Molecular Clues to Understanding Causes of Human-Assisted Reproduction Treatment Failures and Possible Treatment Options
Abstract
1. Introduction
2. Impaired Sperm Function
3. Impaired Oocyte Function
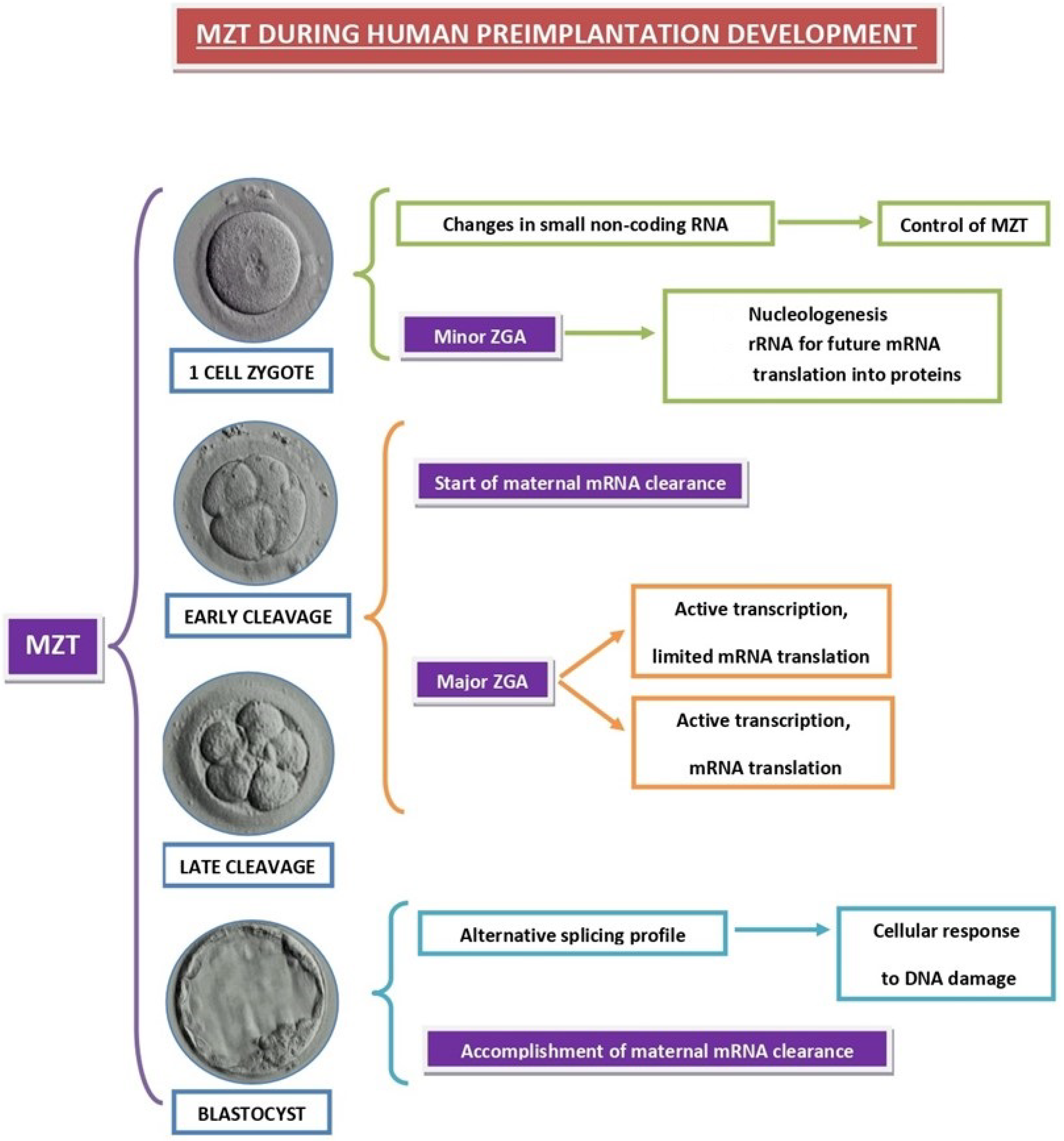
4. Impaired Preimplantation Embryo Development
4.1. Pre-MZT Phase
4.2. Major ZGA Phase
4.3. Accomplished MZT Phase
5. Impaired Uterine Receptivity
5.1. First Messenger Failure
5.2. Failure of Post-Receptor Signal Transduction Pathways
6. Pregnancy Failure after Implantation Stage
7. Clues to Possible Treatment Options
7.1. Male Infertility
7.2. Female Infertility
| Treatment | Mechanism of Action | Target Cells | Reference |
|---|---|---|---|
| ROS scavengers | Re-establishment of redox balance | Oocytes, granulosa cells | [109,116,117,118,119] |
| Protection of mitochondria | Endometriotic cells | [114] | |
| GH | Activation of endogenous antioxidant systems | Oocytes, granulosa cells | [109] |
| Induction of FSHR, LHR, GHR and BMPR1B | Granulosa cells | [130] | |
| Pentoxifylline | Increasing intracellular cAMP | Granulosa cells | [131] |
| Melatonin | Direct ROS scavenger | Oocytes, granulosa cells | [109,133,134] |
| Activation of endogenous antioxidant systems | Oocyte, granulosa cells | [109,133,134] | |
| DNA damage repair | Oocytes | [135] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 2, 366. [Google Scholar] [CrossRef]
- Tesarik, J. Forty years of in vitro fertilization: A history of continuous expansion. In 40 Years after In Vitro Fertilisation; Tesarik, J., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; pp. 1–24. [Google Scholar]
- Gleicher, N.; Kushnir, V.A.; Barad, D.H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open 2019, 2019, hoz017. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, S.A.; Rajkovic, A. Genetics of human female infertility. Biol. Reprod. 2019, 101, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Y.; Yu, Y. Precise Personalized Medicine in Gynecology Cancer and Infertility. Front. Cell Dev. Biol. 2020, 7, 382. [Google Scholar] [CrossRef]
- Tesarik, J. Toward Molecular Medicine in Female Infertility Management: Editorial to the Special Issue “Molecular Mechanisms of Human Oogenesis and Early Embryogenesis”. Int. J. Mol. Sci. 2021, 22, 13517. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C. In vitro fertilization by intracytoplasmic sperm injection. Bioessays 1999, 21, 791–801. [Google Scholar] [CrossRef]
- Mojarrad, M.; Saburi, E.; Golshan, A.; Moghbeli, M. Genetics and molecular biology of male infertility among Iranian population: An update. Am. J. Transl. Res. 2021, 13, 5767–5785. [Google Scholar]
- Tesarik, J.; Testart, J. Treatment of sperm-injected human oocytes with Ca2+ ionophore supports the development of Ca2+ oscillations. Biol. Reprod. 1994, 51, 385–391. [Google Scholar] [CrossRef][Green Version]
- Tesarik, J.; Rienzi, L.; Ubaldi, F.; Mendoza, C.; Greco, E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil. Steril. 2002, 78, 619–624. [Google Scholar] [CrossRef]
