Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula
Abstract
:1. Introduction
2. Results
2.1. Metabolite Identification
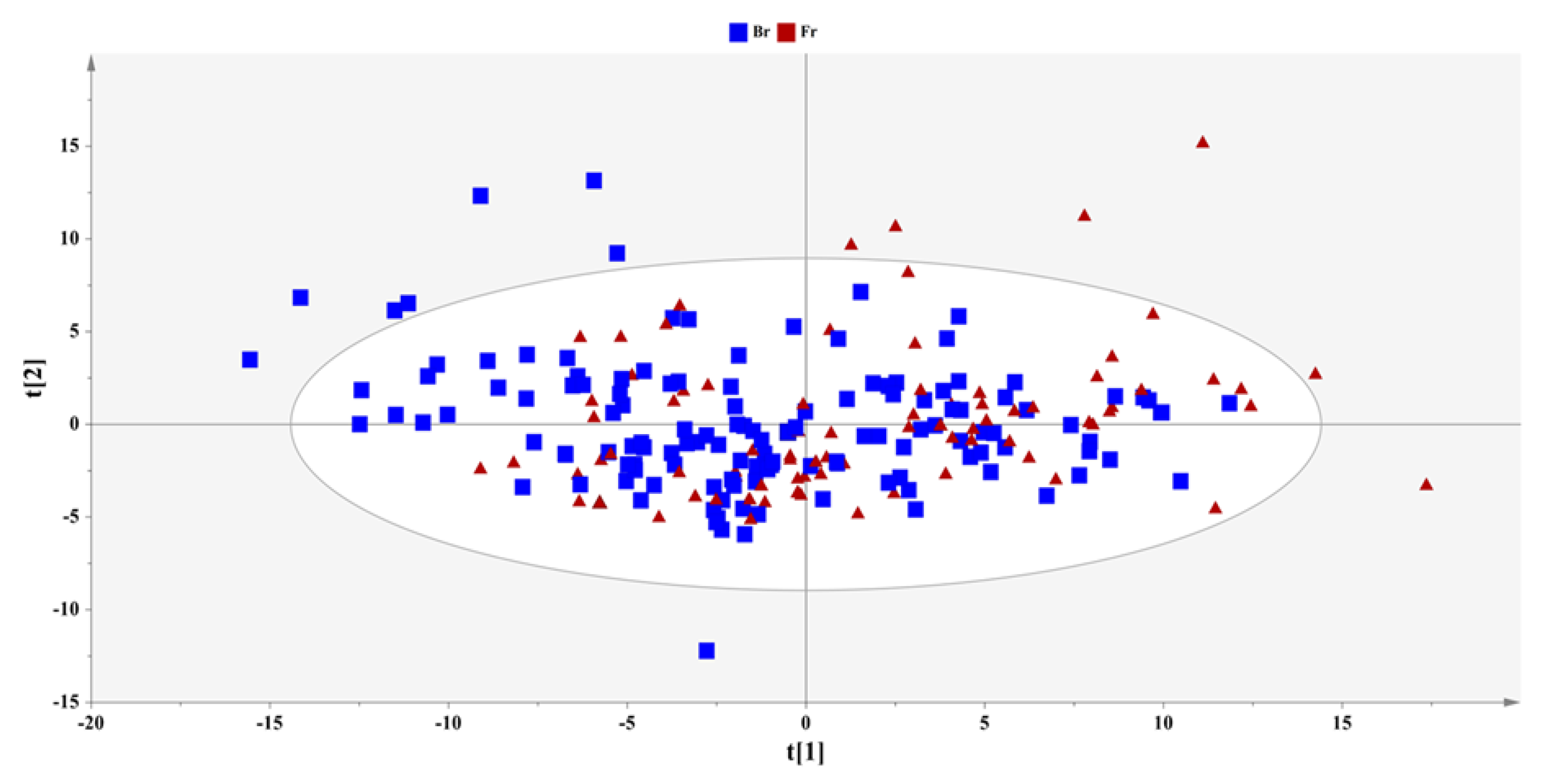
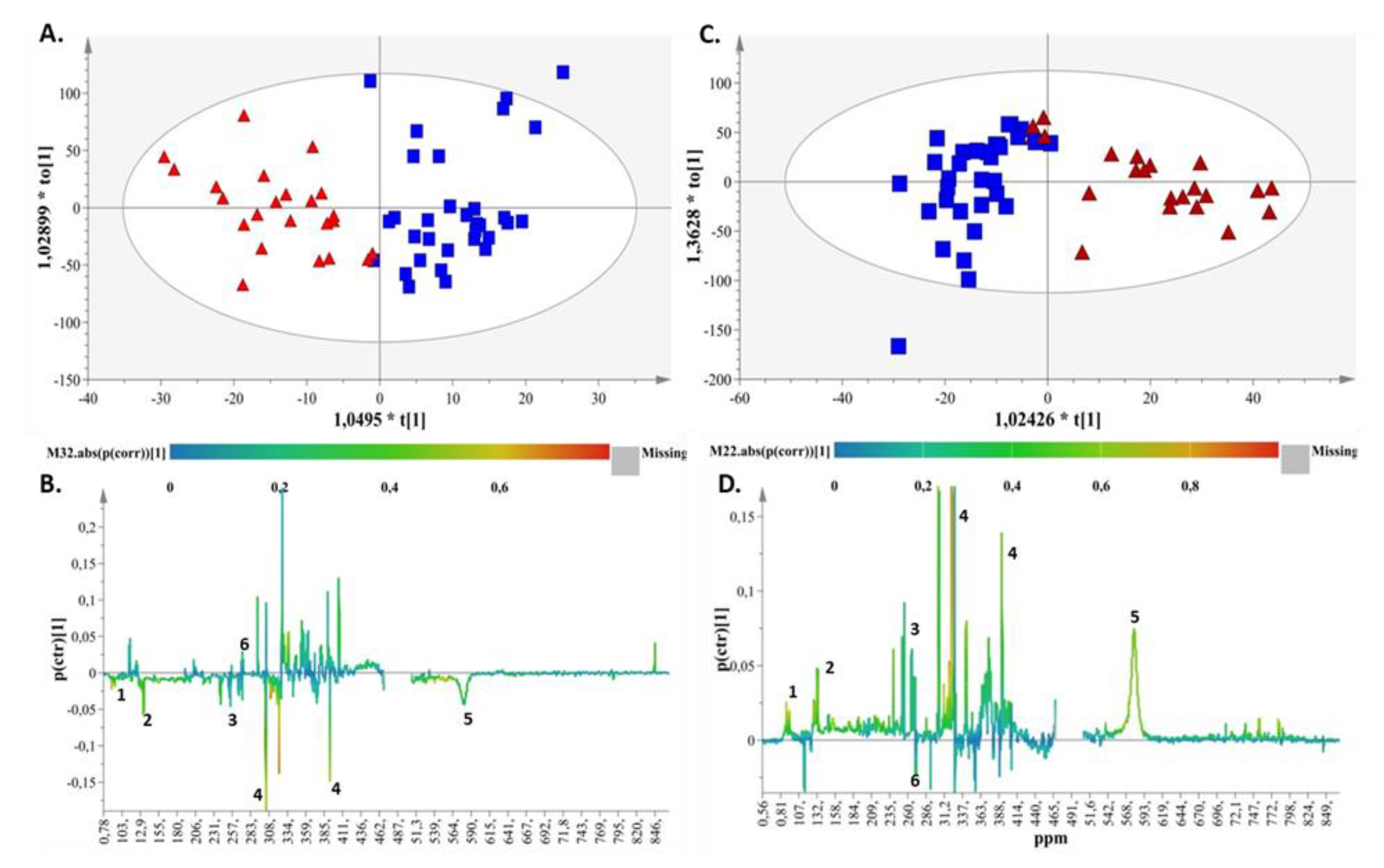
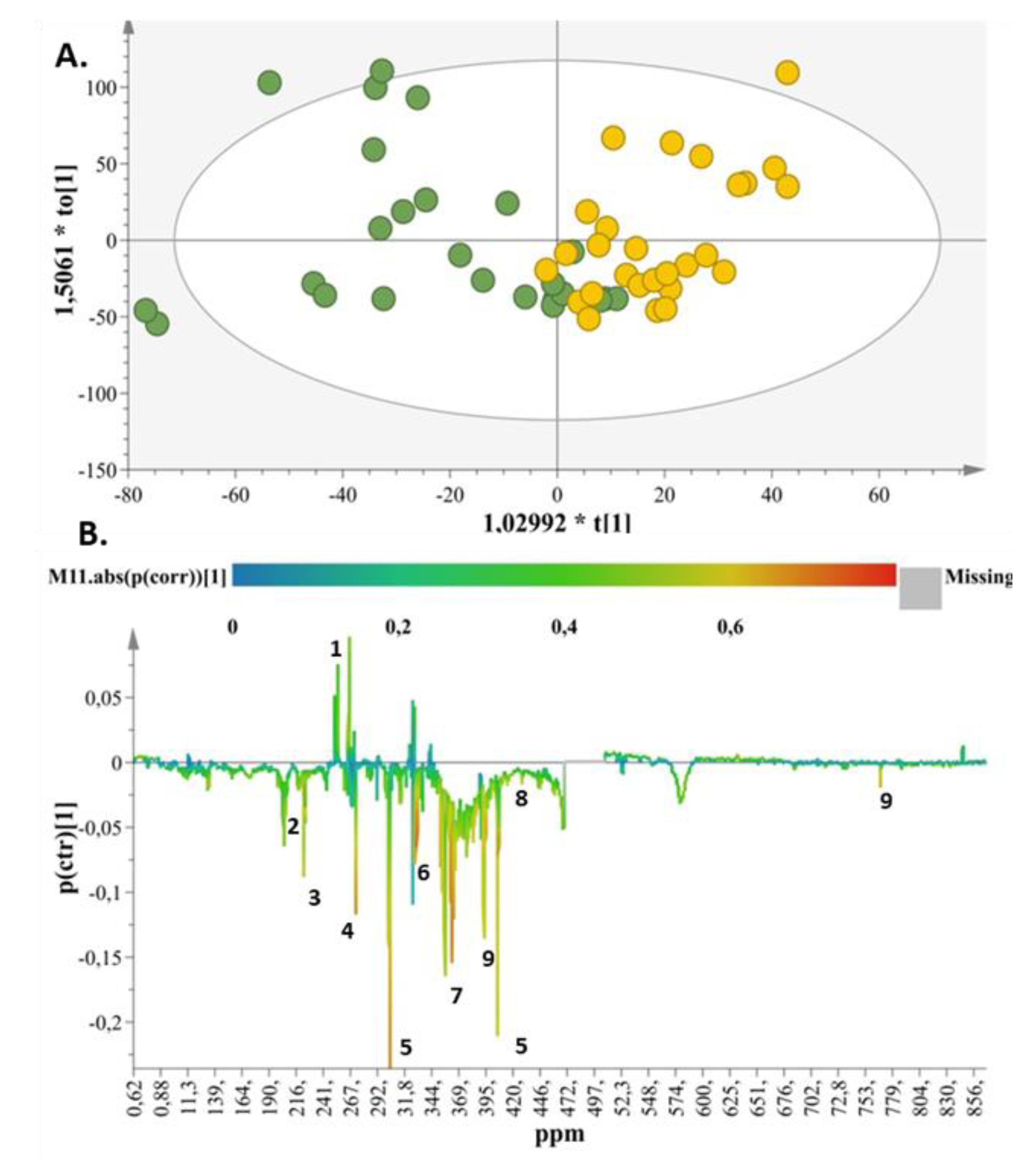
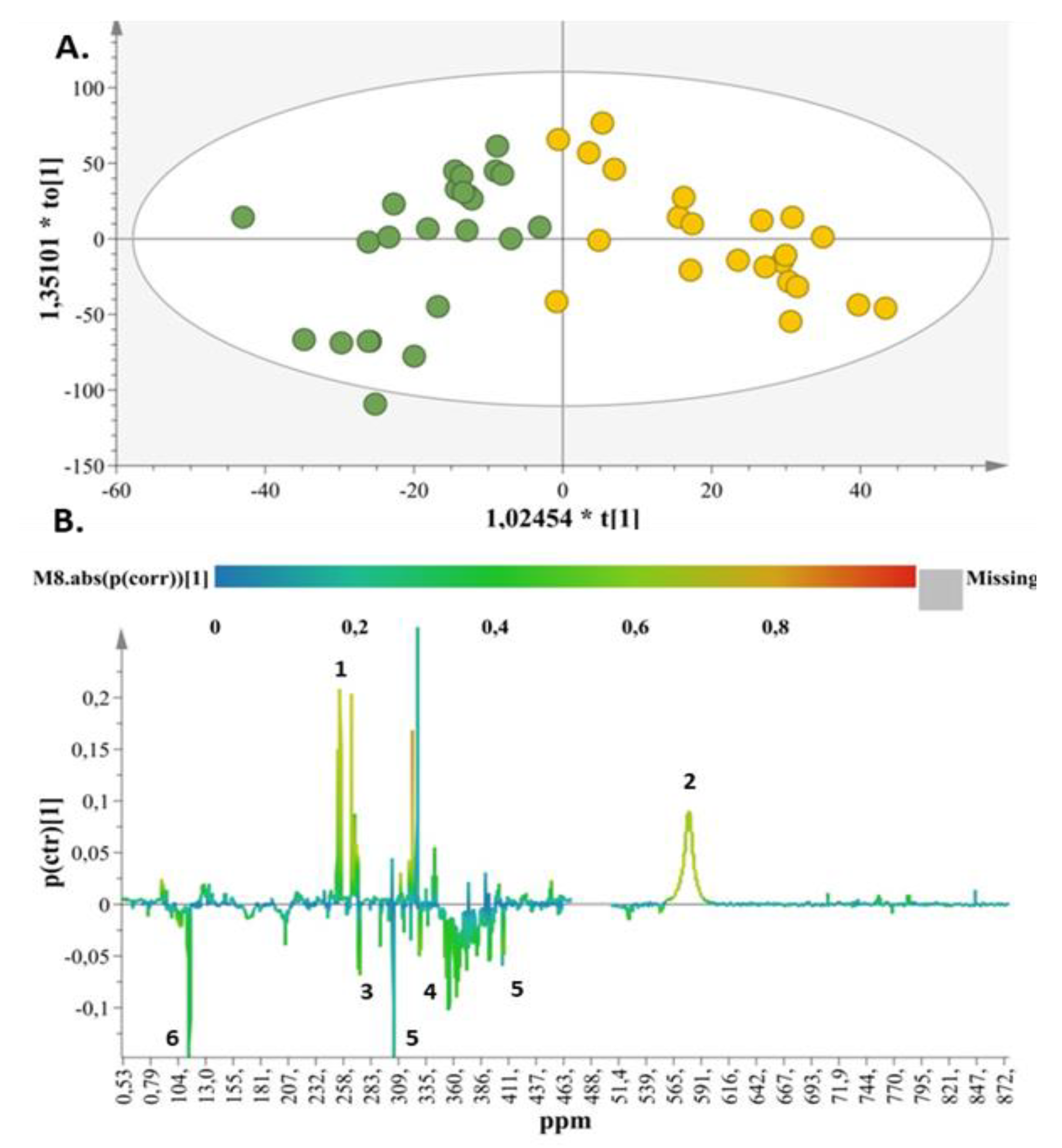
2.2. Metabolic Enquiries
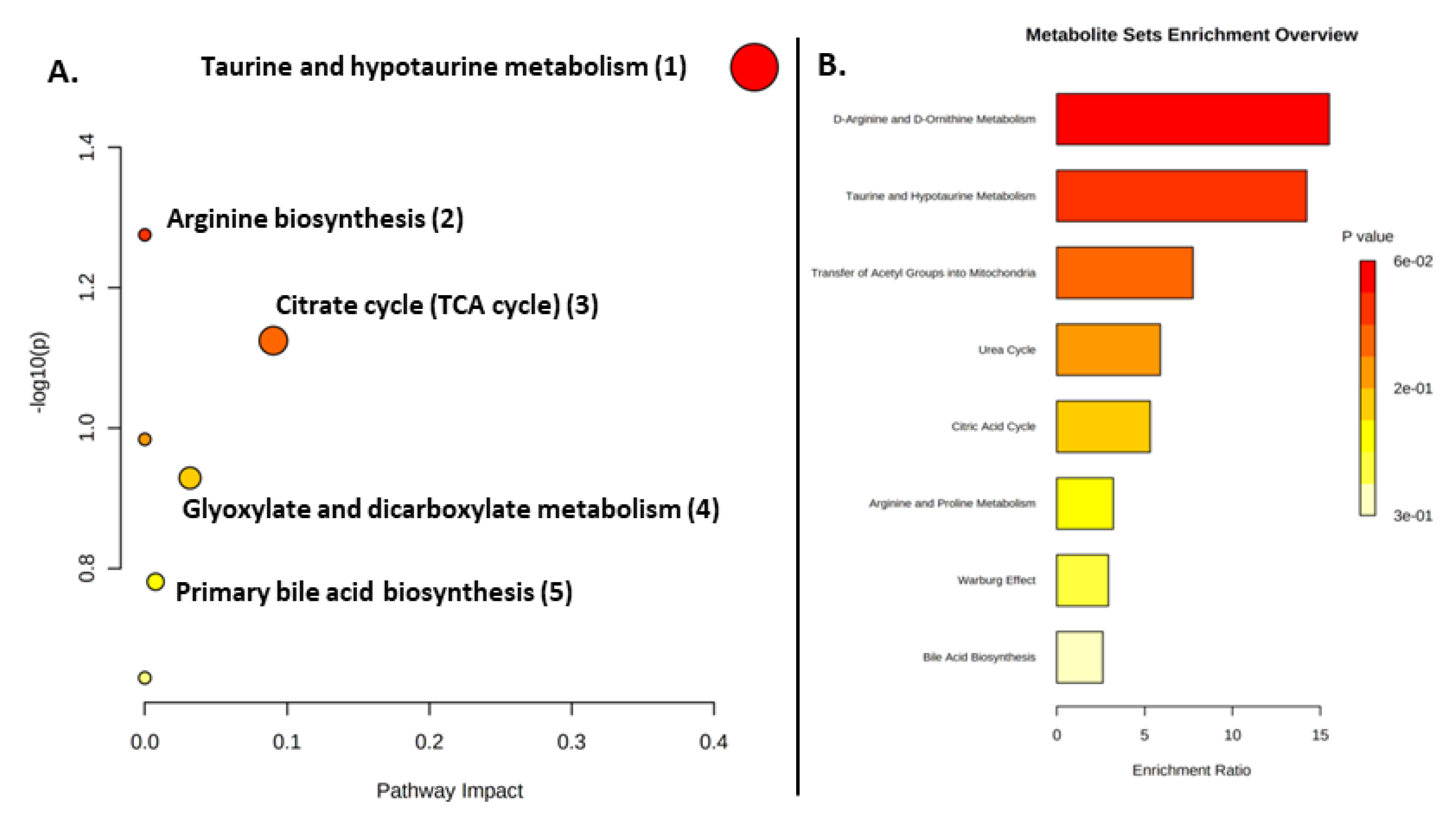
2.3. Metabolite Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Metabolomic Analysis
4.2.1. Sample Preparation
4.2.2. NMR Measurements and Data Processing
4.2.3. Metabolite Identification
4.3. Statistical Analysis
4.3.1. Post Processing of Spectral Data
4.3.2. Identification of Important Features and Model Validation for SIMCA-P
4.3.3. Model Validation for R
4.3.4. Metabolite Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoji, H.; Shimizu, T. Effect of Human Breast Milk on Biological Metabolism in Infants. Pediatr. Int. 2019, 61, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Gianni, M.L.; Canani, R.B.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, F.; Wang, N.; Song, Y.; Yue, Y.; Guan, J.; Li, B.; Huo, G. Distinct Gut Microbiota and Metabolite Profiles Induced by Different Feeding Methods in Healthy Chinese Infants. Front. Microbiol. 2020, 11, 714. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef]
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brüssow, H.; Team, T.S.; et al. Gut Microbiota Analysis Reveals a Marked Shift to Bifidobacteria by a Starter Infant Formula Containing a Synbiotic of Bovine Milk-Derived Oligosaccharides and Bifidobacterium animalis Subsp. Lactis CNCM I-3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef]
- Lee, S.A.; Lim, J.Y.; Kim, B.-S.; Cho, S.J.; Kim, N.Y.; Kim, O.B.; Kim, Y. Comparison of the Gut Microbiota Profile in Breast-Fed and Formula-Fed Korean Infants Using Pyrosequencing. Nutr. Res. Pract. 2014, 9, 242–248. [Google Scholar] [CrossRef]
- Huërou-Luron, I.L.; Blat, S.; Boudry, G. Breast- v. Formula-Feeding: Impacts on the Digestive Tract and Immediate and Long-Term Health Effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bawden, E. Gut microbiome: What we do and don’t know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef]
- Stinson, L.F.; Geddes, D.T. Microbial Metabolites: The next Frontier in Human Milk. Trends Microbiol. 2022, 30, 408–410. [Google Scholar] [CrossRef]
