Antiallergic Effects of N,N-dicoumaroylspermidine Isolated from Lithospermum erythrorhizon on Mast Cells and Ovalbumin-Induced Allergic Rhinitis
Abstract
:1. Introduction
2. Results
2.1. HPLC Analysis of Extracts of L. Erythrorhizon
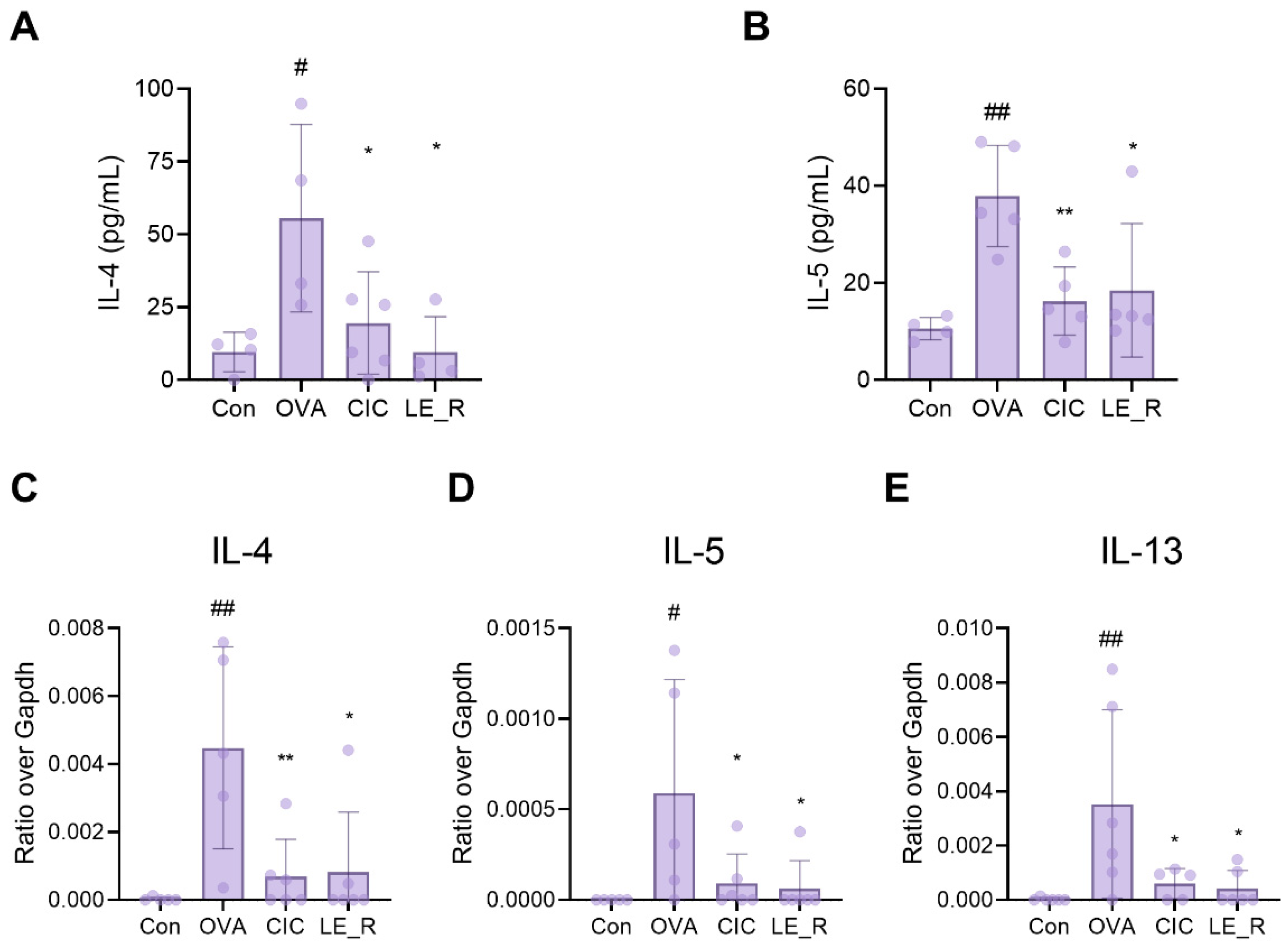
2.2. Effects of L. erythrorhizon Reflux Ethanol Extract on Levels of Th2 Cytokines in Serum and the Nasal Lavage Fluid of OVA-Induced Allergic Rhinitis Mice
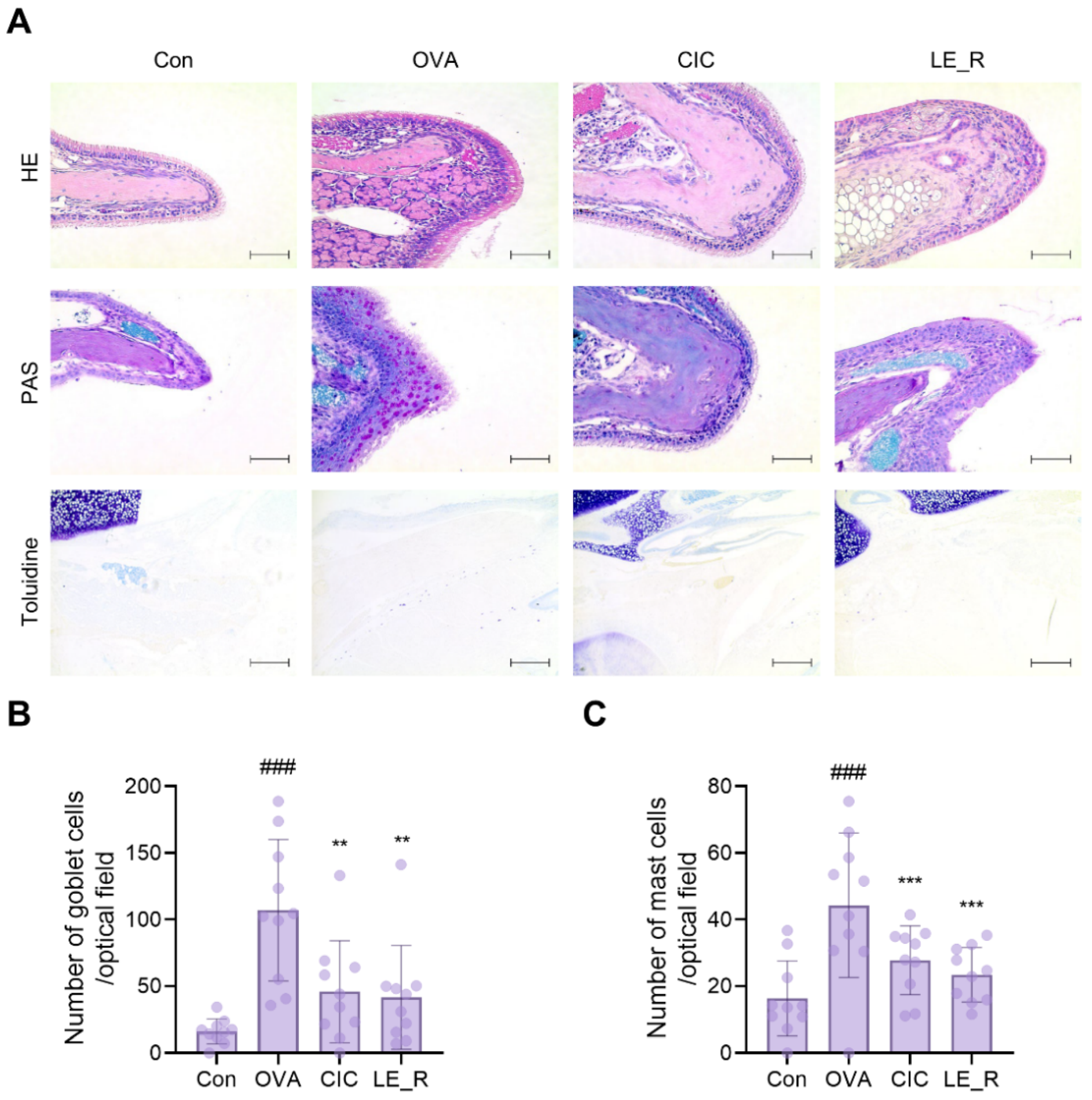
2.3. L. erythrorhizon Reflux Ethanol Extract Causes Histopathological Changes in Tissues from OVA-Induced Allergic Rhinitis Mice Model
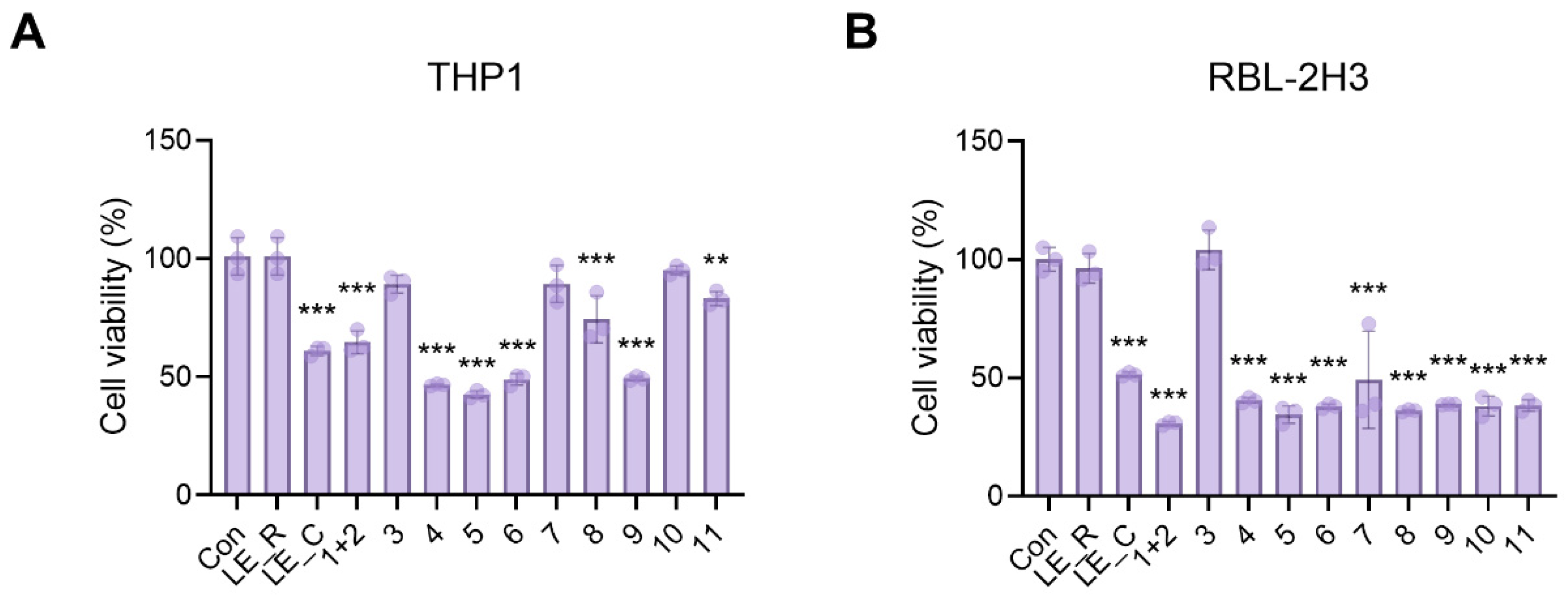
2.4. The Effects of 11 Constituents on Viability of THP1 Macrophages and RBL-2H3 Basophils
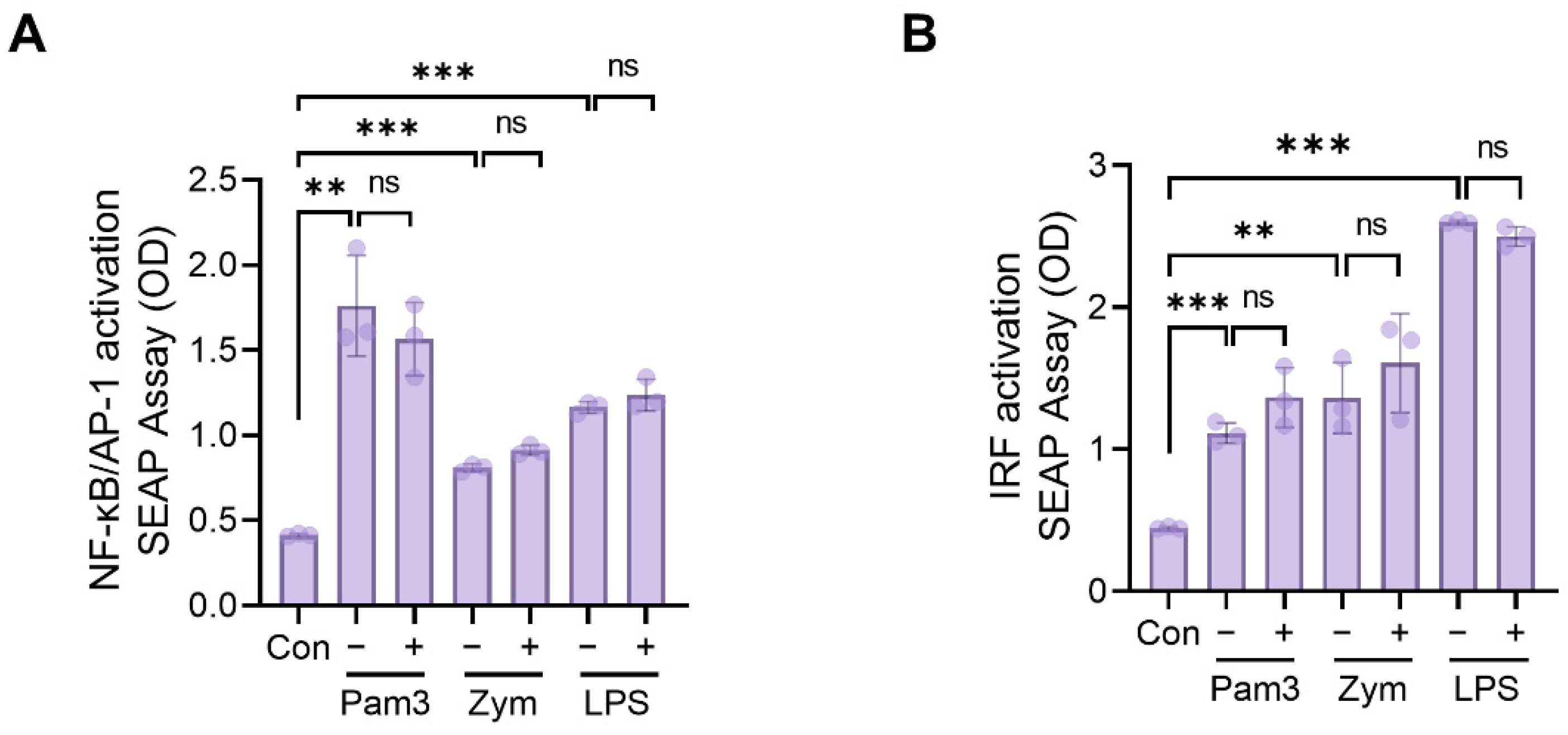
2.5. Assessment of NF-kB, AP1, and IRF Activation
2.6. Antiallergic Effects of N,N′-dicoumaroylspermidine on Mast Cells (RBL-2H3)
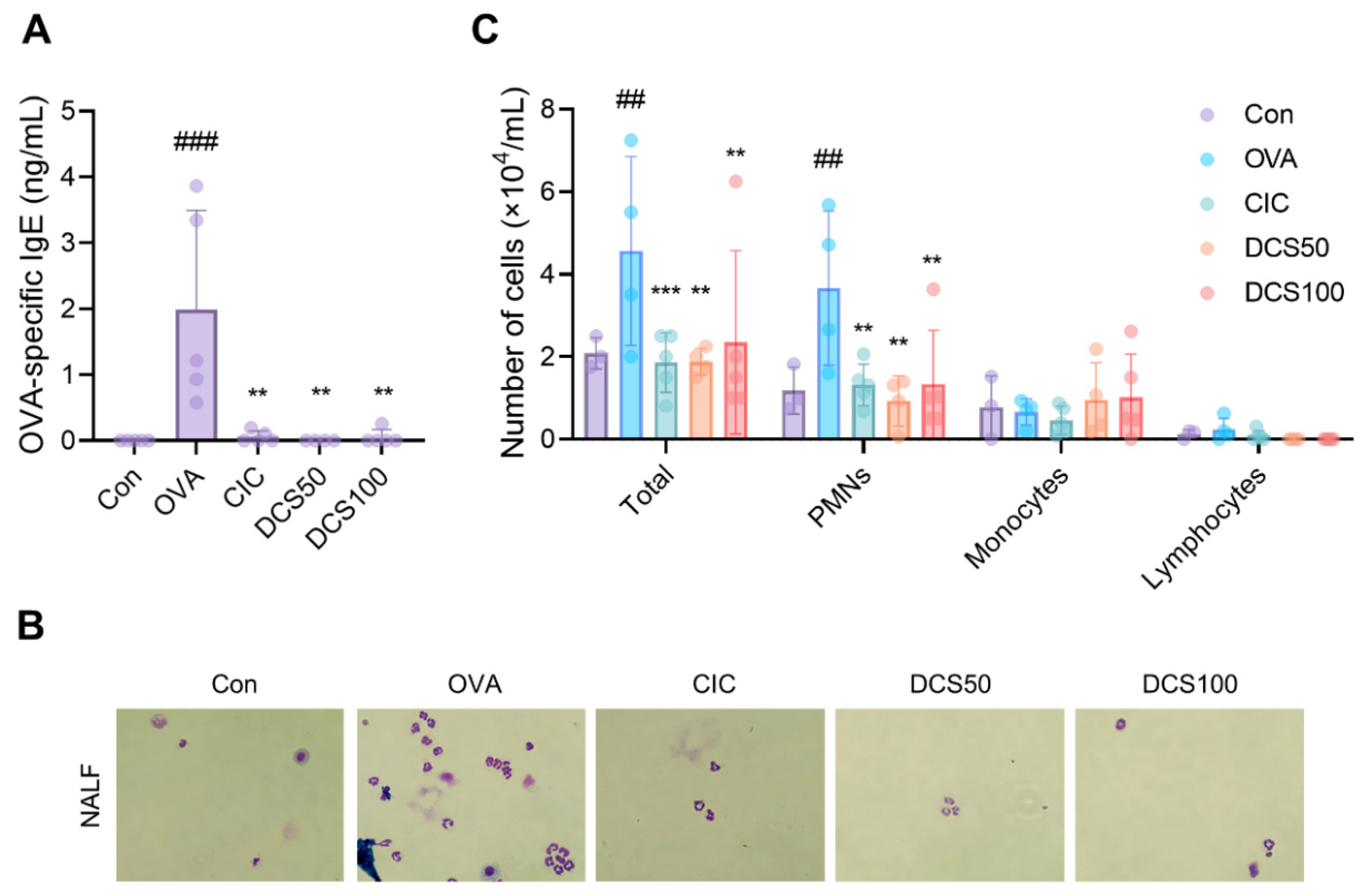
2.7. Effects of N,N′-dicoumaroylspermidine on Serum Levels of OVA-Specific IgE and Inflammatory Cells in the NALF of OVA-Induced Allergic Rhinitis Mice Model
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. HPLC Analysis
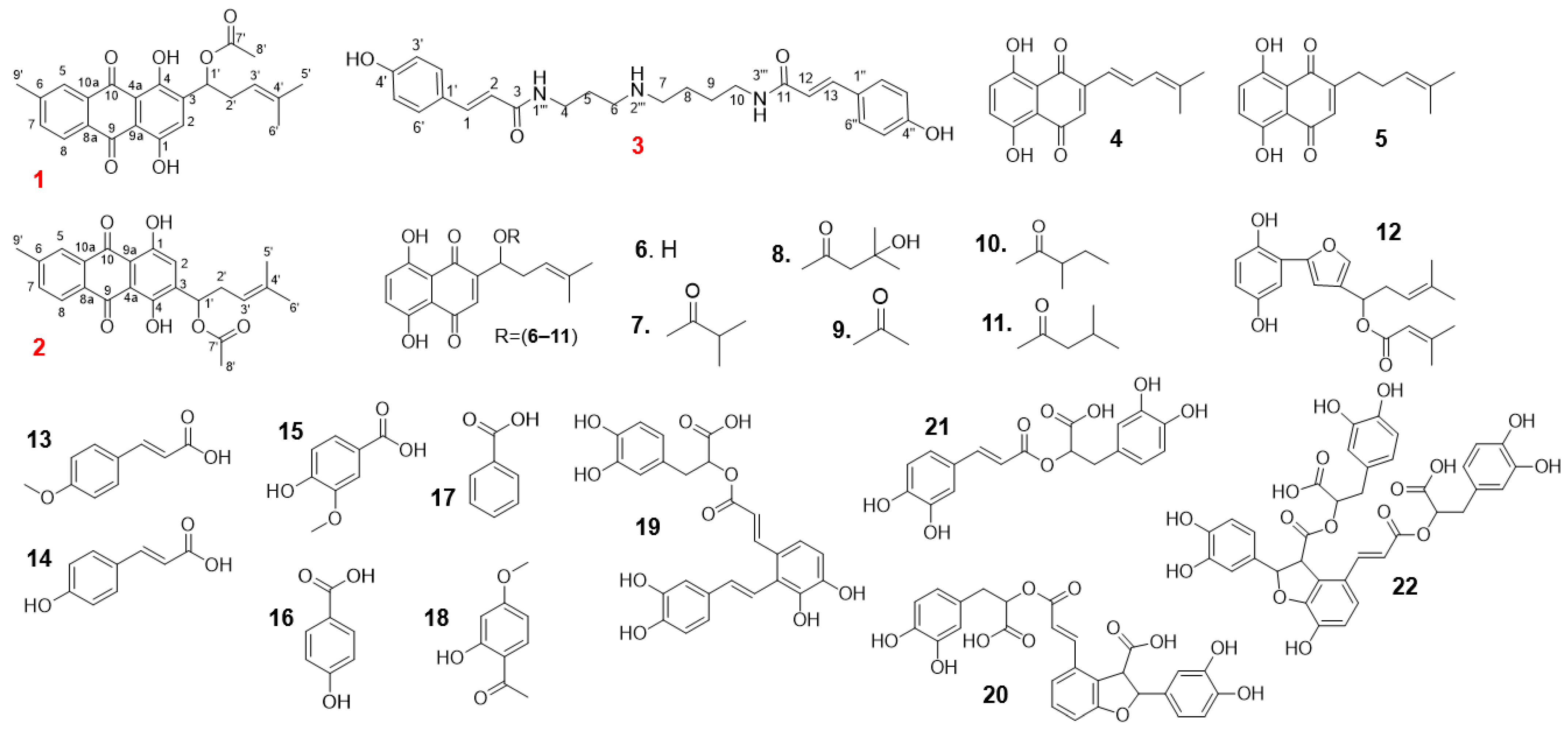
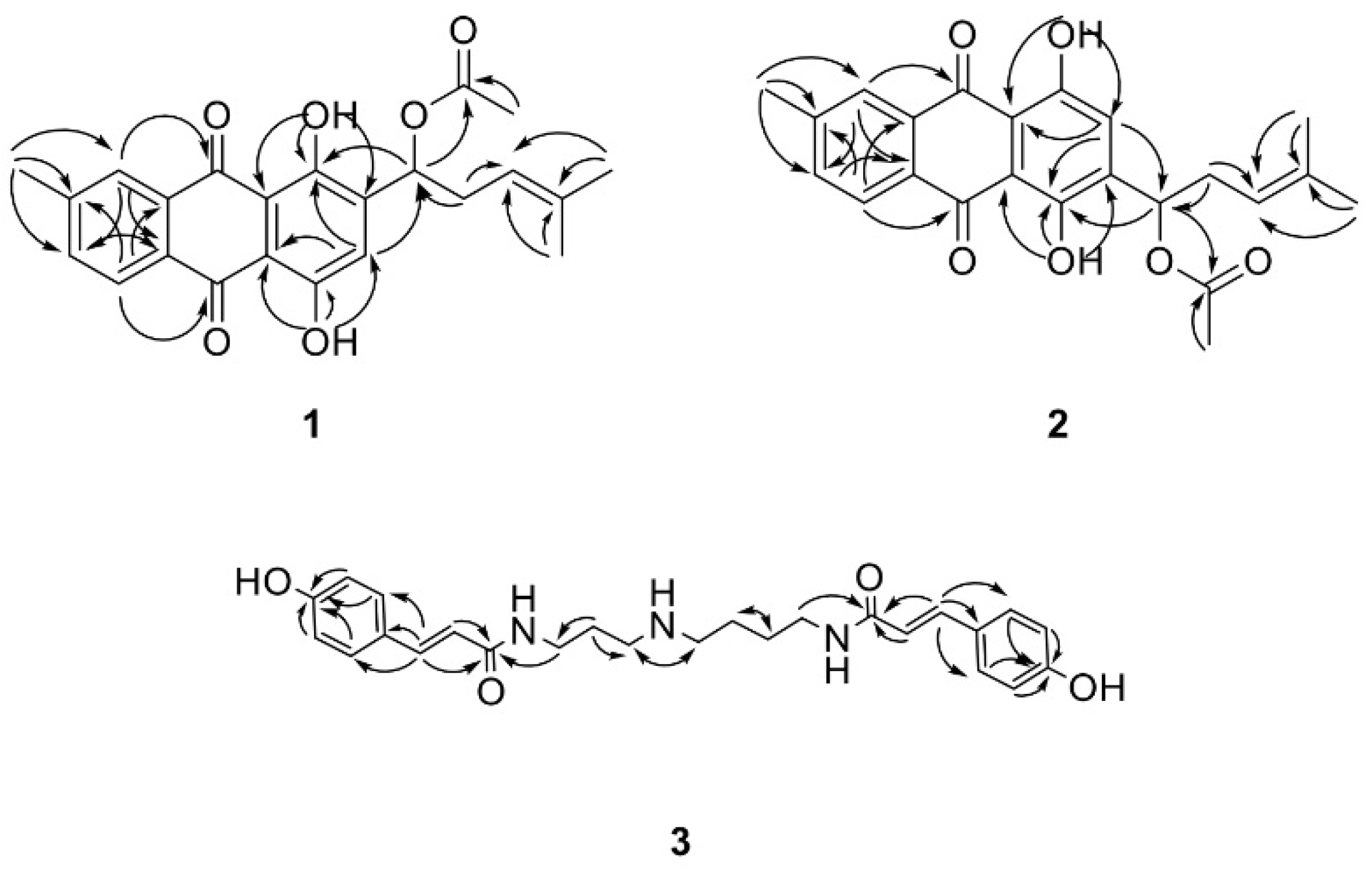
4.3. Isolation and Identification
4.4. Chemicals and Apparatus
4.5. Cell Culture and Viability Assay
4.6. β-Hexosaminidase Assay
4.7. Real-Time Quantitative PCR
4.8. Measurement of NF-κB/AP-1 and IRF Activity
4.9. In Vivo Studies of OVA-Induced Allergic Rhinitis Mouse Model
4.10. Collection and Analysis of Serum and NALF
4.11. Histopathological Evaluation of Nasal Mucosa