- Yanagida, K.; Fujikura, Y.; Katayose, H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod. Med. Biol. 2008, 7, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J. Acquired sperm DNA modifications: Causes, consequences, and potential solutions. Eur. Med. J. 2019, 4, 83–93. [Google Scholar]
- Aitken, R.J.; Clarkson, J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J. Reprod. Fertil. 1987, 81, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S.; Lange, J.; Mohibullah, N. Self-organization of meiotic recombination initiation: General principles and molecular pathways. Annu. Rev. Genet. 2014, 48, 187–214. [Google Scholar] [CrossRef]
- Cooper, T.J.; Wardell, K.; Garcia, V.; Neale, M.J. Homeostatic regulation of meiotic DSB formation by ATM/ATR. Exp. Cell Res. 2014, 329, 124–131. [Google Scholar] [CrossRef]
- Gunes, S.; Al-Sadaan, M.; Agarwal, A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod. Biomed. Online 2015, 31, 309–319. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Gosálvez, J.; Agarwal, A.; Roy, R.; Johnston, S. DNA Damage and Repair in Human Reproductive Cells. Int. J. Mol. Sci. 2018, 20, 31. [Google Scholar] [CrossRef]
- Olsen, A.K.; Lindeman, B.; Wiger, R.; Duale, N.; Brunborg, G. How do male germ cells handle DNA damage? Toxicol. Appl. Pharmacol. 2005, 207 (Suppl. 2), 521–531. [Google Scholar] [CrossRef]
- Tesarik, J.; Ubaldi, F.; Rienzi, L.; Martinez, F.; Iacobelli, M.; Mendoza, C.; Greco, E. Caspase-dependent and -independent DNA fragmentation in Sertoli and germ cells from men with primary testicular failure: Relationship with histological diagnosis. Hum. Reprod. 2004, 19, 254–261. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Feng, H.L.; Zhao, L.; Liu, P.; Li, L.; Yan, J.; Qiao, J. Alteration of ERβ gene RsaI polymorphism may contribute to reduced fertilization rate and embryonic developmental competence. Asian J. Androl. 2011, 13, 317–321. [Google Scholar] [CrossRef]
- Aarabi, M.; Balakier, H.; Bashar, S.; Moskovtsev, S.I.; Sutovsky, P.; Librach, C.L.; Oko, R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014, 28, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.; Balakier, H.; Bashar, S.; Moskovtsev, S.I.; Sutovsky, P.; Librach, C.L.; Oko, R. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil. Steril. 2014, 102, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.I.; Hassold, T.J.; Hunt, P.A. Human aneuploidy: Mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 2012, 13, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef]
- Tesarik, J.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Ovarian aging: Molecular mechanisms and medical management. Int. J. Mol. Sci. 2021, 22, 1371. [Google Scholar] [CrossRef]
- Ottolini, C.S.; Newnham, L.; Capalbo, A.; Natesan, S.A.; Joshi, H.A.; Cimadomo, D.; Griffin, D.K.; Sage, K.; Summers, M.C.; Thornhill, A.R.; et al. Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat. Genet. 2015, 47, 727–735. [Google Scholar] [CrossRef]
