X. (2022). Effects of Tetracycline on Scenedesmus obliquus Microalgae Photosynthetic Processes. International Journal of Molecular Sciences, 23(18), 10544. https://doi.org/10.3390/ijms231810544