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawankar, R.; Mori, S.; Ozu, C.; Kimura, S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy 2011, 1, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Varshney, J.; Varshney, H. Allergic Rhinitis: An Overview. Indian J. Otolaryngol. Head Neck Surg. 2015, 67, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O. Quality of life in adults and children with allergic rhinitis. J. Allergy Clin. Immunol. 2001, 108, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Lotvall, J.; Simoens, S.; Subramanian, S.V.; Church, M.K. Economic burden of inadequate management of allergic diseases in the European Union: A GA(2) LEN review. Allergy 2014, 69, 1275–1279. [Google Scholar] [CrossRef]
- Lee, B.; Shin, Y.W.; Bae, E.A.; Han, S.J.; Kim, J.S.; Kang, S.S.; Kim, D.H. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch. Pharm. Res. 2008, 31, 445–450. [Google Scholar] [CrossRef]
- Pawankar, R.; Bunnag, C.; Khaltaev, N.; Bousquet, J. Allergic Rhinitis and Its Impact on Asthma in Asia Pacific and the ARIA Update 2008. World Allergy Organ. J. 2012, 5, S212–S217. [Google Scholar] [CrossRef] [PubMed]
- Bielory, L. Allergic conjunctivitis and the impact of allergic rhinitis. Curr. Allergy Asthma Rep. 2010, 10, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.L.; Austen, K.F. Recent advances in the cellular and molecular biology of mast cells. Immunol. Today 1989, 10, 381–386. [Google Scholar] [CrossRef]
- Nickoloff, B.J. The cytokine network in psoriasis. Arch. Dermatol. 1991, 127, 871–884. [Google Scholar] [CrossRef]
- Kang, T.K.; Le, T.T.; Kim, K.A.; Kim, Y.J.; Lee, W.B.; Jung, S.H. Roots of Lithospermum erythrorhizon promotes retinal cell survival in optic nerve crush-induced retinal degeneration. Exp. Eye Res. 2021, 203, 108419. [Google Scholar] [CrossRef]