- Bosch, A.A.T.M.; Levin, E.; van Houten, M.A.; Hasrat, R.; Kalkman, G.; Biesbroek, G.; de Steenhuijsen Piters, W.A.A.; de Groot, P.-K.C.M.; Pernet, P.; Keijser, B.J.F.; et al. Development of Upper Respiratory Tract Microbiota in Infancy Is Affected by Mode of Delivery. EBioMedicine 2016, 9, 336–345. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; le Vacon, F.; Potel, G.; de la Cochetiere, M.-F. Development of Intestinal Microbiota in Infants and Its Impact on Health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Brooks, B.; Firek, B.A.; Miller, C.S.; Sharon, I.; Thomas, B.C.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Microbes in the Neonatal Intensive Care Unit Resemble Those Found in the Gut of Premature Infants. Microbiome 2014, 2, 1. [Google Scholar] [CrossRef]
- Knight, K.M.; Moalli, P.A.; Nolfi, A.; Palcsey, S.; Barone, W.R.; Abramowitch, S.D. Impact of Parity on Ewe Vaginal Mechanical Properties Relative to the Nonhuman Primate and Rodent. Int. Urogynecol. J. 2016, 27, 1255–1263. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; de Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Collado, M.C.; Cernada, M.; Baüerl, C.; Vento, M.; Pérez-Martínez, G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012, 3, 352–365. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Selminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Barrett, E.; Deshpandey, A.K.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; O’Sullivan, L.; Watkins, C.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; et al. The neonatal gut harbours distinct bifidobacterial strains. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F405–F410. [Google Scholar] [CrossRef]
- Grüber, C.; van Stuijvenberg, M.; Mosca, F.; Moro, G.; Chirico, G.; Braegger, C.P.; Riedler, J.; Boehm, G.; Wahn, U. Reduced Occurrence of Early Atopic Dermatitis Because of Immunoactive Prebiotics among Low-Atopy-Risk Infants. J. Allergy Clin. Immunol. 2010, 126, 791–797. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections during the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- Rastall, R.A. Gluco and Galacto-Oligosaccharides in Food: Update on Health Effects and Relevance in Healthy Nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 675–678. [Google Scholar] [CrossRef]
- Passeron, T.; Lacour, J.-P.; Fontas, E.; Ortonne, J.-P. Prebiotics and Synbiotics: Two Promising Approaches for the Treatment of Atopic Dermatitis in Children above 2 Years. Allergy 2006, 61, 431–437. [Google Scholar] [CrossRef]