- Wartosch, L.; Schindler, K.; Schuh, M.; Gruhn, J.R.; Hoffmann, E.R.; McCoy, R.C.; Xing, J. Origins and mechanisms leading to aneuploidy in human eggs. Prenat. Diagn. 2021, 41, 620–630. [Google Scholar] [CrossRef]
- Ghevaria, H.; SenGupta, S.; Naja, R.; Odia, R.; Exeter, H.; Serhal, P.; Gonzalez, X.V.; Sun, X.; Delhanty, J. Next generation sequencing detects premeiotic errors in human oocytes. Int. J. Mol. Sci. 2022, 23, 665. [Google Scholar] [CrossRef]
- Wang, S.; Hassold, T.; Hunt, P.; White, M.A.; Zickler, D.; Kleckner, N.; Zhang, L. Inefficient crossover maturation underlies elevated aneuploidy in human female meiosis. Cell 2017, 168, 977–989.e17. [Google Scholar] [CrossRef]
- Fan, C.; Yang, X.; Nie, H.; Wang, S.; Zhang, L. Per-nucleus crossover covariation is regulated by chromosome organization. iScience 2022, 25, 104115. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Maylor-Hagen, H.; Wood, A.; Gruhn, J.; Hoffmann, E.; Broman, K.W.; Hunt, P. Failure to recombine is a common feature of human oogenesis. Am. J. Hum. Genet. 2021, 108, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sfakianoudis, K.; Maziotis, E.; Karantzali, E.; Kokkini, G.; Grigoriadis, S.; Pantou, A.; Giannelou, P.; Petroutsou, K.; Markomichali, C.; Fakiridou, M.; et al. Molecular drivers of developmental arrest in the human preimplantation embryo: A systematic review and critical analysis leading to mapping future research. Int. J. Mol. Sci. 2021, 22, 8353. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, T.A.; Gandolfi, F. The maternal legacy to the embryo: Cytoplasmic components and their effects on early development. Theriogenology 2001, 55, 1255–1276. [Google Scholar] [CrossRef]
- Winata, C.L.; Korzh, V. The translational regulation of maternal mRNAs in time and space. FEBS Lett. 2018, 592, 3007–3023. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Bellusci, M.; Paredes-Fuentes, A.J.; Ruiz-Pesini, E.; Gómez, B.; MITOSPAIN Working Group; Martín, M.A.; Montoya, J.; Artuch, R. The genetic landscape of mitochondrial diseases in Spain: A Nationwide Call. Genes 2021, 12, 1590. [Google Scholar] [CrossRef]
- Tadros, W.; Lipshitz, H.D. The maternal-to-zygotic transition: A play in two acts. Development 2009, 136, 3033–3042. [Google Scholar] [CrossRef]
- Sha, Q.Q.; Zhang, J.; Fan, H.Y. A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammals. Biol. Reprod. 2019, 101, 579–590. [Google Scholar] [CrossRef]
- Latham, K.E. Mechanisms and control of embryonic genome activation in mammalian embryos. Int. Rev. Cytol. 1999, 193, 71–124. [Google Scholar] [CrossRef]
- Abe, K.I.; Funaya, S.; Tsukioka, D.; Kawamura, M.; Suzuki, Y.; Suzuki, M.G.; Schultz, R.M.; Aoki, F. Minor zygotic gene activation is essential for mouse preimplantation development. Proc. Natl. Acad. Sci. USA 2018, 115, E6780–E6788. [Google Scholar] [CrossRef] [PubMed]