- Tanaka, S.; Tajima, M.; Tsukada, M.; Tabata, M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J. Nat. Prod. 1986, 49, 466–469. [Google Scholar] [CrossRef]
- Ito, Y.; Onobori, K.; Yamazaki, T.; Kawamura, Y. Tigloylshikonin, a New Minor Shikonin Derivative, from the Roots and the Commercial Root Extract of Lithospermum erythrorhizon. Chem. Pharm. Bull. 2011, 59, 117–119. [Google Scholar] [CrossRef]
- Brigham, L.A.; Michaels, P.J.; Flores, H.E. Cell-specific production and antimicrobial activity of naphthoquinones in roots of lithospermum erythrorhizon. Plant Physiol. 1999, 119, 417–428. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Zhang, N.; Turpin, J.A.; Buckheit, R.W.; Osterling, C.; Oppenheim, J.J.; Howard, O.M. Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2003, 47, 2810–2816. [Google Scholar] [CrossRef]
- Kang, T.K.; Le, T.T.; Choi, S.-Y.; Song, H.-W.; Lee, W.-B.; Jung, S.H. Roots of Lithospermum erythrorhizon Alleviated Ovalbumin-Induced Allergic Rhinitis and IgE-triggered Degranulation of RBL-2H3 Cells. Appl. Sci. 2022, 12, 6116. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef]
- Cho, M.H.; Paik, Y.S.; Hahn, T.R. Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea. J. Agric. Food Chem. 1999, 47, 4117–4120. [Google Scholar] [CrossRef]
- Kim, D.H.; Hong, S.W.; Kim, B.T.; Bae, E.A.; Park, H.Y.; Han, M.J. Biotransformation of glycyrrhizin by human intestinal bacteria and its relation to biological activities. Arch. Pharm. Res. 2000, 23, 172–177. [Google Scholar] [CrossRef]
