Water-Recyclable Chitosan-Based Ion-Imprinted Thermoresponsive Hydrogel for Rare Earth Metal Ions Accumulation
Abstract
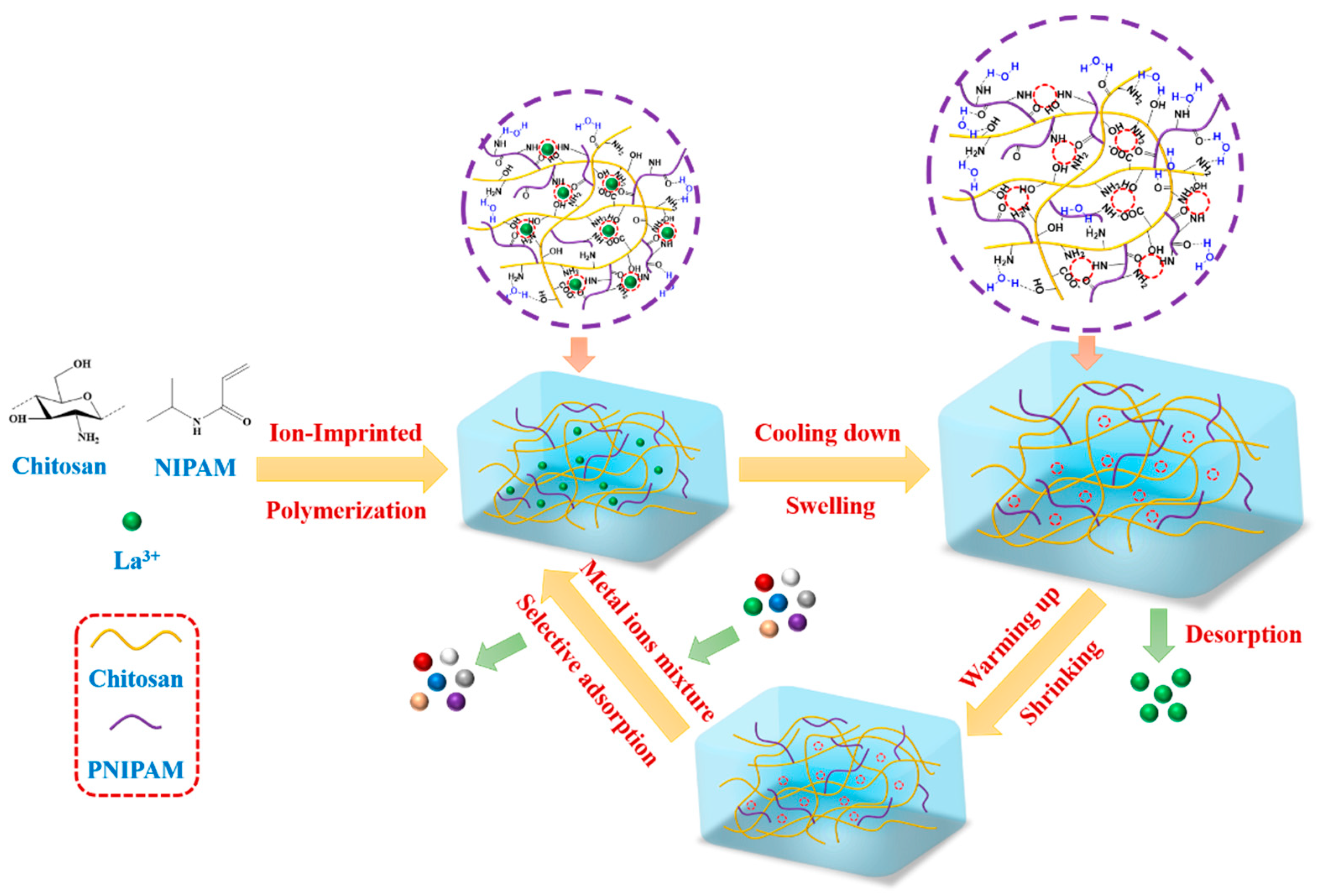
:1. Introduction
2. Results and Discussion
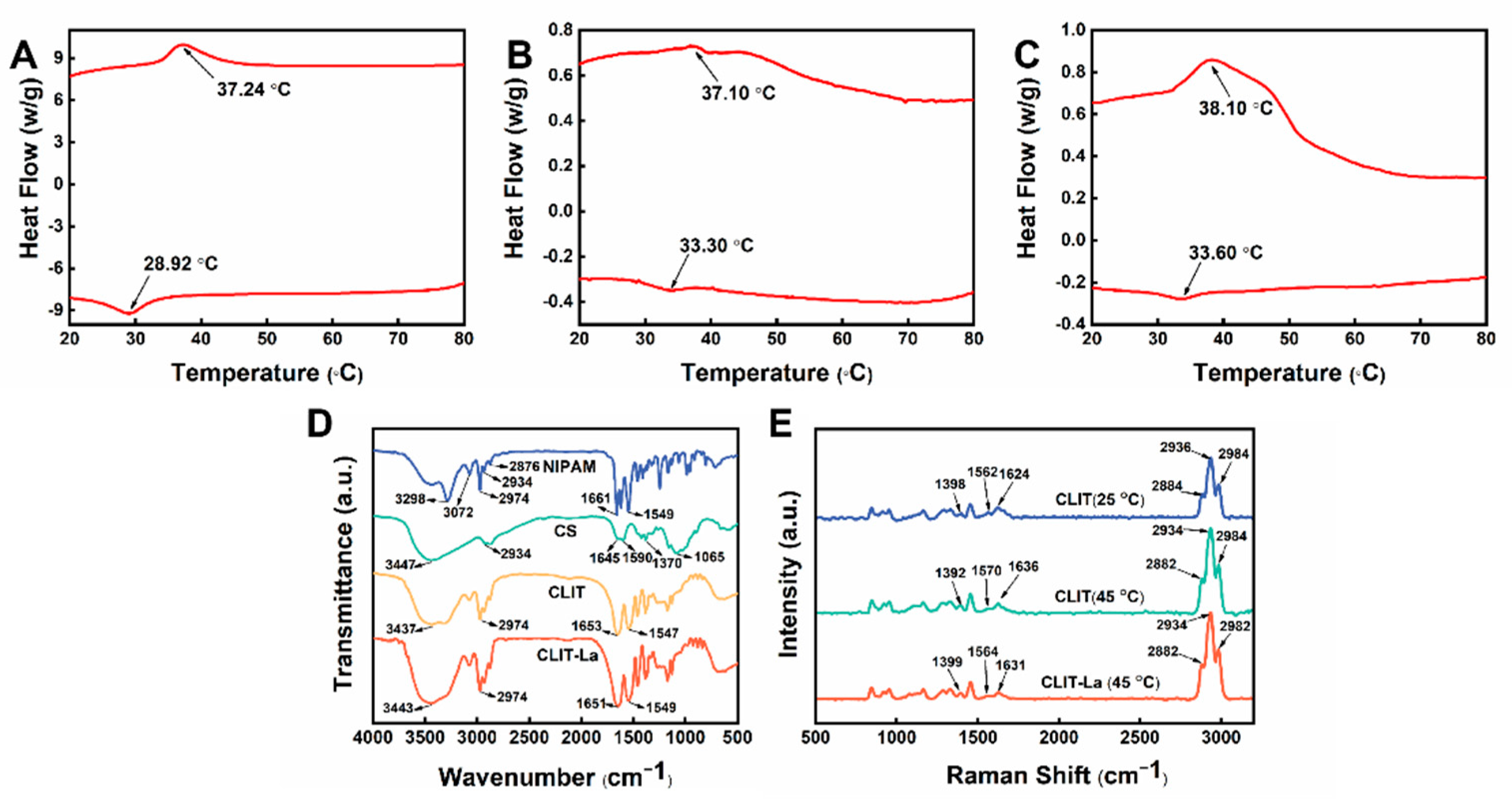
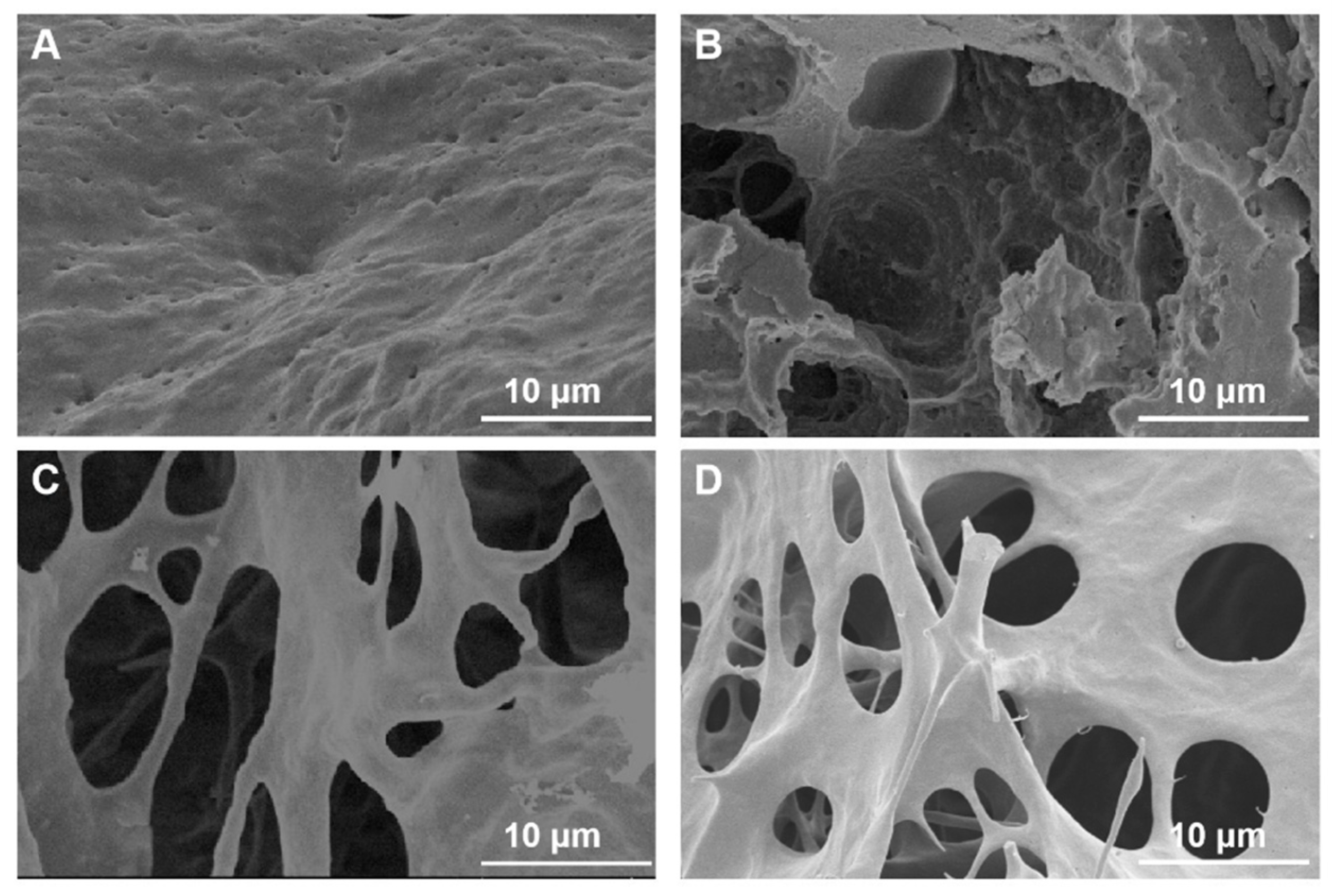
2.1. Characterization of CLIT
2.2. Adsorption Performance of CLIT
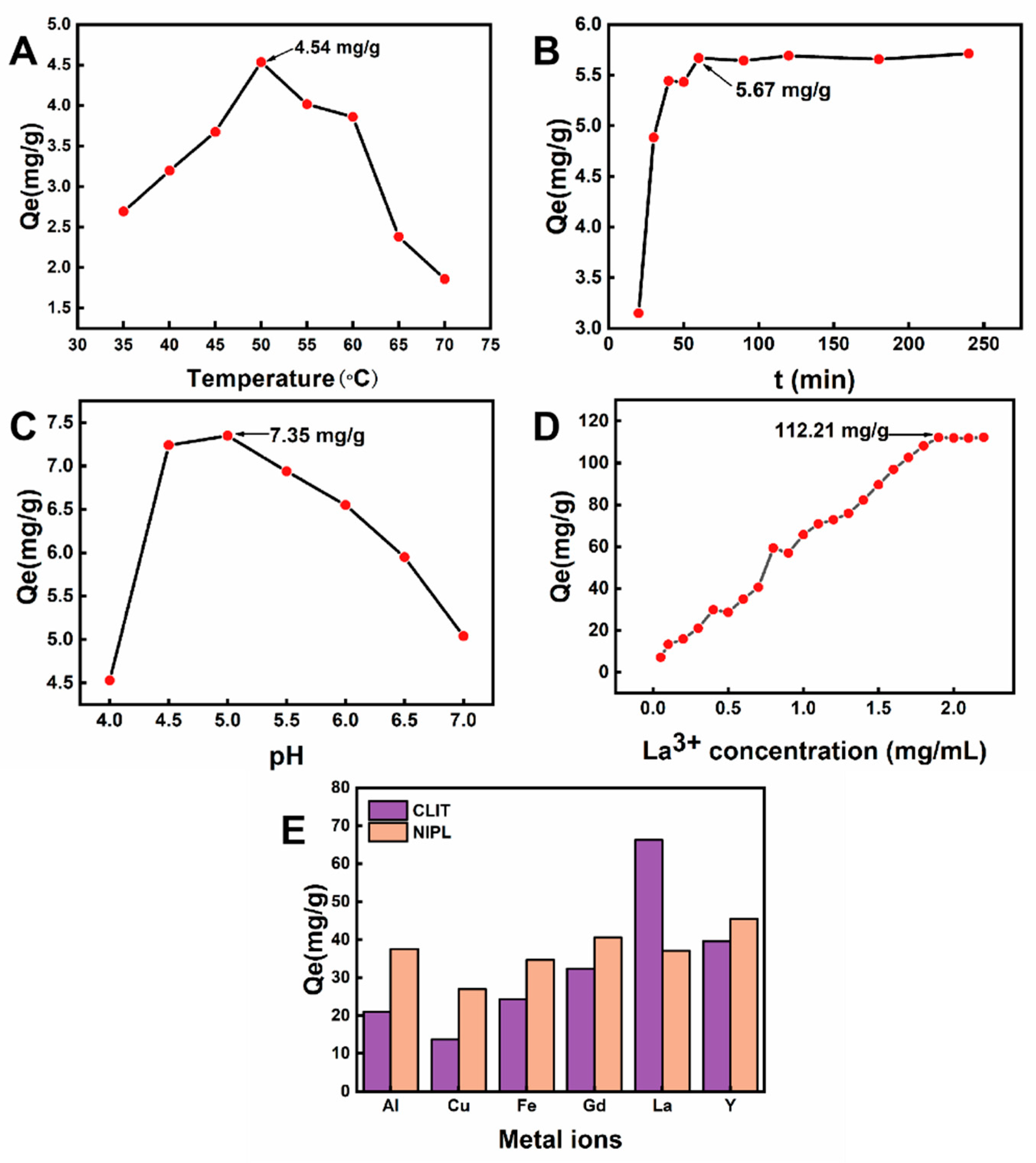
2.2.1. Effects of Adsorption Temperature
2.2.2. Effects of Adsorption Time
2.2.3. Effects of pH Value of La3+ Solution
2.2.4. Effects of Initial La3+ Concentration
2.3. Adsorption Selectivity of CLIT
2.4. Adsorption Mechanism of CLIT
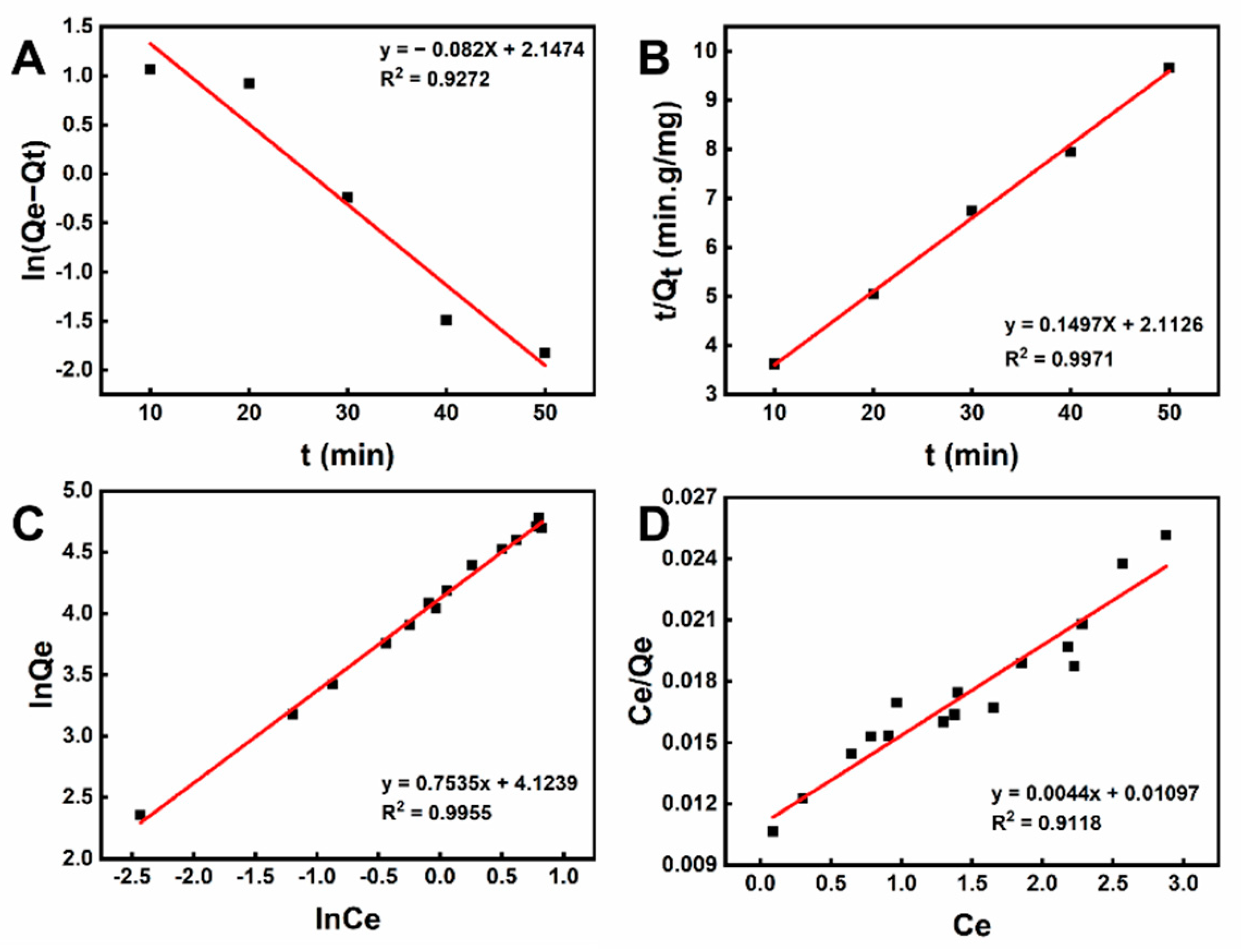
2.4.1. Kinetics Studies
2.4.2. Adsorption Isotherms
2.5. Desorption Performance of CLIT
2.6. Regeneration Performance of CLIT
3. Materials and Methods
3.1. Materials
3.2. Preparation of CLIT
3.3. Characterizations
3.4. La3+ Adsorption Performance and Mechanism of CLIT
3.5. Desorption and Reusability of CLIT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereao, O.; Bode-Aluko, C.; Fatoba, O.; Laatikainen, K.; Petrik, L.J. Rare earth elements removal techniques from water/wastewater: A review. Desalination Water Treat. 2018, 130, 71–86. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Royer-Lavallee, A.; Neculita, C.M.; Coudert, L. Removal and potential recovery of rare earth elements from mine water. J. Ind. Eng. Chem. 2020, 89, 47–57. [Google Scholar] [CrossRef]
- Chen, S.; Yan, J.; Li, J.; Lu, D.J. Magnetic ZnFe2O4 nanotubes for dispersive micro solid-phase extraction of trace rare earth elements prior to their determination by ICP-MS. Mikrochim. Acta 2019, 186, 228. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Debeli, D.K.; Shan, G.; Pan, P. Selective adsorption and high recovery of La3+ using graphene oxide/poly (N-isopropyl acrylamide-maleic acid) cryogel. Chem. Eng. J. 2020, 379, 122335. [Google Scholar] [CrossRef]
