Shrimp Lipid Droplet Protein Perilipin Involves in the Pathogenesis of AHPND-Causing Vibrio parahaemolyticus
Abstract
:1. Introduction
2. Results
2.1. Sequence and Phylogenetic Analysis of LvPerilipin
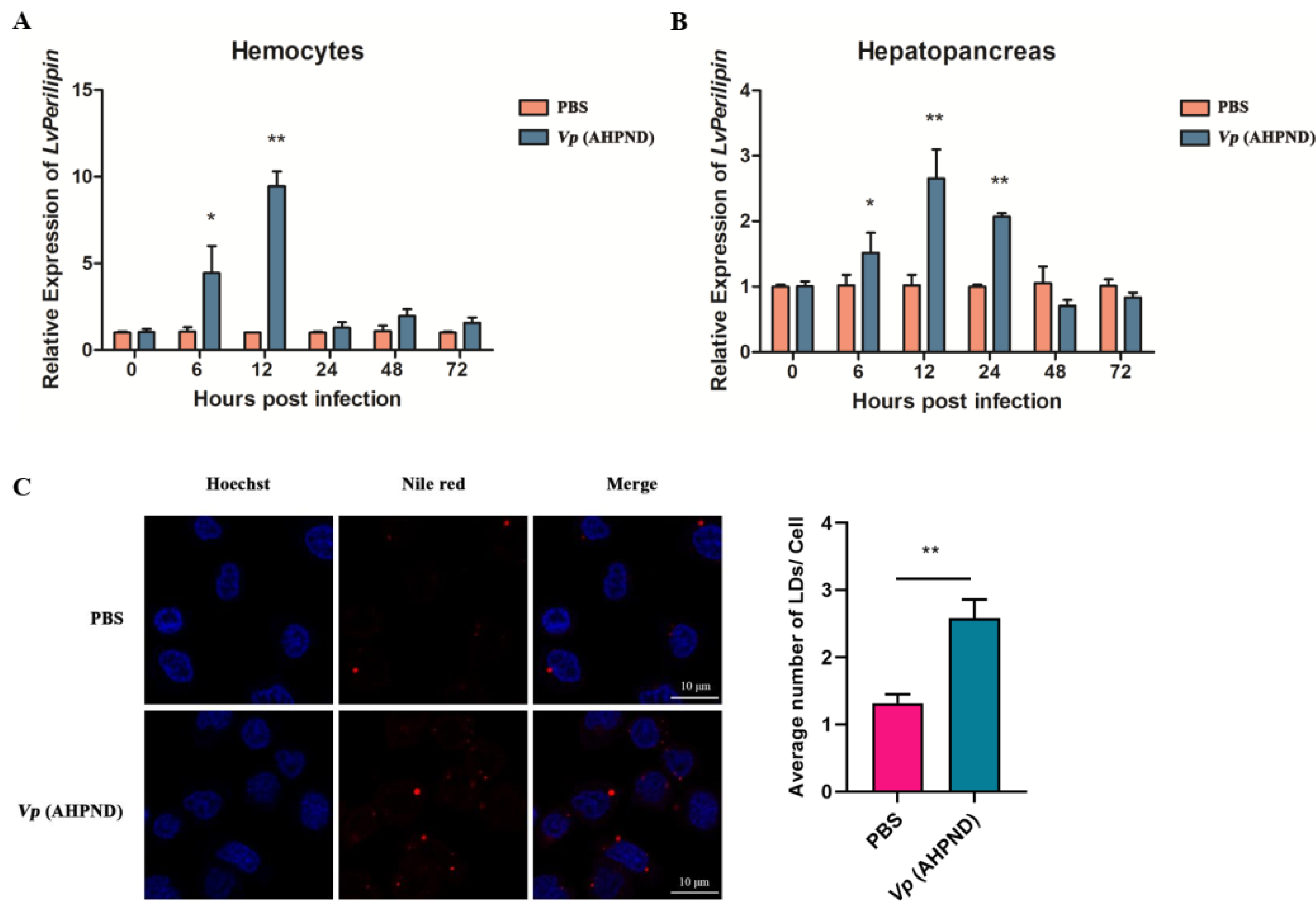
2.2. Vp (AHPND) Infection Up-Regulates LvPerilipin Expression and Increases LDs Biogenesis in Shrimp Hemocytes
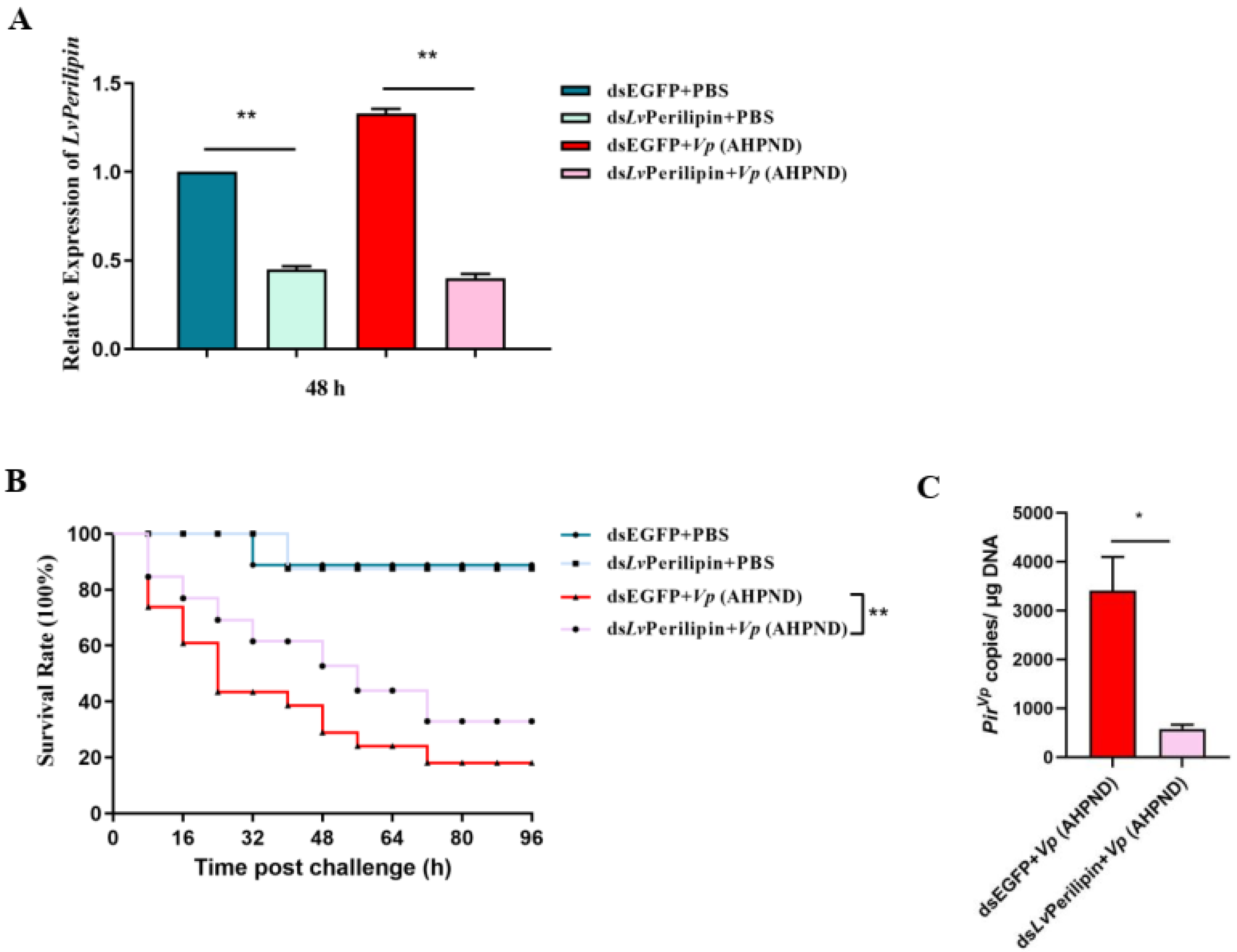
2.3. LvPerilipin Promotes Vp (AHPND) Infection and Shrimp Mortality
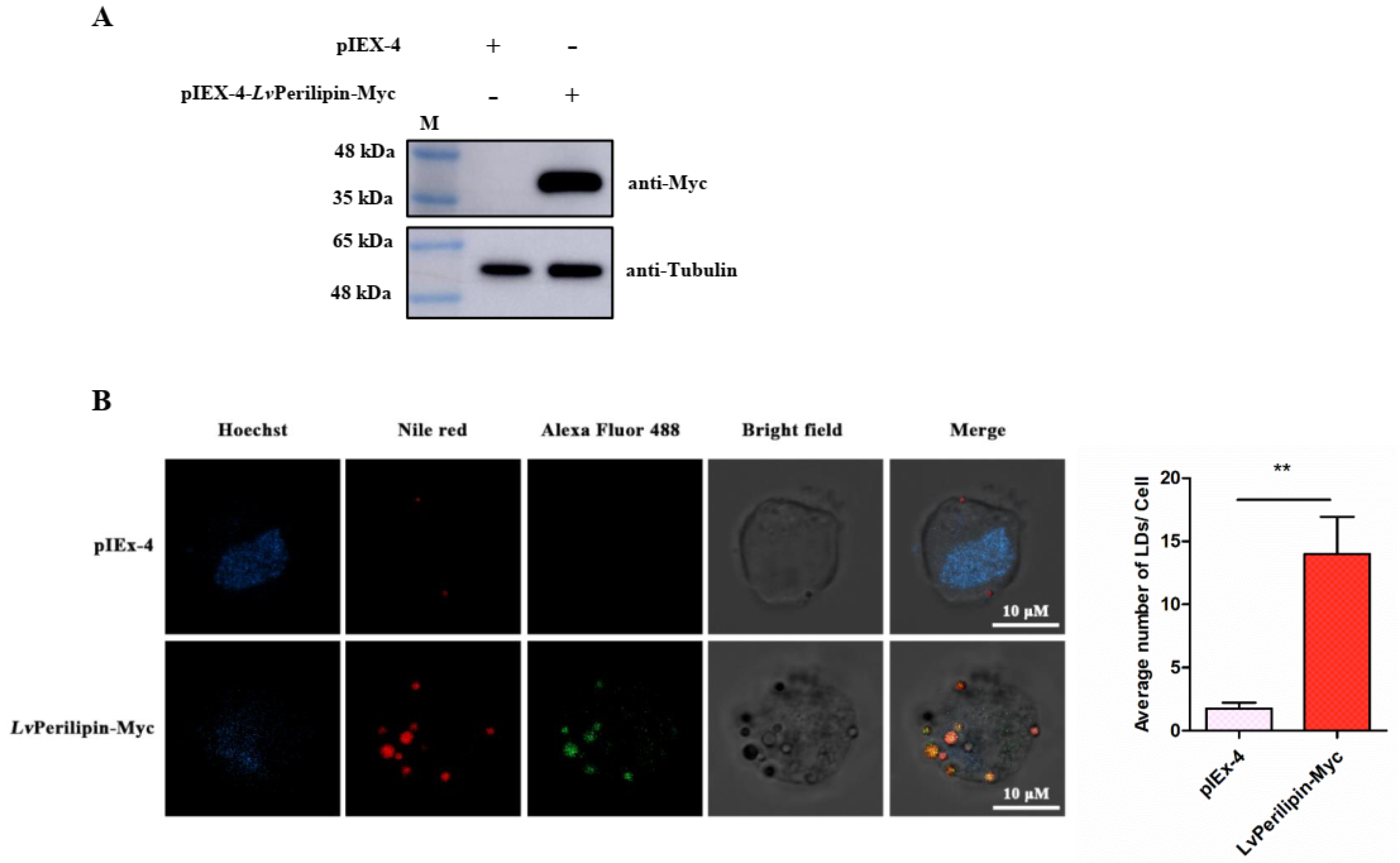
2.4. LvPerilipin Is Located on the Surface of LDs and Promotes LDs Biogenesis
2.5. LvPerilipin Inhibits ROS Production While Vp (AHPND) Infection
3. Discussion
4. Materials and Methods
4.1. Shrimp Culture and Bacteria Challenge
4.2. Total RNA Extraction, cDNA Synthesis, and Genomic DNA Extraction
4.3. Sequence and Phylogenetic Analysis
4.4. RNAi Assay
4.5. qRT-PCR
4.6. Plasmid Construction and Cell Transfection
4.7. Western Blotting Analysis
4.8. Immunofluorescence Analysis
4.9. Lipid Droplets Marking and Observation in Shrimp Hemocytes
4.10. ROS Detection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Roy, S.; Behera, B.K.; Bossier, P.; Das, B.K. Acute hepatopancreatic necrosis disease (AHPND): Virulence, pathogenesis and mitigation strategies in shrimp aquaculture. Toxins 2021, 13, 524. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Organ. 2013, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, L.D.; Cabillon, N.A.; Catedral, D.D.; Amar, E.C.; Usero, R.C.; Monotilla, W.D.; Calpe, A.T.; Fernandez, D.D.; Saloma, C.P. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Dis. Aquat. Organ. 2015, 116, 251–254. [Google Scholar] [CrossRef]