- Han, H.; Weng, X.C.; Bi, K.S. Antioxidants from a Chinese medicinal herb—Lithospermum erythrorhizon. Food Chem. 2008, 106, 2–10. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeong, H.J.; Park, J.Y.; Kim, Y.M.; Park, S.J.; Cho, J.K.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Selective and slow-binding inhibition of shikonin derivatives isolated from Lithospermum erythrorhizon on glycosyl hydrolase 33 and 34 sialidases. Bioorganic Med. Chem. 2012, 20, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Stabile, R.G.; Dicks, A.R. Two-step semi-microscale preparation of a cinnamate ester sunscreen analog. J. Chem. Educ. 2004, 81, 1488–1491. [Google Scholar] [CrossRef]
- Towers, G.H.N.; Maass, W.S.G. Phenolic acids and lignins in the lycopodiales. Phytochemistry 1965, 4, 57–66. [Google Scholar] [CrossRef]
- Tummatorn, J.; Ruchirawat, S.; Ploypradith, P. A Convergent General Strategy for the Functionalized 2-Aryl Cycloalkyl-Fused Chromans: Intramolecular Hetero-Diels-Alder Reactions of ortho-Quinone Methides. Chem. Eur. J. 2010, 16, 1445–1448. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhu, H.F.; Wang, J.H.; Liu, Z.B.; Bi, J.J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2009, 877, 733–737. [Google Scholar] [CrossRef]
- Ly, T.N.; Shimoyamada, M.; Yamauchi, R. Isolation and characterization of rosmarinic acid oligomers in Celastrus hindsii Benth leaves and their antioxidative activity. J. Agric. Food Chem. 2006, 54, 3786–3793. [Google Scholar] [CrossRef]
- Werner, C.; Hu, W.Q.; Lorenziriatsch, A.; Hesse, M. Di-Coumaroylspermidines and Tri-Coumaroylspermidines in Anthers of Different Species of the Genus Aphelandra. Phytochemistry 1995, 40, 461–465. [Google Scholar] [CrossRef]
- Iwanaszko, M.; Kimmel, M. NF-kappaB and IRF pathways: Cross-regulation on target genes promoter level. BMC Genom. 2015, 16, 307. [Google Scholar] [CrossRef]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef]
- Vandenplas, O.; D’Alpaos, V.; Van Brussel, P. Rhinitis and its impact on work. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 145–149. [Google Scholar] [CrossRef]
- Rahim, N.A.; Jantan, I.; Said, M.M.; Jalil, J.; Abd Razak, A.F.; Husain, K. Anti-Allergic Rhinitis Effects of Medicinal Plants and Their Bioactive Metabolites via Suppression of the Immune System: A Mechanistic Review. Front. Pharmacol. 2021, 12, 660083. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Jia, J.; He, M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell Immunol. 2015, 298, 88–95. [Google Scholar] [CrossRef]
- Aswar, U.; Shintre, S.; Chepurwar, S.; Aswar, M. Antiallergic effect of piperine on ovalbumin-induced allergic rhinitis in mice. Pharm. Biol. 2015, 53, 1358–1366. [Google Scholar] [CrossRef]
- Almansouri, H.M.; Yamamoto, S.; Kulkarni, A.D.; Ariizumi, M.; Adjei, A.A.; Yamauchi, K. Effect of dietary nucleosides and nucleotides on murine allergic rhinitis. Am. J. Med. Sci. 1996, 312, 202–205. [Google Scholar] [CrossRef]
- Church, M.K.; Levi-Schaffer, F. The human mast cell. J. Allergy Clin. Immunol. 1997, 99, 155–160. [Google Scholar] [CrossRef]
- Hofmann, A.M.; Abraham, S.N. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr. Opin. Immunol. 2009, 21, 679–686. [Google Scholar] [CrossRef]
- Passante, E.; Ehrhardt, C.; Sheridan, H.; Frankish, N. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm. Res. 2009, 58, 611–618. [Google Scholar] [CrossRef]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India 2010, 27, 66–71. [Google Scholar] [CrossRef]