- da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of rare-earth metals from aqueous solutions by bio/adsorption using non-conventional materials: A review with recent studies and promising approaches in column applications. J. Rare Earths 2020, 38, 339–355. [Google Scholar] [CrossRef]
- Karshigina, Z.; Abisheva, Z.; Bochevskaya, Y.; Akcil, A.; Sargelova, E.; Sukurov, B.; Silachyov, I. Recovery of rare earth metals (REMs) from primary raw material: Sulphatization-leaching-precipitation-extraction. Miner. Process. Extr. Met. Rev. 2018, 39, 319–338. [Google Scholar] [CrossRef]
- Belova, V.J. Tendencies in Application of Ionic Liquids and Binary Extractants in Extraction and Separation of Lanthanides and Actinides. Radiochemistry 2021, 63, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Koivula, R.; Wiikinkoski, E.; Xu, J.; Hietala, S.; Lehto, J.; Harjula, R. Efficient and selective recovery of trace scandium by inorganic titanium phosphate ion-exchangers from leachates of waste bauxite residue. ACS Sustain. Chem. Eng. 2017, 5, 3103–3114. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Chapter 1—Fundamentals of Adsorption Technology. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 1–70. [Google Scholar]
- Han, P.; Li, Z.; Wei, X.; Tang, L.; Li, M.; Liang, Z.; Yin, X.; Wei, S. Ion-imprinted thermosensitive chitosan derivative for heavy metal remediation. Carbohydr. Polym. 2020, 248, 116732. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Chapter 2—Adsorbent. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 71–210. [Google Scholar]
- Cui, J.; Wang, Q.; Gao, J.; Guo, Y.; Cheng, F. The selective adsorption of rare earth elements by modified coal fly ash based SBA-15. Chin. J. Chem. Eng. 2022, 47, 155–164. [Google Scholar] [CrossRef]