- Aranguren Caro, L.F.; Mai, H.N.; Noble, B.; Dhar, A.K. Acute hepatopancreatic necrosis disease (VPAHPND), a chronic disease in shrimp (Penaeus vannamei) population raised in latin America. J. Invertebr. Pathol. 2020, 174, 107424. [Google Scholar] [CrossRef]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci. Rep. 2017, 7, 42177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ng, T.H.; Wang, H.C. Acute hepatopancreatic necrosis disease in penaeid shrimp. Rev. Aquac. 2020, 12, 1867–1880. [Google Scholar] [CrossRef]
- Kumar, R.; Ng, T.H.; Chang, C.C.; Tung, T.C.; Lin, S.S.; Lo, C.F.; Wang, H.C. Bile acid and bile acid transporters are involved in the pathogenesis of acute hepatopancreatic necrosis disease in white shrimp Litopenaeus vannamei. Cell. Microbiol. 2020, 22, e13127. [Google Scholar] [CrossRef]
- Kumar, V.; De Bels, L.; Couck, L.; Baruah, K.; Bossier, P.; Van den Broeck, W. PirABVP toxin binds to epithelial cells of the digestive tract and produce pathognomonic AHPND lesions in germ-free brine shrimp. Toxins 2019, 11, 717. [Google Scholar] [CrossRef]
- Lai, H.C.; Ng, T.H.; Ando, M.; Lee, C.T.; Chen, I.T.; Chuang, J.C.; Mavichak, R.; Chang, S.H.; Yeh, M.D.; Chiang, Y.A.; et al. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish Shellfish Immunol. 2015, 47, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, R.; Aweya, J.J.; Yao, D.; Wang, F.; Li, S.; Tuan, T.N.; Zhang, Y. The PirB toxin protein from Vibrio parahaemolyticus induces apoptosis in hemocytes of Penaeus vannamei. Virulence 2021, 12, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.; Lu, C.-W.; Lin, S.-S.; Chang, C.-C.; Tran, L.H.; Chang, W.-C.; Lo, C.-F.; Wang, H.-C. The Rho signalling pathway mediates the pathogenicity of AHPND-causing V. parahaemolyticus in shrimp. Cell. Microbiol. 2018, 20, e12849. [Google Scholar] [CrossRef]