- Asami, M.; Lam, B.Y.H.; Ma, M.K.; Rainbow, K.; Braun, S.; VerMilyea, M.D.; Yeo, G.S.H.; Perry, A.C.F. Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell 2022, 29, 209–216.e4. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J. Reprod. Fertil. 1986, 78, 463–470. [Google Scholar] [CrossRef]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. Early morphological signs of embryonic genome expression in human preimplantation development as revealed by quantitative electron microscopy. Dev. Biol. 1988, 128, 15–20. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. High-resolution autoradiographic localization of DNA-containing sites and RNA synthesis in developing nucleoli of human preimplantation embryos: A new concept of embryonic nucleologenesis. Development 1987, 101, 777–791. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecny, V. Assembly of the nucleolar precursor bodies in human male pronuclei is correlated with an early RNA synthetic activity. Exp. Cell Res. 1990, 191, 153–156. [Google Scholar] [CrossRef]
- Paloviita, P.; Hydén-Granskog, C.; Yohannes, D.A.; Paluoja, P.; Kere, J.; Tapanainen, J.S.; Krjutško, K.; Tuuri, T.; Võsa, U.; Vuoristo, S. Small RNA expression and miRNA modification dynamics in human oocytes and early embryos. Genome Res. 2021, 31, 1474–1485. [Google Scholar] [CrossRef]
- Lieberfarb, M.E.; Chu, T.; Wreden, C.; Theurkauf, W.; Gergen, J.P.; Strickland, S. Mutations that perturb poly(A)-dependent maternal mRNA activation block the initiation of development. Development 1996, 122, 579–588. [Google Scholar] [CrossRef]
- Potireddy, S.; Vassena, R.; Patel, B.G.; Latham, K.E. Analysis of polysomal mRNA populations of mouse oocytes and zygotes: Dynamic changes in maternal mRNA utilization and function. Dev. Biol. 2006, 298, 155–166. [Google Scholar] [CrossRef]
- Musfee, F.I.; Oluwafemi, O.O.; Agopian, A.J.; Hakonarson, H.; Goldmuntz, E.; Mitchell, L.E. Maternal effect genes as risk factors for congenital heart defects. HGG Adv. 2022, 3, 100098. [Google Scholar] [CrossRef] [PubMed]
- Vuoristo, S.; Bhagat, S.; Hydén-Granskog, C.; Yoshihara, M.; Gawriyski, L.; Jouhilahti, E.M.; Ranga, V.; Tamirat, M.; Huhtala, M.; Kirjanov, I.; et al. DUX4 is a multifunctional factor priming human embryonic genome activation. iScience 2022, 25, 104137. [Google Scholar] [CrossRef] [PubMed]
- Vastenhouw, N.L.; Cao, W.X.; Lipshitz, H.D. The maternal-to-zygotic transition revisited. Development 2019, 146, dev161471. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Franciosi, F. Acquisition of oocyte competence to develop as an embryo: Integrated nuclear and cytoplasmic events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, A.; Planet, E.; Coluccio, A.; Verp, S.; Duc, J.; Trono, D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 2017, 49, 941–945. [Google Scholar] [CrossRef]
- Hendrickson, P.G.; Doráis, J.A.; Grow, E.J.; Whiddon, J.L.; Lim, J.W.; Wike, C.L.; Weaver, B.D.; Pflueger, C.; Emery, B.R.; Wilcox, A.L.; et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 2017, 49, 925–934. [Google Scholar] [CrossRef]