- Liao, C.; Liu, Y.-P.; Ren, H.; Jiang, X.-Y.; Yu, J.-G.; Chen, X.-Q. Rational assembly of GO-based heterocyclic sulfur- and nitrogen-containing aerogels and their adsorption properties toward rare earth elementals. J. Hazard. Mater. 2021, 419, 126484. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zou, J.; Teng, J.; Liu, Q.; Jiang, X.-Y.; Jiao, F.-P.; Yu, J.-G.; Chen, X.-Q. Novel high-gluten flour physically cross-linked graphene oxide composites: Hydrothermal fabrication and adsorption properties for rare earth ions. Ecotoxicol. Environ. Saf. 2018, 166, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, L.; Liao, S.; Liao, X.; Liu, J.; Mao, J.; Chen, Y.; Qiu, T.; Ren, S. Highly efficient enrichment and adsorption of rare earth ions (yttrium (III)) by recyclable magnetic nitrogen functionalized mesoporous expanded perlite. Chin. Chem. Lett. 2020, 31, 2849–2853. [Google Scholar] [CrossRef]
- Yanfei, X.; Huang, L.; Zhiqi, L.; Zongyu, F.; Liangshi, W. Adsorption ability of rare earth elements on clay minerals and its practical performance. J. Rare Earths 2016, 34, 543–548. [Google Scholar]
- Li, K.; Gao, Q.; Yadavalli, G.; Shen, X.; Lei, H.; Han, B.; Xia, K.; Zhou, C. Selective Adsorption of Gd3+ on a Magnetically Retrievable Imprinted Chitosan/Carbon Nanotube Composite with High Capacity. ACS Appl. Mater. Interfaces 2015, 7, 21047–21055. [Google Scholar] [CrossRef]
- Jumadilov, T.; Totkhuskyzy, B.; Malimbayeva, Z.; Kondaurov, R.; Imangazy, A.; Khimersen, K.; Grazulevicius, J. Impact of Neodymium and Scandium Ionic Radii on Sorption Dynamics of Amberlite IR120 and AB-17-8 Remote Interaction. Materials 2021, 14, 5402. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Hammouda, S.B.; Sillanpää, M. Fabrication of novel metal ion imprinted xanthan gum-layered double hydroxide nanocomposite for adsorption of rare earth elements. Carbohydr. Polym. 2018, 194, 274–284. [Google Scholar] [CrossRef]
- Nik Mustapa, N.R.; Malek, N.F.A.; Yusoff, M.M.; Rahman, M.L. Ion imprinted polymers for selective recognition and separation of lanthanum and cerium ions from other lanthanide. Sep. Sci. Technol. 2016, 51, 2762–2771. [Google Scholar] [CrossRef]