- Grunig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef]
- Yannai, S. Dictionary of Food Compounds with CD-ROM, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013; p. xxvi. 2320p. [Google Scholar]
- Wang, W.; Yu, Z.; Meng, J.; Zhou, P.; Luo, T.; Zhang, J.; Wu, J.; Lou, Y. Rice phenolamindes reduce the survival of female adults of the white-backed planthopper Sogatella furcifera. Sci. Rep. 2020, 10, 5778. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, L.P.; Liu, G.M.; Zhang, D.S.; He, H.J. Evaluation of the Nutritional Quality of Chinese Kale (Brassica alboglabra Bailey) Using UHPLC-Quadrupole-Orbitrap MS/MS-Based Metabolomics. Molecules 2017, 22, 1262. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Fawzi Mahomoodally, M. RP-UHPLC-MS Chemical Profiling, Biological and In Silico Docking Studies to Unravel the Therapeutic Potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Yoo, Y.; Hahn, T.R.; Bhoo, S.H.; Lee, S.W.; Cho, M.H. Antimicrobial Activity of UV-Induced Phenylamides from Rice Leaves. Molecules 2014, 19, 18139–18151. [Google Scholar] [CrossRef]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Yogendra, K.N.; Kushalappa, A.C.; Sarmiento, F.; Rodriguez, E.; Mosquera, T. Metabolomics deciphers quantitative resistance mechanisms in diploid potato clones against late blight. Funct. Plant Biol. 2015, 42, 284–298. [Google Scholar] [CrossRef]
- McLusky, S.R.; Bennett, M.H.; Beale, M.H.; Lewis, M.J.; Gaskin, P.; Mansfield, J.W. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J. 1999, 17, 523–534. [Google Scholar] [CrossRef]
- Zhu, F.X.; Xu, Z.M.; Yonekura, L.; Yang, R.H.; Tamura, H. Antiallergic activity of rosmarinic acid esters is modulated by hydrophobicity, and bulkiness of alkyl side chain. Biosci. Biotech. Bioch. 2015, 79, 1178–1182. [Google Scholar] [CrossRef]


| Position | 1 | 2 | ||
|---|---|---|---|---|
| δ C | δ H | δ C | δ H | |
| 1 | 157.40, C | 157.34, C | ||
| 2 | 125.29, CH | 7.31, s | 125.04, CH | 7.30, s |
| 3 | 142.36, C | 142.11, C | ||
| 4 | 154.99, C | 154.95, C | ||
| 5 | 127.39, CH | 8.16, m | 127.20, CH | 8.16, m |
| 6 | 145.97, C | 145.89, C | ||
| 7 | 135.36, CH | 7.65, m | 135.43, CH | 7.65, m |
| 8 | 127.3, CH | 8.26, d (7.96) | 127.30, CH | 8.24, d (7.96) |
| 9 | 186.63, C | 186.97, C | ||
| 10 | 187.18, C | 187.33, C | ||
| 4a | 112.68, C | 112.52, C | ||
| 8a | 131.21, C | 131.20, C | ||
| 9a | 112.23, C | 112.08, C | ||
| 10a | 133.41, C | 133.38, C | ||
| 1′ | 70.09, CH | 6.20, m | 70.09, CH | 6.20, m |
| 2′ | 33.01, CH2 | 2.49–2.73, m | 33.01, CH2 | 2.49–2.73, m |
| 3′ | 118.13, CH | 5.17, m | 118.13, CH | 5.17, m |
| 4′ | 135.67, C | 135.67, C | ||
| 5′ | 17.88, CH3 | 1.59, s | 17.88, CH3 | 1.59, s |
| 6′ | 25.81, CH3 | 1.71, s | 25.81, CH3 | 1.71, s |
| 7′ | 170.10, C | 170.10, C | ||
| 8′ | 21.10, CH3 | 2.16, s | 21.10, CH3 | 2.16, s |
| 9′ | 22.03, CH3 | 2.56, s | 22.03, CH3 | 2.56, s |
| OH-1 | 12.98 | 12.94 | ||
| OH-4 | 13.39 | 13.43 | ||
| Position | 3 | |
|---|---|---|
| δ C | δ H | |
| 1 | 140.73, CH | 7.51, d (15.56) |
| 2 | 116.24, CH | 6.42, d (15.68) |
| 3 | 168.93, C | |
| 4 | 35.56, CH2 | 3.43, t (6.40) |
| 5 | 26.58, CH2 | 1.89–1.99, m |
| 6 | 44.96, CH2 | 2.99–3.08, m |
| 7 | 47.62, CH2 | 2.99–3.08, m |
| 8 | 26.32, CH2 | 1.65–1.82, m |
| 9 | 23.28, CH2 | 1.65–1.82, m |
| 10 | 38.10, CH2 | 3.37, t (6.72) |
| 11 | 168.05, C | |
| 12 | 116.91, CH | 6.42, d (15.68) |
| 13 | 141.40, CH | 7.47, d (15.56) |
| 1′ | 126.02, C | |
| 2′ | 129.17, CH | 7.41, d (8.64) |
| 3′ | 115.36, CH | 6.80, d (8.56) |
| 4′ | 159.26, C | |
| 5′ | 115.36, CH | 6.80, d (8.56) |
| 6′ | 129.17, CH | 7.41, d (8.64) |
| 1″ | 126.18, C | |
| 2″ | 129.30, CH | 7.42, d (8.68) |
| 3″ | 115.39, CH | 6.80, d (8.56) |
| 4″ | 159.42, C | |
| 5″ | 115.39, CH | 6.80, d (8.56) |
| 6″ | 129.30, CH | 7.42, d (8.68) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.T.; Kang, T.K.; Lee, W.-B.; Jung, S.H. Antiallergic Effects of N,N-dicoumaroylspermidine Isolated from Lithospermum erythrorhizon on Mast Cells and Ovalbumin-Induced Allergic Rhinitis. Int. J. Mol. Sci. 2022, 23, 10403. https://doi.org/10.3390/ijms231810403
Le TT, Kang TK, Lee W-B, Jung SH. Antiallergic Effects of N,N-dicoumaroylspermidine Isolated from Lithospermum erythrorhizon on Mast Cells and Ovalbumin-Induced Allergic Rhinitis. International Journal of Molecular Sciences. 2022; 23(18):10403. https://doi.org/10.3390/ijms231810403
Chicago/Turabian StyleLe, Tam Thi, Tae Kyeom Kang, Wook-Bin Lee, and Sang Hoon Jung. 2022. "Antiallergic Effects of N,N-dicoumaroylspermidine Isolated from Lithospermum erythrorhizon on Mast Cells and Ovalbumin-Induced Allergic Rhinitis" International Journal of Molecular Sciences 23, no. 18: 10403. https://doi.org/10.3390/ijms231810403
APA StyleLe, T. T., Kang, T. K., Lee, W.-B., & Jung, S. H. (2022). Antiallergic Effects of N,N-dicoumaroylspermidine Isolated from Lithospermum erythrorhizon on Mast Cells and Ovalbumin-Induced Allergic Rhinitis. International Journal of Molecular Sciences, 23(18), 10403. https://doi.org/10.3390/ijms231810403