- Wyatt, C.D.R.; Pernaute, B.; Gohr, A.; Miret-Cuesta, M.; Goyeneche, L.; Rovira, Q.; Salzer, M.C.; Boke, E.; Bogdanovic, O.; Bonnal, S.; et al. A developmentally programmed splicing failure contributes to DNA damage response attenuation during mammalian zygotic genome activation. Sci. Adv. 2022, 8, eabn4935. [Google Scholar] [CrossRef]
- Sha, Q.Q.; Zheng, W.; Wu, Y.W.; Li, S.; Guo, L.; Zhang, S.; Lin, G.; Ou, X.H.; Fan, H.Y. Dynamics and clinical relevance of maternal mRNA clearance during the oocyte-to-embryo transition in humans. Nat. Commun. 2020, 11, 4917. [Google Scholar] [CrossRef]
- Yan, L.; Yang, M.; Guo, H.; Yang, L.; Wu, J.; Li, R.; Liu, P.; Lian, Y.; Zheng, X.; Yan, J.; et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 2013, 20, 1131–1139. [Google Scholar] [CrossRef]
- Wu, J.; Xu, J.; Liu, B.; Yao, G.; Wang, P.; Lin, Z.; Huang, B.; Wang, X.; Li, T.; Shi, S.; et al. Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature 2018, 557, 256–260. [Google Scholar] [CrossRef]
- Tesarik, J. Involvement of oocyte-coded message in cell differentiation control of early human embryos. Development 1989, 105, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Winata, C.L.; Łapiński, M.; Pryszcz, L.; Vaz, C.; Bin Ismail, M.H.; Nama, S.; Hajan, H.S.; Lee, S.G.P.; Korzh, V.; Sampath, P.; et al. Cytoplasmic polyadenylation-mediated translational control of maternal mRNAs directs maternal-to-zygotic transition. Development 2018, 145, dev159566. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.; Drapkina, Y.; Fedorov, I.; Chagovets, V.; Makarova, N.; Shamina, M.; Kalinina, E.; Sukhikh, G. Small noncoding RNA signatures for determining the developmental potential of an embryo at the morula stage. Int. J. Mol. Sci. 2020, 21, 9399. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Ran, H.; Zhang, S.; Xia, G.; Wang, B.; Wang, H. Molecular determinants of uterine receptivity. Int. J. Dev. Biol. 2014, 58, 147–154. [Google Scholar] [CrossRef]
- Dey, S.K. How we are born. J. Clin. Investig. 2010, 120, 952–955. [Google Scholar] [CrossRef][Green Version]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Su, R.W.; Fazleabas, A.T. Implantation and establishment of pregnancy in human and nonhuman primates. Adv. Anat. Embryol. Cell Biol. 2015, 216, 189–213. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Liu, J.; Kong, N.; Jiang, Y.; Jiang, R.; Zhen, X.; Zhou, J.; Li, C.; Sun, H.; et al. ATF3 deficiency impairs the proliferative-secretory phase transition and decidualization in RIF patients. Cell Death Dis. 2021, 12, 387. [Google Scholar] [CrossRef]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef]
- Paria, B.C.; Song, H.; Dey, S.K. Implantation: Molecular basis of embryo-uterine dialogue. Int. J. Dev. Biol. 2001, 45, 597–605. [Google Scholar]