- Shi, M.; Lu, T.; Li, X.; Yang, Y. Preparation and properties of GO-based lanthanum ion-imprinted polymer, La-IIP-MAA/Fe3O4-GO. J. Rare Earths 2020, 40, 135–142. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.; Youssef, I. Preparation of ruthenium (III) ion-imprinted beads based on 2-pyridylthiourea modified chitosan. J. Colloid Interface Sci. 2018, 513, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xue, K.; Loh, X. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, Y.; Ju, B.; Tian, Y. Preparation of thermoresponsive alginate/starch ether composite hydrogel and its application to the removal of Cu(II) from aqueous solution. Bioresour. Technol. 2019, 294, 122192. [Google Scholar] [CrossRef]
- Zidan, T.; Abdelhamid, A.E.; Zaki, E. N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions. Int. J. Biol. Macromol. 2020, 158, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Tong, X.; Dai, K.; Xu, Q. A super-stretchable and tough functionalized boron nitride/PEDOT: PSS/poly (N-isopropylacrylamide) hydrogel with self-healing, adhesion, conductive and photothermal activity. J. Mater. Chem. A 2019, 7, 8204–8209. [Google Scholar] [CrossRef]
- Franco, S.; Buratti, E.; Nigro, V.; Bertoldo, M.; Ruzicka, B.; Angelini, R. Thermal Behaviour of Microgels Composed of Interpenetrating Polymer Networks of Poly(N-isopropylacrylamide) and Poly(acrylic acid): A Calorimetric Study. Polymers 2022, 14, 115. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, S.; Gao, Y.; Cui, X. Thermo-responsive poly(N-isopropylacrylamide)-hyaluronic acid nano-hydrogel and its multiple applications. Int. J. Biol. Macromol. 2022, 194, 811–818. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Yang, D.; Qin, X.; Gao, H.; Bai, X.; Wen, S. Application of a new depressant dithiocarbamate chitosan in separation of chalcopyrite and molybdenite. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 634, 127920. [Google Scholar] [CrossRef]