- Kumar, R.; Tung, T.C.; Ng, T.H.; Chang, C.C.; Chen, Y.L.; Chen, Y.M.; Lin, S.S.; Wang, H.C. Metabolic alterations in shrimp stomach during acute hepatopancreatic necrosis disease and effects of taurocholate on Vibrio parahaemolyticus. Front. Microbiol. 2021, 12, 631468. [Google Scholar] [CrossRef]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Londos, C.; Sztalryd, C.; Tansey, J.T.; Kimmel, A.R. Role of PAT proteins in lipid metabolism. Biochimie 2005, 87, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gong, J.; Wu, H.; Xu, W.; Wu, L.; Xu, D.; Gao, J.; Wu, J.W.; Yang, H.; Yang, M.; et al. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun. 2013, 4, 1594. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, R.E.; Ramos, S.V.; Vandenboom, R.; Roy, B.D.; Peters, S.J. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R644–R650. [Google Scholar] [CrossRef]
- Chen, F.L.; Yang, Z.H.; Wang, X.C.; Liu, Y.; Yang, Y.H.; Li, L.X.; Liang, W.C.; Zhou, W.B.; Hu, R.M. Adipophilin affects the expression of TNF-alpha, MCP-1, and IL-6 in THP-1 macrophages. Mol. Cell. Biochem. 2010, 337, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tan, Y.; Chen, L.; Liu, Y.; Ren, Z. Reactive oxygen species induces lipid droplet accumulation in HepG2 cells by increasing perilipin 2 expression. Int. J. Mol. Sci. 2018, 19, 3445. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy 2016, 12, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell. Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.D.; Noland, R.C.; Hebert, R.C.; Masinter, B.S.; Smith, S.R.; Rustan, A.C.; Ravussin, E.; Bajpeyi, S. Perilipin 3 differentially regulates skeletal muscle lipid oxidation in active, sedentary, and type 2 diabetic males. J. Clin. Endocrinol. Metab. 2015, 100, 3683–3692. [Google Scholar] [CrossRef]
- Covington, J.D.; Galgani, J.E.; Moro, C.; LaGrange, J.M.; Zhang, Z.; Rustan, A.C.; Ravussin, E.; Bajpeyi, S. Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PloS ONE 2014, 9, e91675. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jin, Y.; Wang, Q.; Huang, J.; Wu, X.; Ren, Z. Perilipin 5 protects against cellular oxidative stress by enhancing mitochondrial function in HepG2 Cells. Cells 2019, 8, 1241. [Google Scholar] [CrossRef]
- Allen, P.E.; Martinez, J.J. Modulation of host lipid pathways by pathogenic intracellular bacteria. Pathogens 2020, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Roingeard, P.; Melo, R.C. Lipid droplet hijacking by intracellular pathogens. Cell. Microbiol. 2017, 19, e12688. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Sánchez-Álvarez, M.; Fajardo, A.; Kapetanovic, R.; Steiner, B.; Dutra, F.; Moreira, L.; López, J.A.; Campo, R.; Marí, M.; et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 2020, 370, 8085. [Google Scholar] [CrossRef] [PubMed]
- Abuaita, B.H.; Schultz, T.L.; O’Riordan, M.X. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized staphylococcus aureus. Cell Host Microbe 2018, 24, 625–636. [Google Scholar] [CrossRef]
- Pinegin, B.; Vorobjeva, N.; Pashenkov, M.; Chernyak, B. The role of mitochondrial ROS in antibacterial immunity. J. Cell. Physiol. 2018, 233, 3745–3754. [Google Scholar] [CrossRef]
- Barisch, C.; Soldati, T. Breaking fat! How mycobacteria and other intracellular pathogens manipulate host lipid droplets. Biochimie 2017, 141, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.; Bozza, F.A.; Gomes, R.N.; Bozza, M.; Weller, P.F.; Castro-Faria-Neto, H.C.; Bozza, P.T. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: Innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J. Immunol. 2002, 169, 6498–6506. [Google Scholar] [CrossRef]