- Nikniaz, L.; Ostadrahimi, A.; Mahdavi, R.; Hejazi, M.A.; Salekdeh, G.H. Effects of synbiotic supplementation on breast milk levels of IgA, TGF-β1, and TGF-β2. J. Hum. Lact. 2013, 29, 591–596. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Nikniaz, L.; Mahdavi, R.; Hejazi, M.A.; Nikniaz, Z. Effects of synbiotic supplementation on lactating mothers’ energy intake and BMI, and infants’ growth. Int. J. Food Sci. Nutr. 2013, 64, 711–714. [Google Scholar] [CrossRef]
- Diaz Heijtz, R. Fetal, Neonatal, and Infant Microbiome: Perturbations and Subsequent Effects on Brain Development and Behavior. Semin. Fetal Neonatal. Med. 2016, 21, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; German, J.B. Food in an Evolutionary Context: Insights from Mother’s Milk. J. Sci. Food Agric. 2012, 92, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.; Siega-Riz, A.M. Trends in Food and Beverage Consumption Among Infants and Toddlers: 2005–2012. Pediatrics 2017, 139, e20163290. [Google Scholar] [CrossRef]
- Hsieh, A.T.; Brenna, J.T. Dietary Docosahexaenoic Acid but Not Arachidonic Acid Influences Central Nervous System Fatty Acid Status in Baboon Neonates. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Keim, S.A. How Research on Charitable Giving Can Inform Strategies to Promote Human Milk Donations to Milk Banks. J. Hum. Lact. 2015, 31, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.; Javanparast, S.; Newman, L. Mothers’ Knowledge of and Attitudes toward Human Milk Banking in South Australia: A Qualitative Study. J. Hum. Lact. 2013, 29, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Colombo, L.; Crippa, B.L.; Consonni, D.; Bettinelli, M.E.; Agosti, V.; Mangino, G.; Bezze, E.N.; Mauri, P.A.; Zanotta, L.; Roggero, P.; et al. Breastfeeding Determinants in Healthy Term Newborns. Nutrients 2018, 10, 48. [Google Scholar] [CrossRef]
- Busck-Rasmussen, M.; Villadsen, S.F.; Norsker, F.N.; Mortensen, L.; Andersen, A.-M.N. Breastfeeding Practices in Relation to Country of Origin Among Women Living in Denmark: A Population-Based Study. Matern. Child Health J. 2014, 18, 2479–2488. [Google Scholar] [CrossRef]
- Ahern, G.J.; Hennessy, A.A.; Ryan, C.A.; Ross, R.P.; Stanton, C. Advances in Infant Formula Science. Annu. Rev. Food Sci. Technol. 2019, 10, 75–102. [Google Scholar] [CrossRef]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Greef, E.D.; Veereman, G. Prebiotics in Infant Formula. Gut Microbes 2014, 5, 681–687. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Lönnerdal, B.; Timby, N.; Domellöf, M.; Hernell, O.; Slupsky, C.M. Fecal Microbiome and Metabolome of Infants Fed Bovine MFGM Supplemented Formula or Standard Formula with Breast-Fed Infants as Reference: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 11589. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczyński, M.; Szymański, H.; Sadowska-Krawczenko, I.; Piwowarczyk, A.; Rasmussen, P.B.; Kristensen, M.B.; West, C.E.; Hernell, O. Effects of Infant Formula Supplemented with Prebiotics Compared with Synbiotics on Growth up to the Age of 12 Mo: A Randomized Controlled Trial. Pediatr. Res. 2017, 81, 752–758. [Google Scholar] [CrossRef]
- Chua, M.C.; Ben-Amor, K.; Lay, C.; Goh, A.E.N.; Chiang, W.C.; Rao, R.; Chew, C.; Chaithongwongwatthana, S.; Khemapech, N.; Knol, J.; et al. Effect of Synbiotic on the Gut Microbiota of Cesarean Delivered Infants: A Randomized, Double-Blind, Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 102–106. [Google Scholar] [CrossRef]
- Schouten, B.; van Esch, B.C.; Hofman, G.A.; van Doorn, S.A.; Knol, J.; Nauta, A.J.; Garssen, J.; Willemsen, L.E.; Knippels, L.M. Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J. Nutr. 2009, 139, 1398–1403. [Google Scholar] [CrossRef]
- Jain, A.; Li, X.H.; Chen, W.N. An Untargeted Fecal and Urine Metabolomics Analysis of the Interplay between the Gut Microbiome, Diet and Human Metabolism in Indian and Chinese Adults. Sci. Rep. 2019, 9, 9191. [Google Scholar] [CrossRef]
- Picaud, J.-C.; de Magistris, A.; Mussap, M.; Corbu, S.; Dessì, A.; Noto, A.; Fanos, V.; Cesare Marincola, F. Urine NMR Metabolomics Profile of Preterm Infants with Necrotizing Enterocolitis Over the First Two Months of Life: A Pilot Longitudinal Case-Control Study. Front. Mol. Biosci. 2021, 8, 680159. [Google Scholar] [CrossRef]
- Muhle-Goll, C.; Eisenmann, P.; Luy, B.; Kölker, S.; Tönshoff, B.; Fichtner, A.; Westhoff, J.H. Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study. Int. J. Mol. Sci. 2020, 21, 1187. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Stanton, C.; Ross, R.P.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium and Lactobacillus Composition at Species Level and Gut Microbiota Diversity in Infants before 6 Weeks. Int. J. Mol. Sci. 2019, 20, 3306. [Google Scholar] [CrossRef] [Green Version]
- Giallourou, N.; Fardus-Reid, F.; Panic, G.; Veselkov, K.; McCormick, B.J.J.; Olortegui, M.P.; Ahmed, T.; Mduma, E.; Yori, P.P.; Mahfuz, M.; et al. Metabolic maturation in the first 2 years of life in resource-constrained settings and its association with postnatal growths. Sci. Adv. 2020, 6, eaay5969. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.; Momin, S.R.; Senn, M.K.; Wood, A.C. Metabolomic Insights into the Effects of Breast Milk Versus Formula Milk Feeding in Infants. Curr. Nutr. Rep. 2019, 8, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Valles, Y.; Gosalbes, M.J.; de Vries, L.E.; Abelián, J.J.; Francino, M.P. Metagenomics and Development of the Gut Microbiota in Infants. Clin. Microbiol. Infect. 2012, 18, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zöhrer, E.; Resch, B.; Scharnagl, H.; Schlagenhauf, A.; Fauler, G.; Stojakovic, T.; Hofer, N.; Lang, U.; Jahnel, J. Serum Bile Acids in Term and Preterm Neonates. Medicine 2016, 95, e5219. [Google Scholar] [CrossRef]
- Van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Sillner, N.; Walker, A.; Lucio, M.; Maier, T.V.; Bazanella, M.; Rychlik, M.; Haller, D.; Schmitt-Kopplin, P. Longitudinal Profiles of Dietary and Microbial Metabolites in Formula- and Breastfed Infants. Front. Mol. Biosci. 2021, 8, 660456. [Google Scholar] [CrossRef]
- Haschke-Becher, E.; Kainz, A.; Bachmann, C. Reference Values of Amino Acids and of Common Clinical Chemistry in Plasma of Healthy Infants Aged 1 and 4 Months. J. Inherit. Metab. Dis. 2016, 39, 25–37. [Google Scholar] [CrossRef]
- Giribaldi, M.; Peila, C.; Coscia, A.; Cavallarin, L.; Antoniazzi, S.; Corbu, S.; Maiocco, G.; Sottemano, S.; Cresi, F.; Moro, G.E.; et al. Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers. Nutrients 2020, 12, 2247. [Google Scholar] [CrossRef]
- Dessì, A.; Murgia, A.; Agostino, R.; Pattumelli, M.G.; Schirru, A.; Scano, P.; Fanos, V.; Caboni, P. Exploring the Role of Different Neonatal Nutrition Regimens during the First Week of Life by Urinary GC-MS Metabolomics. Int. J. Mol. Sci. 2016, 17, 265. [Google Scholar] [CrossRef]
- Schimmel, P.; Kleinjans, L.; Bongers, R.S.; Knol, J.; Belzer, C. Breast Milk Urea as a Nitrogen Source for Urease Positive Bifidobacterium Infantis. FEMS Microbiol. Ecol. 2021, 97, fiab019. [Google Scholar] [CrossRef]
- He, X.; Sotelo-Orozco, J.; Rudolph, C.; Lönnerdal, B.; Slupsky, C.M. The Role of Protein and Free Amino Acids on Intake, Metabolism, and Gut Microbiome: A Comparison Between Breast-Fed and Formula-Fed Rhesus Monkey Infants. Front. Pediatr. 2020, 7, 563. [Google Scholar] [CrossRef]
- Slupsky, C.M.; He, X.; Hernell, O.; Andersson, Y.; Rudolph, C.; Lönnerdal, B.; West, C.E. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs. regular infant formula: A randomized controlled trial. Sci. Rep. 2017, 7, 3640. [Google Scholar] [CrossRef]
- Wu, T.-C.; Huang, I.-F.; Chen, Y.-C.; Chen, P.-H.; Yang, L.-Y. Differences in Serum Biochemistry between Breast-Fed and Formula-Fed Infants. J. Chin. Med. Assoc. 2011, 74, 511–515. [Google Scholar] [CrossRef]
- Echeverri-Peña, O.; Guevara-Morales, J.; Barbosa, A.C.; Cortes, S.C.; Pulido, N.; Rodriguez-Lopez, A.; Parra, M. Metabolic Impact of Infant Formulas in Young Infants. An Outlook from the Urine Metabolome. Heliyon 2020, in press. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Arboleya, S.; Nikpoor, N.; Auger, J.; Salazar, N.; Cuesta, I.; Alvarez-Buylla, J.R.; Mantecón, L.; Solís, G.; Gueimonde, M.; et al. In Vitro Probiotic Modulation of the Intestinal Microbiota and 2′Fucosyllactose Consumption in Fecal Cultures from Infants at Two Months of Age. Microorganisms 2022, 10, 318. [Google Scholar] [CrossRef]
- Lee, H.; Slupsky, C.M.; Heckmann, A.B.; Christensen, B.; Peng, Y.; Li, X.; Hernell, O.; Lönnerdal, B.; Li, Z. Milk Fat Globule Membrane as a Modulator of Infant Metabolism and Gut Microbiota: A Formula Supplement Narrowing the Metabolic Differences between Breastfed and Formula-Fed Infants. Mol. Nutr. Food Res. 2021, 65, 2000603. [Google Scholar] [CrossRef]
- Georgakopoulou, I.; Chasapi, S.A.; Bariamis, S.E.; Varvarigou, A.; Spraul, M.; Spyroulias, G.A. Metabolic Changes in Early Neonatal Life: NMR Analysis of the Neonatal Metabolic Profile to Monitor Postnatal Metabolic Adaptations. Metabolomics 2020, 16, 58. [Google Scholar] [CrossRef]
- O’Sullivan, A.; He, X.; McNiven, E.M.S.; Haggarty, N.W.; Lönnerdal, B.; Slupsky, C.M. Early Diet Impacts Infant Rhesus Gut Microbiome, Immunity, and Metabolism. J. Proteome Res. 2013, 12, 2833–2845. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Swann, J.R. Metabolic Phenotyping of Malnutrition during the First 1000 Days of Life. Eur. J. Nutr. 2019, 58, 909–930. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Petrucca, A.; Paci, P.; Fuentes, S.; Praticò, G.; Capuani, G.; Masotti, A.; Reddel, S.; Russo, A.; et al. Phylogenetic and Metabolic Tracking of Gut Microbiota during Perinatal Development. PLoS ONE 2015, 10, e0137347. [Google Scholar] [CrossRef] [Green Version]
- Bardanzellu, F.; Fanos, V. Omics in Neonatology: The Future? Innov. Front. Neonatol. 2020, 22, 169–183. [Google Scholar] [CrossRef]
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human Milk Composition Promotes Optimal Infant Growth, Development and Health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef] [PubMed]
- Pintus, M.C.; Lussu, M.; Dessì, A.; Pintus, R.; Noto, A.; Masile, V.; Marcialis, M.A.; Puddu, M.; Fanos, V.; Atzori, L. Urinary 1H-NMR Metabolomics in the First Week of Life Can Anticipate BPD Diagnosis. Oxid. Med. Cell. Longev. 2018, 2018, e7620671. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Herrera-Van Oostdam, A.S.; Zheng, J.; Chi Guo, A.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The Urinary Metabolome of Healthy Newborns. Metabolites 2020, 10, 165. [Google Scholar] [CrossRef]
- Ribo, S.; Sánchez-Infantes, D.; Martinez-Guino, L.; García-Mantrana, I.; Ramon-Krauel, M.; Tondo, M.; Arning, E.; Nofrarías, M.; Osorio-Conles, Ó.; Fernández-Pérez, A.; et al. Increasing Breast Milk Betaine Modulates Akkermansia Abundance in Mammalian Neonates and Improves Long-Term Metabolic Health. Sci. Transl. Med. 2021, 13, eabb0322. [Google Scholar] [CrossRef]
- Shoji, H.; Taka, H.; Kaga, N.; Ikeda, N.; Kitamura, T.; Miura, Y.; Shimizu, T. A Pilot Study of the Effect of Human Breast Milk on Urinary Metabolome Analysis in Infants. J. Pediatr. Endocrinol. Metab. 2017, 30, 939–946. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Kvistgaard, A.S.; Peerson, J.M.; Donovan, S.M.; Peng, Y. Growth, Nutrition, and Cytokine Response of Breast-Fed Infants and Infants Fed Formula with Added Bovine Osteopontin. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 28–40. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of Human Breast Milk: From Macronutrient to Microbiome and MicroRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.A.N.; et al. Recommendations and Standardization of Biomarker Quantification Using NMR-Based Metabolomics with Particular Focus on Urinary Analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, G.; van den Berg, F.; Andersson, C. Correlation Optimized Warping and Dynamic Time Warping as Preprocessing Methods for Chromatographic Data. J. Chemom. 2004, 18, 231–241. [Google Scholar] [CrossRef]
- Fotiou, M.; Fotakis, C.; Tsakoumaki, F.; Athanasiadou, E.; Kyrkou, C.; Dimitropoulou, A.; Tsiaka, T.; Chatziioannou, A.C.; Sarafidis, K.; Menexes, G.; et al. 1H NMR-Based Metabolomics Reveals the Effect of Maternal Habitual Dietary Patterns on Human Amniotic Fluid Profile. Sci. Rep. 2018, 8, 4076. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Olival, A.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Untargeted Urinary 1H NMR-Based Metabolomic Pattern as a Potential Platform in Breast Cancer Detection. Metabolites 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Filntisi, A.; Fotakis, C.; Asvestas, P.; Matsopoulos, G.K.; Zoumpoulakis, P.; Cavouras, D. Automated metabolite identification from biological fluid 1H NMR spectra. Metabolomics 2017, 13, 146. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Eriksson, L.; Antti, H.; Gottfries, J.; Holmes, E.; Johansson, E.; Lindgren, F.; Long, I.; Lundstedt, T.; Trygg, J.; Wold, S. Using Chemometrics for Navigating in the Large Data Sets of Genomics, Proteomics, and Metabonomics (Gpm). Anal. Bioanal. Chem. 2004, 380, 419–429. [Google Scholar] [CrossRef]
- Pearson, F.R.S.K. LIII. On Lines and Planes of Closest Fit to Systems of Points in Space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef]
- Ståhle, L.; Wold, S. Partial Least Squares Analysis with Cross-Validation for the Two-Class Problem: A Monte Carlo Study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial Least Squares for Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [Optimized Potentials for Liquid Simulations] Potential Functions for Proteins, Energy Minimizations for Crystals of Cyclic Peptides and Crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falaina, V.; Fotakis, C.; Boutsikou, T.; Tsiaka, T.; Moros, G.; Ouzounis, S.; Andreou, V.; Iliodromiti, Z.; Xanthos, T.; Vandenplas, Y.; et al. Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula. Int. J. Mol. Sci. 2022, 23, 10476. https://doi.org/10.3390/ijms231810476
Falaina V, Fotakis C, Boutsikou T, Tsiaka T, Moros G, Ouzounis S, Andreou V, Iliodromiti Z, Xanthos T, Vandenplas Y, et al. Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula. International Journal of Molecular Sciences. 2022; 23(18):10476. https://doi.org/10.3390/ijms231810476
Chicago/Turabian StyleFalaina, Vasiliki, Charalambos Fotakis, Theodora Boutsikou, Thalia Tsiaka, Georgios Moros, Sotirios Ouzounis, Vasiliki Andreou, Zoi Iliodromiti, Theodoros Xanthos, Yvan Vandenplas, and et al. 2022. "Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula" International Journal of Molecular Sciences 23, no. 18: 10476. https://doi.org/10.3390/ijms231810476
APA StyleFalaina, V., Fotakis, C., Boutsikou, T., Tsiaka, T., Moros, G., Ouzounis, S., Andreou, V., Iliodromiti, Z., Xanthos, T., Vandenplas, Y., Iacovidou, N., & Zoumpoulakis, P. (2022). Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula. International Journal of Molecular Sciences, 23(18), 10476. https://doi.org/10.3390/ijms231810476