- Pedrali, D.; Barbarito, S.; Lavelli, V. Encapsulation of grape seed phenolics from winemaking byproducts in hydrogel microbeads–Impact of food matrix and processing on the inhibitory activity towards α-glucosidase. LWT 2020, 133, 109952. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Tun, T.; Liu, S.; Hu, J.; Zhou, G. Cu(II) and Cd(II) capture using novel thermosensitive hydrogel microspheres: Adsorption behavior study and mechanism investigation. J. Chem. Technol. Biotechnol. 2021, 96, 2382–2389. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Moskalets, A.P.; Dubovik, A.S.; Plashchina, I.G.; Khokhlov, A.R. Energetics and Mechanisms of poly(N-isopropylacrylamide) Phase Transitions in Water–Methanol Solutions. Macromolecules 2020, 53, 10765–10772. [Google Scholar] [CrossRef]
- Wu, N.; Pan, C.; Zhang, B.; Rao, Y.; Yu, D. Preparation and properties of a thermo-sensitive hydrogel based on oxidized sodium alginate. Acta Polym. Sin. 2007, 6, 497. [Google Scholar] [CrossRef]
- Mirkin, N.G.; Krimm, S. Structural Dependence of NH Stretch Mode Frequency Shifts in Amide and Peptide Systems. J. Phys. Chem. A 2004, 108, 5438–5448. [Google Scholar] [CrossRef]
- Bai, W.; Fan, L.; Zhou, Y.; Zhang, Y.; Shi, J.; Lv, G.; Wu, Y.; Liu, Q.; Song, J. Removal of Cd2+ions from aqueous solution using cassava starch-based superabsorbent polymers. J. Appl. Polym. Sci. 2017, 134, 17. [Google Scholar] [CrossRef]
- Chen, L.; Dai, J.; Hu, B.; Wang, J.; Wu, Y.; Dai, J.; Meng, M.; Li, C.; Yan, Y. Recent Progresses on the Adsorption and Separation of Ions by Imprinting Routes. Sep. Purif. Rev. 2020, 49, 265–293. [Google Scholar] [CrossRef]
- Zhang, H.; Omer, A.; Hu, Z.; Yang, L.-Y.; Ji, C.; Ouyang, X.-K. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef]
- Baghbadorani, N.B.; Behzad, T.; Etesami, N.; Heidarian, P. Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly(acrylic acid) superadsorbent hydrogels. Compos. Part B Eng. 2019, 176, 107084. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Li, W.; Zheng, X.; Chen, Y.; Wang, Q. Selective Adsorption of La3+ Using a Tough Alginate-Clay-Poly(n-isopropylacrylamide) Hydrogel with Hierarchical Pores and Reversible Re-Deswelling/Swelling Cycles. ACS Sustain. Chem. Eng. 2016, 4, 6732–6743. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Li, L.; Guo, W.; Xing, J.; Wang, H.; Hu, X.; Lyu, W.; Chen, R.; Song, J.; et al. Novel talc encapsulated lanthanum alginate hydrogel for efficient phosphate adsorption and fixation. Chemosphere 2020, 256, 127124. [Google Scholar] [CrossRef]
- Li, Z.; Tian, H.; Yuan, Y.; Yin, X.; Wei, X.; Tang, L.; Wei, S. Metal-ion-imprinted thermo-responsive materials obtained from bacterial cellulose: Synthesis, characterization, and adsorption evaluation. J. Mater. Chem. A 2019, 7, 11742–11755. [Google Scholar] [CrossRef]
- Li, X.; Yang, M.; Shi, X.; Chu, X.; Chen, L.; Wu, Q.; Wang, Y. Effect of the intramolecular hydrogen bond on the spectral and optical properties in chitosan oligosaccharide. Phys. E Low-Dimens. Syst. Nanostructures 2015, 69, 237–242. [Google Scholar] [CrossRef]
- Qi, Z.W.; Xi, G.C.; Nan, L.; Shi, X.W.; Cheng, S.L.; Xiang, H.M.; Chen, G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.M.; Mostapa, N.R.N.; Sarkar, M.S.; Biswas, T.K.; Rahman, M.L.; Arshad, S.E.; Sarjadi, M.S.; Kulkarni, A.D. Synthesis of ion imprinted polymers for selective recognition and separation of rare earth metals. J. Rare Earths 2017, 35, 177–186. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Jia, G.; Tang, X.; Xu, J. Synthesis of hydrochar supported zero-valent iron composites through hydrothermal carbonization of granatum and zero-valent iron: Potential applications for Pb2+ removal. Water Sci. Technol. 2021, 84, 1873–1884. [Google Scholar] [CrossRef]
- Obilko, V.; Spasonova, L.; Kovalchuk, I.; Kornilovych, B.; Kholodko, Y. Adsorption of Uranium (VI) from Aqueous Solutions by Amino-functionalized Clay Minerals. Colloids Interfaces 2019, 3, 41. [Google Scholar] [CrossRef]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Equilibrium and kinetic studies for silver removal from aqueous solution by hybrid hydrogels. J. Hazard. Mater. 2019, 365, 237–244. [Google Scholar] [CrossRef]