- Li, Q.; Ruan, L.; Zhu, L.; Yang, Z.; Zhu, M.; Luo, Y. Elevated estradiol levels in frozen embryo transfer have different effects on pregnancy outcomes depending on the stage of transferred embryos. Sci. Rep. 2022, 12, 5592. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Conde-López, C.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Luteal Phase in Assisted Reproductive Technology. Front. Reprod. Health 2020, 2, 595183. [Google Scholar] [CrossRef]
- Altmäe, S.; Mendoza-Tesarik, R.; Mendoza, C.; Mendoza, N.; Cucinelli, F.; Tesarik, J. Effect of growth hormone on uterine receptivity in women with repeated implantation failure in an oocyte donation program: A randomized controlled trial. J. Endocr. Soc. 2017, 2, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Wu, Z.; Yan, J.; Norman, R.J.; Li, R. The potential role of growth hormone on the endometrium in assisted reproductive technology. Front. Endocrinol. 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lv, M.; Wang, P.; Guo, C.; Ni, Z.; Bao, H.; Tang, Y.; Cai, H.; Lu, J.; Deng, W.; et al. Sequential activation of uterine epithelial IGF1R by stromal IGF1 and embryonic IGF2 directs normal uterine preparation for embryo implantation. J. Mol. Cell Biol. 2021, 13, 646–661. [Google Scholar] [CrossRef]
- Song, H.; Lim, H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction 2006, 131, 341–349. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, J.W.; Wang, J.; Ma, L.; Martin, J.F.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. Bmp2 is critical for the murine uterine decidual response. Mol. Cell. Biol. 2007, 27, 5468–5478. [Google Scholar] [CrossRef]
- Yu, H.F.; Yang, Z.Q.; Xu, M.Y.; Huang, J.C.; Yue, Z.P.; Guo, B. Yap is essential for uterine decidualization through Rrm2/GSH/ROS pathway in response to Bmp2. Int. J. Biol. Sci. 2022, 18, 2261–2276. [Google Scholar] [CrossRef]
- Berneau, S.C.; Ruane, P.T.; Brison, D.R.; Kimber, S.J.; Westwood, M.; Aplin, J.D. Characterisation of osteopontin in an in vitro model of embryo implantation. Cells 2019, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, A.; Vilella, F. Mother and Embryo Cross-Communication. Genes 2020, 11, 376. [Google Scholar] [CrossRef]
- Illera, M.J.; Cullinan, E.; Gui, Y.; Yuan, L.; Beyler, S.A.; Lessey, B.A. Blockade of the alpha(v)beta(3) integrin adversely affects implantation in the mouse. Biol. Reprod. 2000, 62, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Dhar, R.; Das, C. Steroids modulate the expression of alpha4 integrin in mouse blastocysts and uterus during implantation. Biol. Reprod. 2002, 66, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Das, S.K.; Dey, S.K. erbB genes in the mouse uterus: Cell-specific signaling by epidermal growth factor (EGF) family of growth factors during implantation. Dev. Biol. 1998, 204, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Anim-Somuah, M.; Kalumbi, C.; Farquharson, R.; Aplin, J.D. Different types of recurrent miscarriage are associated with varying patterns of adhesion molecule expression in endometrium. Reprod. Biomed. Online 2007, 14, 224–234. [Google Scholar] [CrossRef]
- Genbacev, O.D.; Prakobphol, A.; