New Inhibitors of the Human p300/CBP Acetyltransferase Are Selectively Active against the Arabidopsis HAC Proteins
Abstract
1. Introduction
2. Results
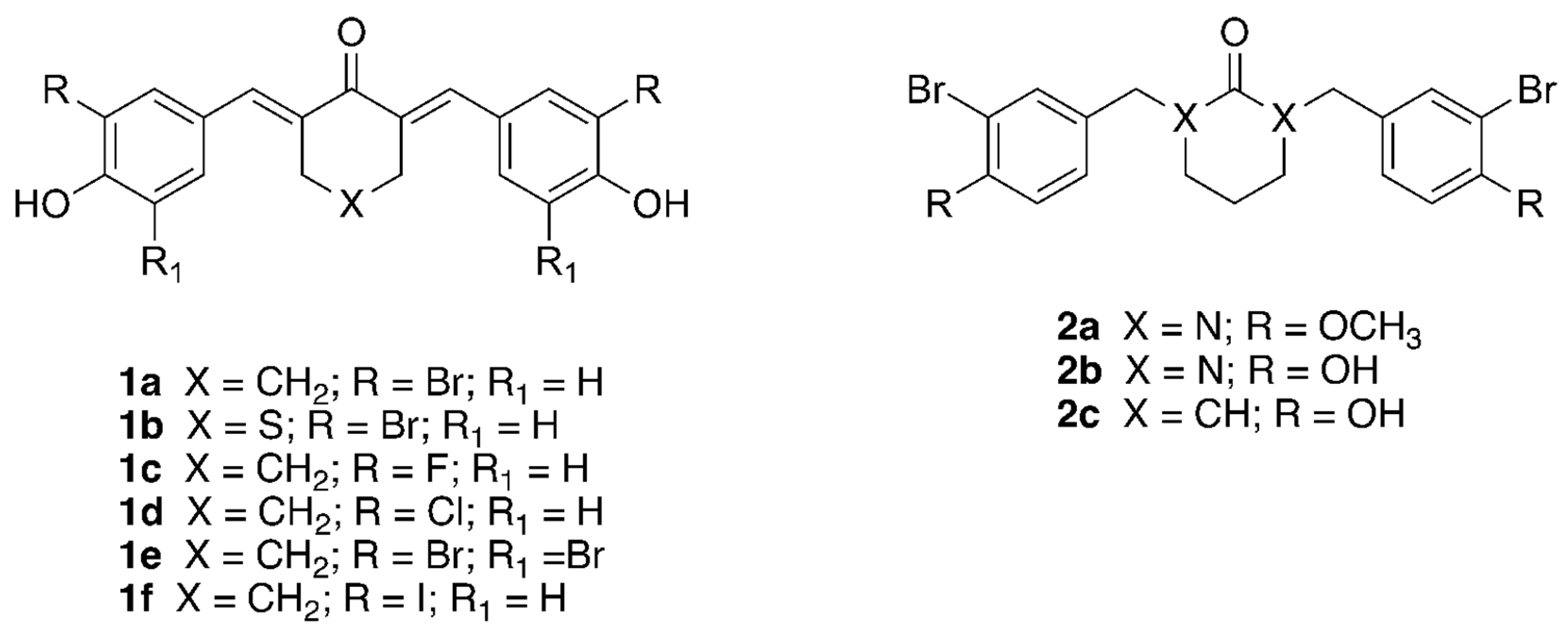
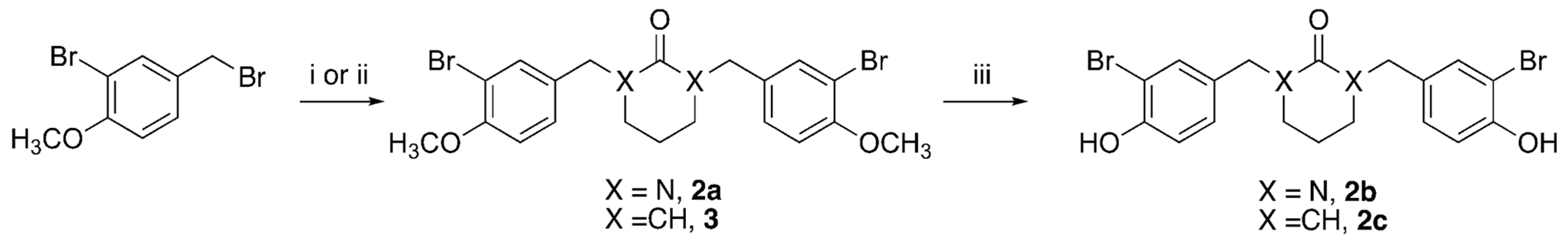
2.1. Synthesis of HAC Inhibitors
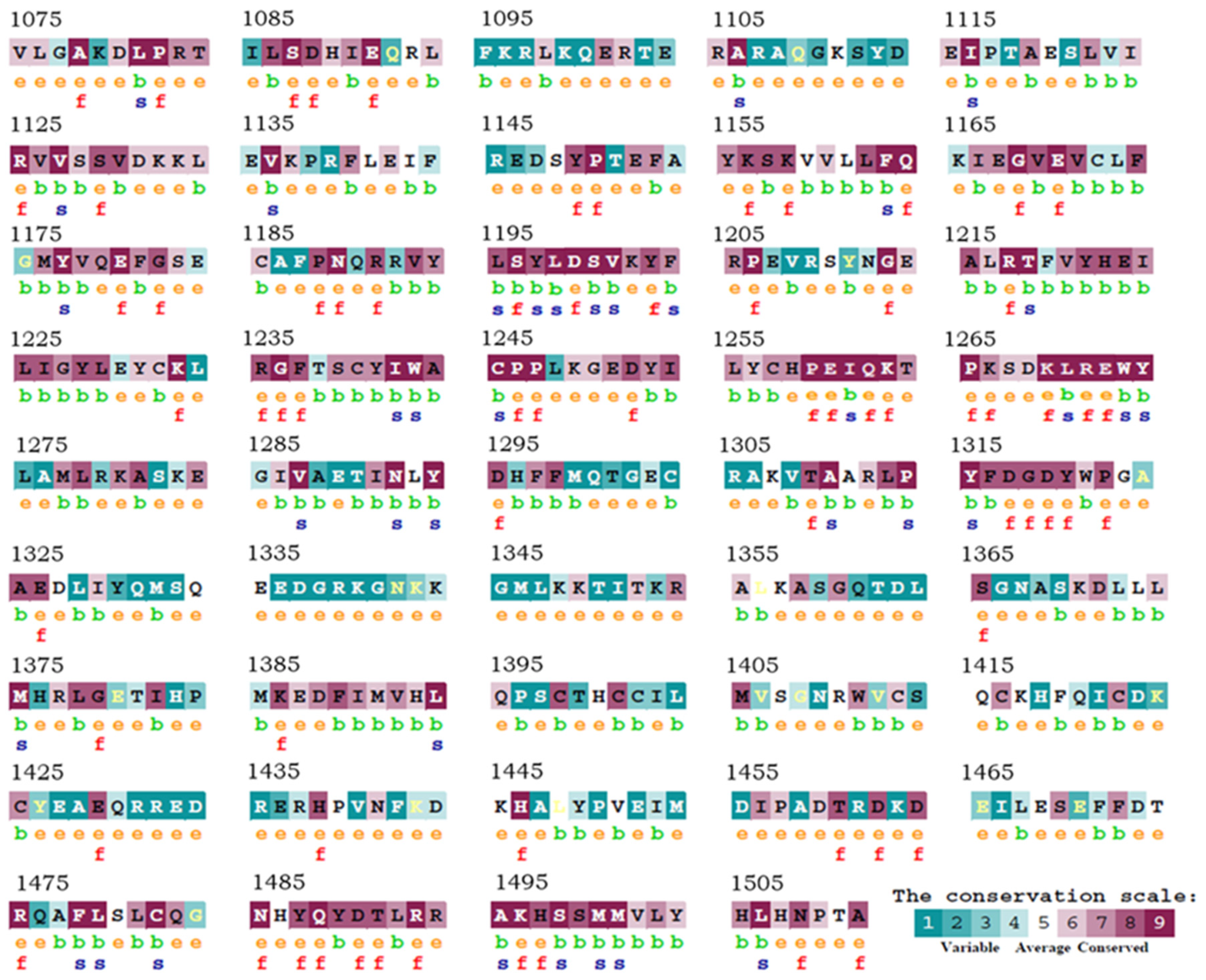
2.2. Evolutive Conservation Analysis of the HAC1 Catalytic Site
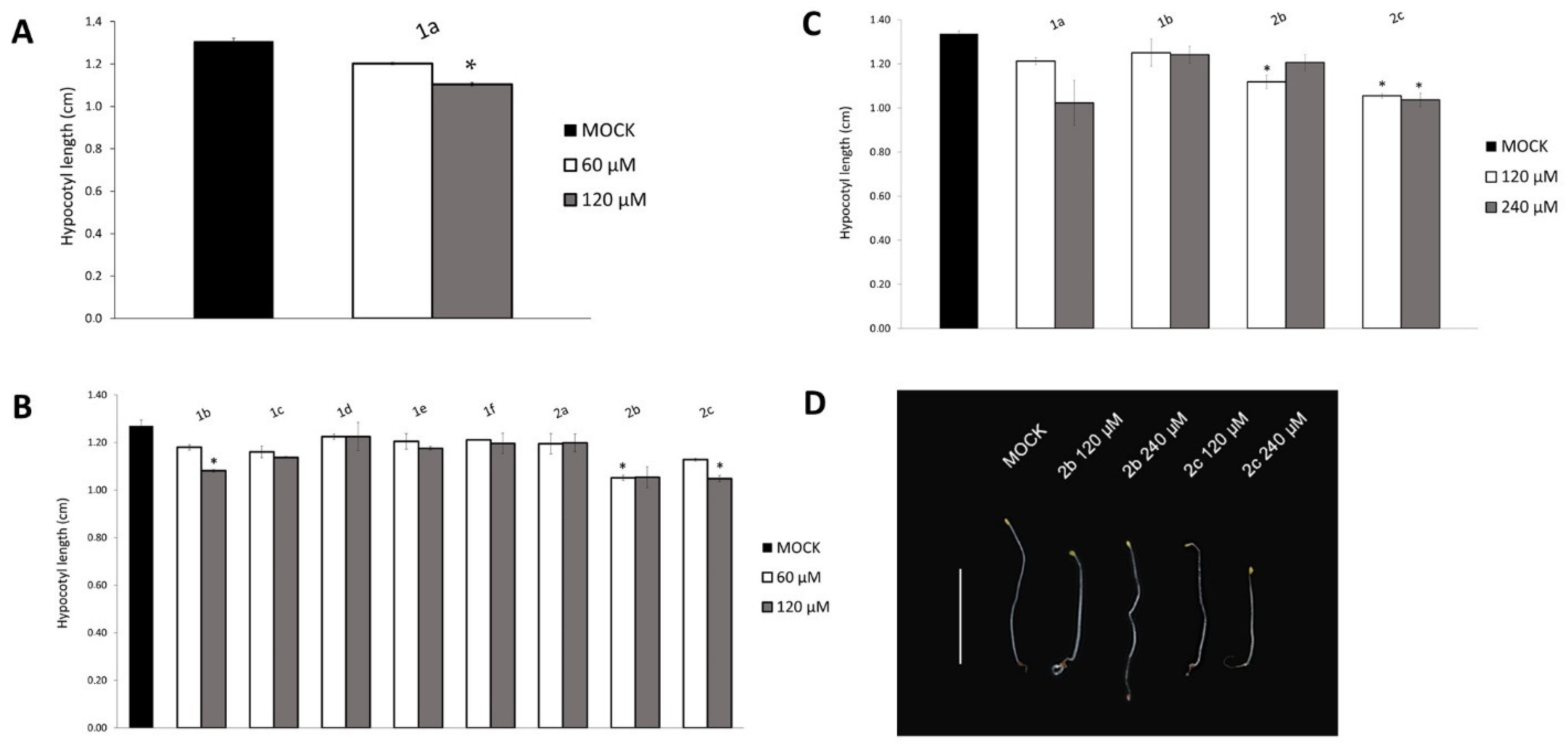
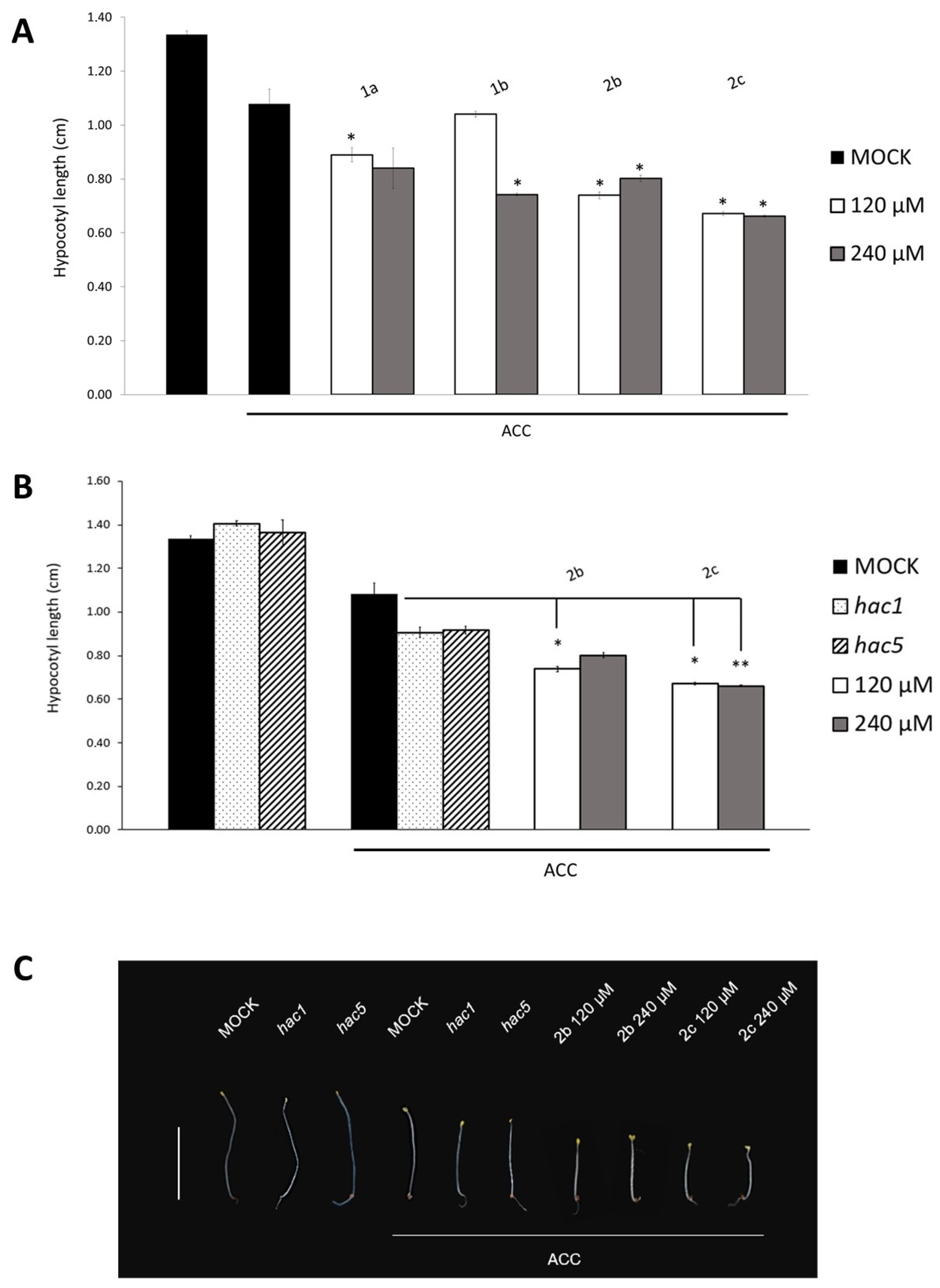
2.3. The p300/CBP Inhibitor 1a Affects Hypocotyl Elongation in Arabidopsis Seedlings
2.4. Screening and Selection of New HAC Inhibitors
2.5. Treatment with Compounds 2b and 2c Mimics Ethylene Effect on hac Mutants
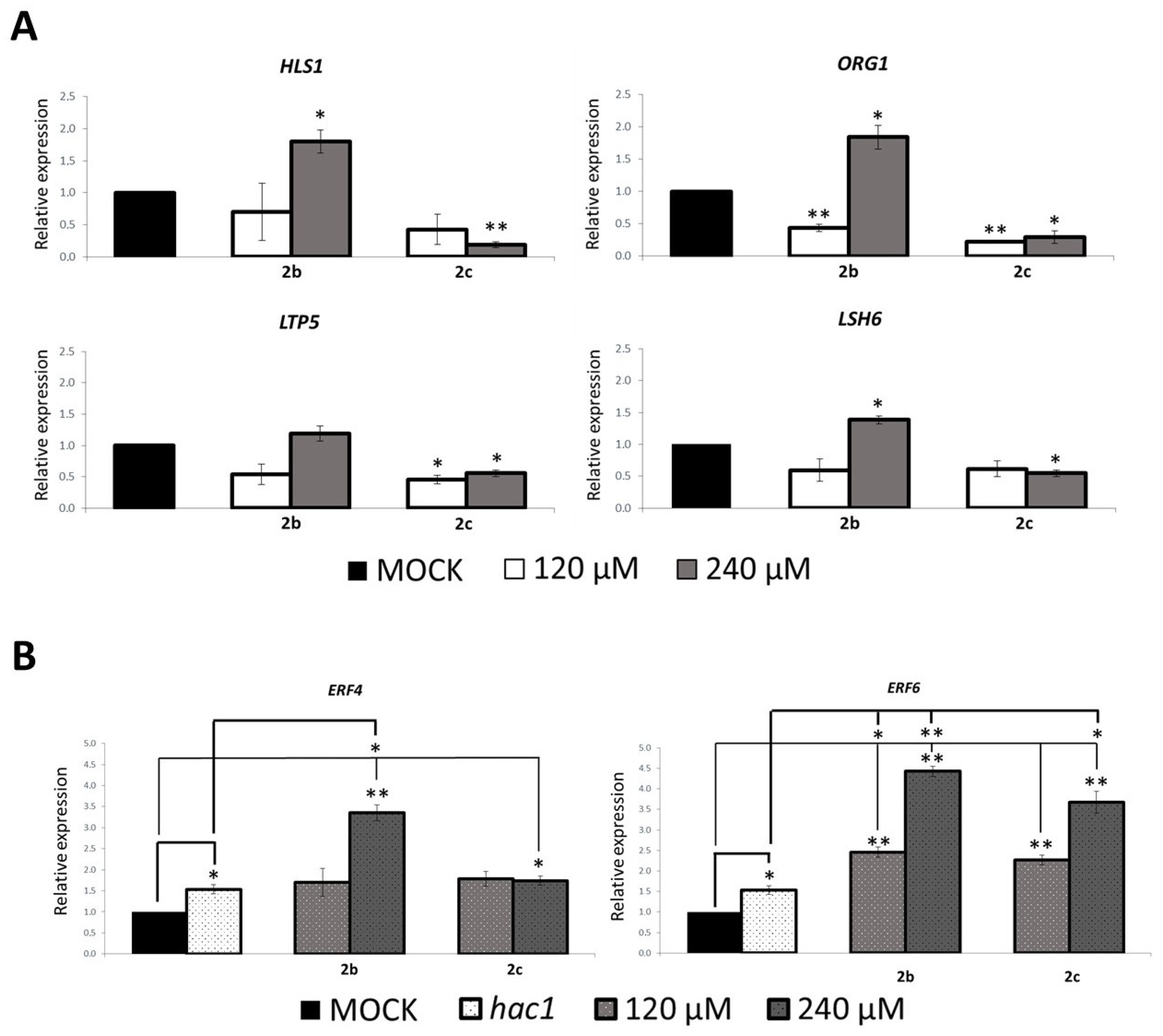
2.6. Treatment with 2b and 2c Affects Expression of HAC Target Genes
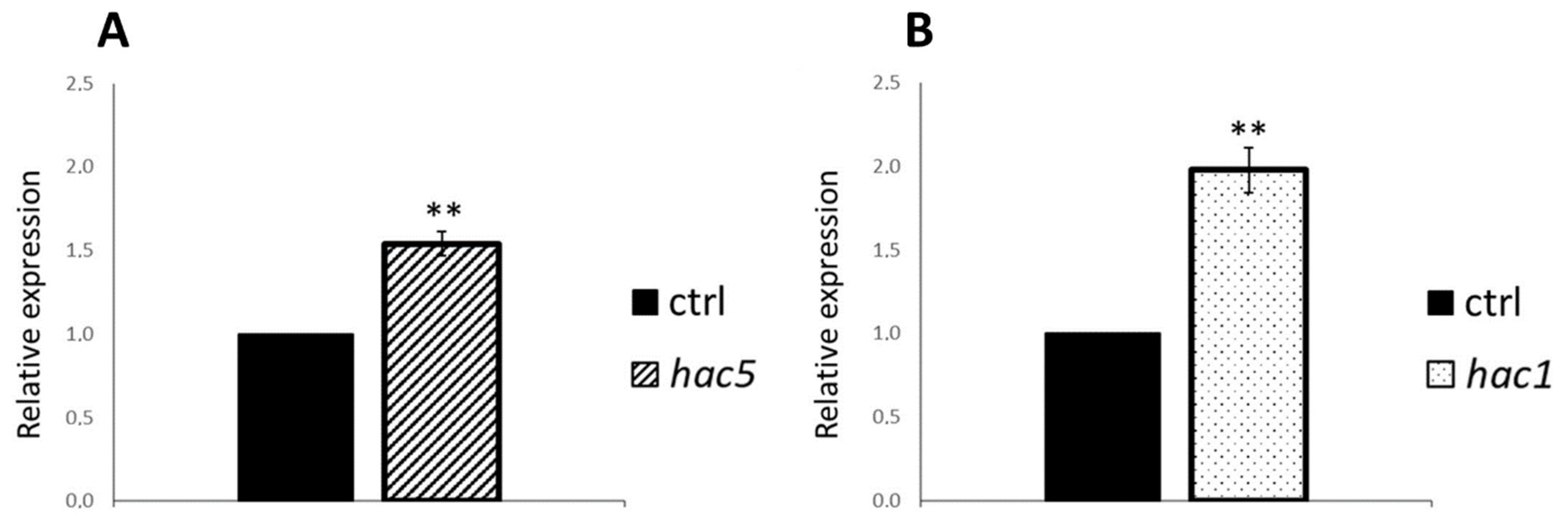
2.7. Expression of HAC1 and HAC5 Is Mutually Regulated
2.8. Seedlings Effectively Uptake HAC Inhibitors
2.9. Compound 2c Selectively Inhibits HAC1 Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Instrumentation
4.1.2. General Experimental Procedure
General Procedure A (GP-A) for the Synthesis of Derivatives 2b and 2c
4.1.3. Specific Procedures and Characterization
1,3-Bis(3-bromo-4-methoxybenzyl)tetrahydropyrimidin-2(1H)-one (2a)
1,3-Bis(3-bromo-4-hydroxybenzyl)tetrahydropyrimidin-2(1H)-one (2b)
2,6-Bis(3-bromo-4-hydroxybenzyl)cyclohexanone (2c)
2,6-Bis(3-bromo-4-methoxybenzyl)cyclohexanone (3)
4.2. Plant Material and Growth Conditions
4.3. RNA Extraction
4.4. Expression Analysis
4.5. Phenotypic Analysis
4.6. HPLC Analysis
4.7. Evolutive Conservation Analysis
4.8. Expression and Purification of Recombinant HAC1
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shahbazian, M.D.; Grunstein, M. Functions of Site-Specific Histone Acetylation and Deacetylation. Annu. Rev. Biochem. 2007, 76, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Drazic, A.; Myklebust, L.M.; Ree, R.; Arnesen, T. The World of Protein Acetylation. Biochim. Biophys. Acta 2016, 1864, 1372–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, H.; Wang, G.; Ren, H. Imbalance of Lysine Acetylation Contributes to the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 7182. [Google Scholar] [CrossRef]
- Koutelou, E.; Farria, A.T.; Dent, S.Y.R. Complex Functions of Gcn5 and Pcaf in Development and Disease. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194609. [Google Scholar] [CrossRef] [PubMed]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and Deacetylation of Non-Histone Proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Kong, F.; Ma, L.; Wang, X.; You, H.; Zheng, K.; Tang, R. Regulation of Epithelial-Mesenchymal Transition by Protein Lysine Acetylation. Cell Commun. Signal. 2022, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Roeder, R.G. Activation of P53 Sequence-Specific DNA Binding by Acetylation of the P53 C-Terminal Domain. Cell 1997, 90, 595–606. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, H.; Vo, N.; Goodman, R.H. Acetylation of Adenovirus E1A Regulates Binding of the Transcriptional Corepressor CtBP. Proc. Natl. Acad. Sci. USA 2000, 97, 14323–14328. [Google Scholar] [CrossRef]
- Holmqvist, P.-H.; Mannervik, M. Genomic Occupancy of the Transcriptional Co-Activators P300 and CBP. Transcription 2013, 4, 18–23. [Google Scholar] [CrossRef]
- Bedford, D.C.; Kasper, L.H.; Fukuyama, T.; Brindle, P.K. Target Gene Context Influences the Transcriptional Requirement for the KAT3 Family of CBP and P300 Histone Acetyltransferases. Epigenetics 2010, 5, 9–15. [Google Scholar] [CrossRef]
- Acevedo, M.L.; Kraus, W.L. Mediator and P300/CBP-Steroid Receptor Coactivator Complexes Have Distinct Roles, but Function Synergistically, during Estrogen Receptor Alpha-Dependent Transcription with Chromatin Templates. Mol. Cell. Biol. 2003, 23, 335–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallberg, A.E.; Yamamura, S.; Malik, S.; Spiegelman, B.M.; Roeder, R.G. Coordination of P300-Mediated Chromatin Remodeling and TRAP/Mediator Function through Coactivator PGC-1alpha. Mol. Cell 2003, 12, 1137–1149. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, H.; Chen, F.; Liu, Y. The Roles of Histone Acetylation in Seed Performance and Plant Development. Plant Physiol. Biochem. 2014, 84, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Müller, A.; Napoli, C.A.; Selinger, D.A.; Pikaard, C.S.; Richards, E.J.; Bender, J.; Mount, D.W.; Jorgensen, R.A. Analysis of Histone Acetyltransferase and Histone Deacetylase Families of Arabidopsis Thaliana Suggests Functional Diversification of Chromatin Modification among Multicellular Eukaryotes. Nucleic Acids Res. 2002, 30, 5036–5055. [Google Scholar] [CrossRef] [PubMed]
- Bordoli, L.; Netsch, M.; Lüthi, U.; Lutz, W.; Eckner, R. Plant Orthologs of P300/CBP: Conservation of a Core Domain in Metazoan P300/CBP Acetyltransferase-Related Proteins. Nucleic Acids Res. 2001, 29, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Earley, K.W.; Shook, M.S.; Brower-Toland, B.; Hicks, L.; Pikaard, C.S. In Vitro Specificities of Arabidopsis Co-Activator Histone Acetyltransferases: Implications for Histone Hyperacetylation in Gene Activation. Plant J. 2007, 52, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Liu, C.; Pei, Y.; Deng, X.; Niu, L.; Cao, X. Involvement of the Histone Acetyltransferase AtHAC1 in the Regulation of Flowering Time via Repression of FLOWERING LOCUS C in Arabidopsis. Plant Physiol. 2007, 143, 1660–1668. [Google Scholar] [CrossRef]
- Boycheva, I.; Vassileva, V.; Iantcheva, A. Histone Acetyltransferases in Plant Development and Plasticity. Curr. Genom. 2014, 15, 28–37. [Google Scholar] [CrossRef]