- Han, D.; Lu, Z.; Chester, S.A.; Lee, H. Micro 3D Printing of a Temperature-Responsive Hydrogel Using Projection Micro-Stereolithography. Sci. Rep. 2018, 8, 1963. [Google Scholar] [CrossRef]
- Xiaogang, L.; Yuwen, C.; Xiu, W.; Minglei, T.; Xiancai, L.; Yifeng, Y. Preparation and Adsorption Properties of Lanthanum Ion MCM-41 Imprinted Polymers. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1483–1489. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Shi, M.; Huang, Y.; Liu, X.; Li, X. Preparation and Adsorption Properties of Lanthanide Ion Surface-Imprinted Polymer Based on Reaming MCM-41. J. Inorg. Organomet. Polym. Mater. 2021, 32, 161–168. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Li, J. Straw-supported ion imprinted polymer sorbent prepared by surface imprinting technique combined with AGET ATRP for selective adsorption of La 3+ ions. Chem. Eng. J. 2016, 293, 24–33. [Google Scholar] [CrossRef]
- Ni, C.; Liu, Q.; Ren, Z.; Hu, H.; Sun, B.; Liu, C.; Shao, P.; Yang, L.; Pavlostathis, S.G.; Luo, X. Selective removal and recovery of La (III) using a phosphonic-based ion imprinted polymer: Adsorption performance, regeneration, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106701. [Google Scholar] [CrossRef]
- Jančář, L.; Slezáčková, B.; Sommer, L. Spectrophotometric determination of trace uranium with Eriochrome Azurol B and Chrome Azurol S in the presence of the cationic surfactant Septonex. Talanta 1989, 36, 549–555. [Google Scholar] [CrossRef]
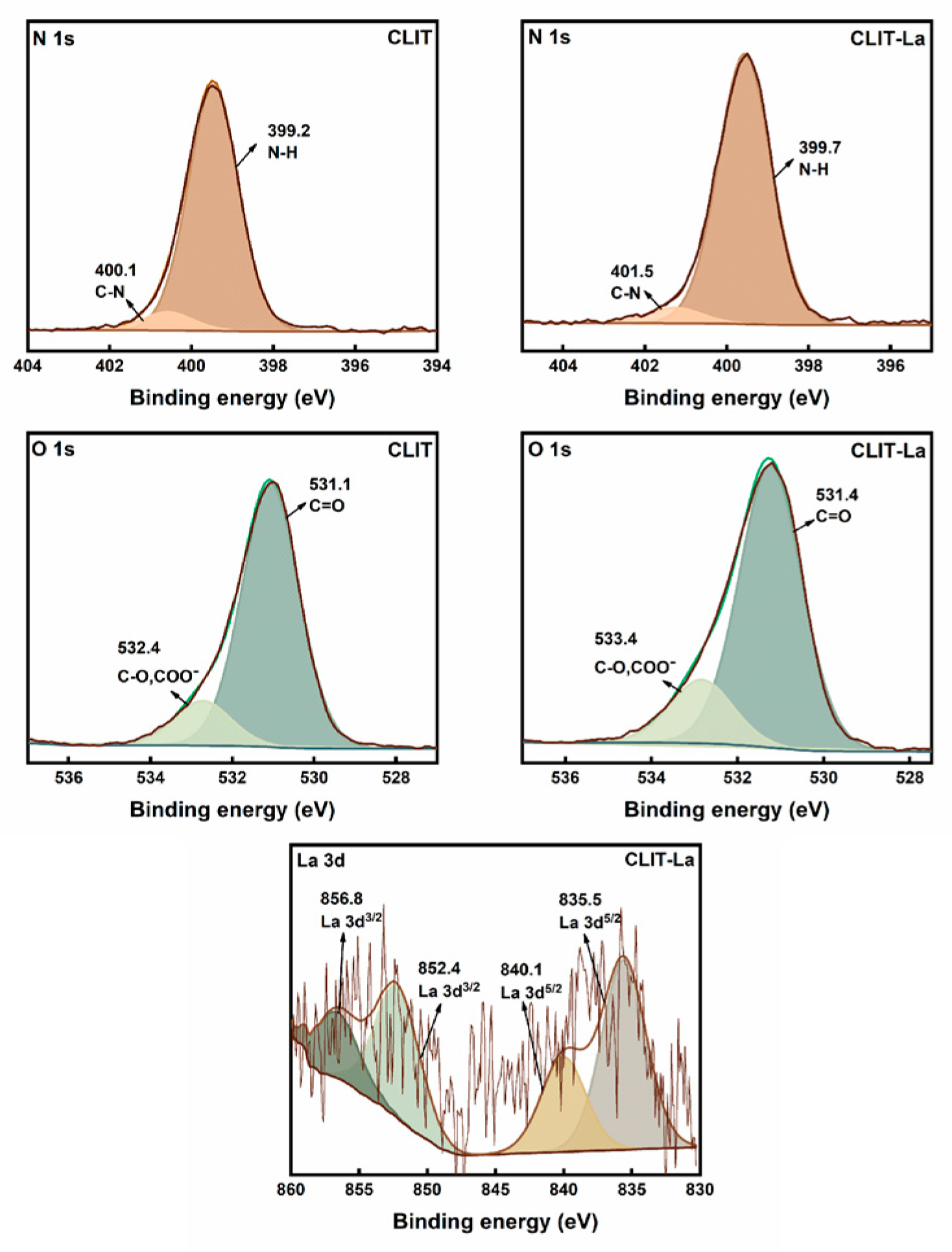

| Samples | N1s (eV) | O1s (eV) | La3d3/2 (eV) | La3d5/2 (eV) | ||||
|---|---|---|---|---|---|---|---|---|
| C-N | N-H | C-O, COO− | C=O | |||||
| CLIT | 400.1 | 399.2 | 532.4 | 531.1 | - | - | ||
| CLIT-La | 401.5 | 399.7 | 533.4 | 531.4 | 852.4 | 856.8 | 835.5 | 840.1 |
| Samples | kLa3+/Al3+ | kLa3+/Cu2+ | kLa3+/Fe3+ | kLa3+/Gd3+ | kLa3+/Y3+ |
|---|---|---|---|---|---|