- D’Avila, H.; Melo, R.C.; Parreira, G.G.; Werneck-Barroso, E.; Castro-Faria-Neto, H.C.; Bozza, P.T. Mycobacterium bovis bacillus Calmette-Guérin induces TLR2-mediated formation of lipid bodies: Intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 2006, 176, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, Z.; Rao, C.; Chen, J.; Yuan, S.; Zhang, J.; Mao, C.; Yan, J.; Xia, Y.; Zhang, M.; et al. Burkholderia pseudomallei interferes with host lipid metabolism via NR1D2-mediated PNPLA2/ATGL suppression to block autophagy-dependent inhibition of infection. Autophagy 2021, 17, 1918–1933. [Google Scholar] [CrossRef]
- Almeida, P.E.; Silva, A.R.; Maya-Monteiro, C.M.; Töröcsik, D.; D’Avila, H.; Dezsö, B.; Magalhães, K.G.; Castro-Faria-Neto, H.C.; Nagy, L.; Bozza, P.T. Mycobacterium bovis bacillus Calmette-Guérin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: Functions in inflammation, lipid metabolism, and pathogenesis. J. Immunol. 2009, 183, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.S.G.; Soares, V.C.; Ferreira, A.C.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Teixeira, L.; Nunes da Silva, M.A.; Barreto, E.; Mattos, M.; et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020, 16, e1009127. [Google Scholar] [CrossRef] [PubMed]
- Tansey, J.T.; Sztalryd, C.; Hlavin, E.M.; Kimmel, A.R.; Londos, C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004, 56, 379–385. [Google Scholar] [CrossRef]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Herms, A.; Bosch, M.; Ariotti, N.; Reddy, B.J.; Fajardo, A.; Fernández-Vidal, A.; Alvarez-Guaita, A.; Fernández-Rojo, M.A.; Rentero, C.; Tebar, F.; et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr. Biol. 