- Boycheva, I.; Vassileva, V.; Revalska, M.; Zehirov, G.; Iantcheva, A. Different Functions of the Histone Acetyltransferase HAC1 Gene Traced in the Model Species Medicago Truncatula, Lotus Japonicus and Arabidopsis Thaliana. Protoplasma 2017, 254, 697–711. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Li, J.; Li, Q.; Yang, H. Involvement of Arabidopsis Histone Acetyltransferase HAC Family Genes in the Ethylene Signaling Pathway. Plant Cell Physiol. 2014, 55, 426–435. [Google Scholar] [CrossRef]
- Hinckley, W.E.; Keymanesh, K.; Cordova, J.A.; Brusslan, J.A. The HAC1 Histone Acetyltransferase Promotes Leaf Senescence and Regulates the Expression of ERF022. Plant Direct 2019, 3, e00159. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, L.; Zhai, Q.; You, Y.; Deng, L.; Wu, F.; Chen, R.; Jiang, H.; Wang, H.; Chen, Q.; et al. Mediator Subunit MED25 Links the Jasmonate Receptor to Transcriptionally Active Chromatin. Proc. Natl. Acad. Sci. USA 2017, 114, E8930–E8939. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wei, L.; Chen, S.-S.; Cai, X.-W.; Su, Y.-N.; Li, L.; Chen, S.; He, X.-J. The CBP/P300 Histone Acetyltransferases Function as Plant-Specific MEDIATOR Subunits in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Horinouchi, S.; Beppu, T. Trichostatin A and Trapoxin: Novel Chemical Probes for the Role of Histone Acetylation in Chromatin Structure and Function. Bioessays 1995, 17, 423–430. [Google Scholar] [CrossRef]
- Fujii, S.; Okinaga, T.; Ariyoshi, W.; Takahashi, O.; Iwanaga, K.; Nishino, N.; Tominaga, K.; Nishihara, T. Mechanisms of G1 Cell Cycle Arrest and Apoptosis in Myeloma Cells Induced by Hybrid-Compound Histone Deacetylase Inhibitor. Biochem. Biophys. Res. Commun. 2013, 434, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-A.; Han, G.; Kim, H.-J.; Kim, H.-M.; Cho, S.-D. Chemopreventive and Chemotherapeutic Effect of a Novel Histone Deacetylase Inhibitor, by Specificity Protein 1 in MDA-MB-231 Human Breast Cancer Cells. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2014, 23, 277–285. [Google Scholar] [CrossRef]
- Sako, K.; Kim, J.-M.; Matsui, A.; Nakamura, K.; Tanaka, M.; Kobayashi, M.; Saito, K.; Nishino, N.; Kusano, M.; Taji, T.; et al. Ky-2, a Histone Deacetylase Inhibitor, Enhances High-Salinity Stress Tolerance in Arabidopsis Thaliana. Plant Cell Physiol. 2016, 57, 776–783. [Google Scholar] [CrossRef]
- Biel, M.; Kretsovali, A.; Karatzali, E.; Papamatheakis, J.; Giannis, A. Design, Synthesis, and Biological Evaluation of a Small-Molecule Inhibitor of the Histone Acetyltransferase Gcn5. Angew. Chem. Int. Ed. Engl. 2004, 43, 3974–3976. [Google Scholar] [CrossRef]
- Aquea, F.; Timmermann, T.; Herrera-Vásquez, A. Chemical Inhibition of the Histone Acetyltransferase Activity in Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2017, 483, 664–668. [Google Scholar] [CrossRef]
- Ruta, V.; Longo, C.; Boccaccini, A.; Madia, V.N.; Saccoliti, F.; Tudino, V.; Di Santo, R.; Lorrai, R.; Dello Ioio, R.; Sabatini, S.; et al. Inhibition of Polycomb Repressive Complex 2 Activity Reduces Trimethylation of H3K27 and Affects Development in Arabidopsis Seedlings. BMC Plant Biol. 2019, 19, 429. [Google Scholar] [CrossRef]
- Costi, R.; Di Santo, R.; Artico, M.; Miele, G.; Valentini, P.; Novellino, E.; Cereseto, A. Cinnamoyl Compounds as Simple Molecules That Inhibit P300 Histone Acetyltransferase. J. Med. Chem. 2007, 50, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Madia, V.N.; Benedetti, R.; Barreca, M.L.; Ngo, L.; Pescatori, L.; Messore, A.; Pupo, G.; Saccoliti, F.; Valente, S.; Mai, A.; et al. Structure-Activity Relationships on Cinnamoyl Derivatives as Inhibitors of P300 Histone Acetyltransferase. ChemMedChem 2017, 12, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Usuki, T. Total Synthesis of Acerogenins E, G and K, and Centrolobol. Tetrahedron 2013, 69, 2807–2815. [Google Scholar] [CrossRef]
- Kaiser, S.; Smidt, S.P.; Pfaltz, A. Iridium Catalysts with Bicyclic Pyridine-Phosphinite Ligands: Asymmetric Hydrogenation of Olefins and Furan Derivatives. Angew. Chem. Int. Ed. Engl. 2006, 45, 5194–5197. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An Improved Methodology to Estimate and Visualize Evolutionary Conservation in Macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Shi, H.; Xue, C.; Wang, L.; Xi, Y.; Li, J.; Quail, P.H.; Deng, X.W.; Guo, H. A Molecular Framework of Light-Controlled Phytohormone Action in Arabidopsis. Curr. Biol. 2012, 22, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shen, X.; Liu, R.; Xue, C.; Wei, N.; Deng, X.W.; Zhong, S. The Red Light Receptor Phytochrome B Directly Enhances Substrate-E3 Ligase Interactions to Attenuate Ethylene Responses. Dev. Cell 2016, 39, 597–610. [Google Scholar] [CrossRef]
- Lehman, A.; Black, R.; Ecker, J.R. HOOKLESS1, an Ethylene Response Gene, Is Required for Differential Cell Elongation in the Arabidopsis Hypocotyl. Cell 1996, 85, 183–194. [Google Scholar] [CrossRef]
- Kang, H.-G.; Foley, R.C.; Oñate-Sánchez, L.; Lin, C.; Singh, K.B. Target Genes for OBP3, a Dof Transcription Factor, Include Novel Basic Helix-Loop-Helix Domain Proteins Inducible by Salicylic Acid. Plant J. 2003, 35, 362–372. [Google Scholar] [CrossRef]
- Segura, A.; Moreno, M.; García-Olmedo, F. Purification and Antipathogenic Activity of Lipid Transfer Proteins (LTPs) from the Leaves of Arabidopsis and Spinach. FEBS Lett. 1993, 332, 243–246. [Google Scholar] [CrossRef]