| CLIT | 3.61 | 5.66 | 3.09 | 2.27 | 1.83 |
| NIPL | 0.99 | 1.42 | 1.08 | 0.91 | 0.80 |
| Sample | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| Kf (L/mg) | n | Rf2 | Kl (L/mg) | Qm (mg/g) | Rl2 | |
| CLIT | 2.0662 | 1.4914 | 0.9955 | 0.7713 | 130.01 | 0.9118 |
| La3+ Ion-Imprinted Adsorbent | Adsorption Capacity (mg/g) | Regeneration Times | Regeneration Efficiency | Eluents | Ref. |
|---|---|---|---|---|---|
| La-IIP-Schiff | 25.0 | 10 cycles | About 92% | HCl | [21] |
| La-IIP-Azo | 24.3 | ||||
| La (III)-IIP-PEI/MCM-41 | 212.69 | Not reported | Not reported | HCl | [53] |
| La-IIP/MCM-41 | 272.2 | 5 cycles | About 81% | HCl | [54] |
| La-IIP-MAA/Fe3O4-GO | 110.98 | 5 cycles | About 90% | HCl | [22] |
| La-IIP | 62.8 | 5 cycles | About 100% | HCl | [56] |
| CLIT | 112.21 | 10 cycles | About 83% | deionized water | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Ding, K.; Tang, L.; Qin, Z.; Li, M.; Yin, X. Water-Recyclable Chitosan-Based Ion-Imprinted Thermoresponsive Hydrogel for Rare Earth Metal Ions Accumulation. Int. J. Mol. Sci. 2022, 23, 10542. https://doi.org/10.3390/ijms231810542
Qiu Y, Ding K, Tang L, Qin Z, Li M, Yin X. Water-Recyclable Chitosan-Based Ion-Imprinted Thermoresponsive Hydrogel for Rare Earth Metal Ions Accumulation. International Journal of Molecular Sciences. 2022; 23(18):10542. https://doi.org/10.3390/ijms231810542
Chicago/Turabian StyleQiu, Yuheng, Kaiqi Ding, Liwen Tang, Ziyu Qin, Mengting Li, and Xueqiong Yin. 2022. "Water-Recyclable Chitosan-Based Ion-Imprinted Thermoresponsive Hydrogel for Rare Earth Metal Ions Accumulation" International Journal of Molecular Sciences 23, no. 18: 10542. https://doi.org/10.3390/ijms231810542
APA StyleQiu, Y., Ding, K., Tang, L., Qin, Z., Li, M., & Yin, X. (2022). Water-Recyclable Chitosan-Based Ion-Imprinted Thermoresponsive Hydrogel for Rare Earth Metal Ions Accumulation. International Journal of Molecular Sciences, 23(18), 10542. https://doi.org/10.3390/ijms231810542