2013, 23, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Hinson, E.R.; Cresswell, P. The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic alpha-helix. Proc. Natl. Acad. Sci. USA 2009, 106, 20452–20457. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Cermelli, S.; Li, Z.; Kassan, A.; Bosch, M.; Sigua, R.; Huang, L.; Ouellette, A.J.; Pol, A.; Welte, M.A.; et al. A novel role for lipid droplets in the organismal antibacterial response. eLife 2012, 1, e00003. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yao, D.; Zhao, M.; Lu, K.; Lin, Z.; Chen, X.; Zhao, Y.; Zhang, Y. Shrimp Lipid Droplet Protein Perilipin Involves in the Pathogenesis of AHPND-Causing Vibrio parahaemolyticus. Int. J. Mol. Sci. 2022, 23, 10520. https://doi.org/10.3390/ijms231810520
Wang C, Yao D, Zhao M, Lu K, Lin Z, Chen X, Zhao Y, Zhang Y. Shrimp Lipid Droplet Protein Perilipin Involves in the Pathogenesis of AHPND-Causing Vibrio parahaemolyticus. International Journal of Molecular Sciences. 2022; 23(18):10520. https://doi.org/10.3390/ijms231810520
Chicago/Turabian StyleWang, Chuanqi, Defu Yao, Mingming Zhao, Kaiyu Lu, Zhongyang Lin, Xiuli Chen, Yongzhen Zhao, and Yueling Zhang. 2022. "Shrimp Lipid Droplet Protein Perilipin Involves in the Pathogenesis of AHPND-Causing Vibrio parahaemolyticus" International Journal of Molecular Sciences 23, no. 18: 10520. https://doi.org/10.3390/ijms231810520
APA StyleWang, C., Yao, D., Zhao, M., Lu, K., Lin, Z., Chen, X., Zhao, Y., & Zhang, Y. (2022). Shrimp Lipid Droplet Protein Perilipin Involves in the Pathogenesis of AHPND-Causing Vibrio parahaemolyticus. International Journal of Molecular Sciences, 23(18), 10520. https://doi.org/10.3390/ijms231810520