- Zhao, L.; Nakazawa, M.; Takase, T.; Manabe, K.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Matsui, M. Overexpression of LSH1, a Member of an Uncharacterised Gene Family, Causes Enhanced Light Regulation of Seedling Development. Plant J. 2004, 37, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.H.; Smolik, S. CBP/P300 in Cell Growth, Transformation, and Development. Genes Dev. 2000, 14, 1553–1577. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.G.; Ozdag, H.; Caldas, C. P300/CBP and Cancer. Oncogene 2004, 23, 4225–4231. [Google Scholar] [CrossRef]
- Roelfsema, J.H.; Peters, D.J.M. Rubinstein-Taybi Syndrome: Clinical and Molecular Overview. Expert Rev. Mol. Med. 2007, 9, 1–16. [Google Scholar] [CrossRef]
- Huang, Y.; Mouttet, B.; Warnatz, H.-J.; Risch, T.; Rietmann, F.; Frommelt, F.; Ngo, Q.A.; Dobay, M.P.; Marovca, B.; Jenni, S.; et al. The Leukemogenic TCF3-HLF Complex Rewires Enhancers Driving Cellular Identity and Self-Renewal Conferring EP300 Vulnerability. Cancer Cell 2019, 36, 630–644.e9. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-X.; Wei, B.-F.; Zhang, X.; Gong, Y.-P.; Ma, L.-Y.; Zhao, W. Current Development of CBP/P300 Inhibitors in the Last Decade. Eur. J. Med. Chem. 2021, 209, 112861. [Google Scholar] [CrossRef]
- Hogg, S.J.; Motorna, O.; Cluse, L.A.; Johanson, T.M.; Coughlan, H.D.; Raviram, R.; Myers, R.M.; Costacurta, M.; Todorovski, I.; Pijpers, L.; et al. Targeting Histone Acetylation Dynamics and Oncogenic Transcription by Catalytic P300/CBP Inhibition. Mol. Cell 2021, 81, 2183–2200.e13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, B.; Liu, X.; Zhang, X.D.; Zhang, L.; Liu, T. Histone Acetyltransferases CBP/P300 in Tumorigenesis and CBP/P300 Inhibitors as Promising Novel Anticancer Agents. Theranostics 2022, 12, 4935–4948. [Google Scholar] [CrossRef] [PubMed]
- Vittorioso, P.; Cowling, R.; Faure, J.D.; Caboche, M.; Bellini, C. Mutation in the Arabidopsis PASTICCINO1 Gene, Which Encodes a New FK506-Binding Protein-like Protein, Has a Dramatic Effect on Plant Development. Mol. Cell. Biol. 1998, 18, 3034–3043. [Google Scholar] [CrossRef]

| Control | Compound 2c (mg) | Sample | Compound 2c (mg) |
|---|---|---|---|
| C.1 | 0.91157 | S2c.1 | 0.67533 |
| C.2 | 0.83752 | S2c.2 | 0.62548 |
| C.3 | 0.90223 | S2c.3 | 0.59607 |
| average | 0.88377 | 0.63229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, C.; Lepri, A.; Paciolla, A.; Messore, A.; De Vita, D.; Bonaccorsi di Patti, M.C.; Amadei, M.; Madia, V.N.; Ialongo, D.; Di Santo, R.; et al. New Inhibitors of the Human p300/CBP Acetyltransferase Are Selectively Active against the Arabidopsis HAC Proteins. Int. J. Mol. Sci. 2022, 23, 10446. https://doi.org/10.3390/ijms231810446
Longo C, Lepri A, Paciolla A, Messore A, De Vita D, Bonaccorsi di Patti MC, Amadei M, Madia VN, Ialongo D, Di Santo R, et al. New Inhibitors of the Human p300/CBP Acetyltransferase Are Selectively Active against the Arabidopsis HAC Proteins. International Journal of Molecular Sciences. 2022; 23(18):10446. https://doi.org/10.3390/ijms231810446
Chicago/Turabian StyleLongo, Chiara, Andrea Lepri, Andrea Paciolla, Antonella Messore, Daniela De Vita, Maria Carmela Bonaccorsi di Patti, Matteo Amadei, Valentina Noemi Madia, Davide Ialongo, Roberto Di Santo, and et al. 2022. "New Inhibitors of the Human p300/CBP Acetyltransferase Are Selectively Active against the Arabidopsis HAC Proteins" International Journal of Molecular Sciences 23, no. 18: 10446. https://doi.org/10.3390/ijms231810446
APA StyleLongo, C., Lepri, A., Paciolla, A., Messore, A., De Vita, D., Bonaccorsi di Patti, M. C., Amadei, M., Madia, V. N., Ialongo, D., Di Santo, R., Costi, R., & Vittorioso, P. (2022). New Inhibitors of the Human p300/CBP Acetyltransferase Are Selectively Active against the Arabidopsis HAC Proteins. International Journal of Molecular Sciences, 23(18), 10446. https://doi.org/10.3390/ijms231810446











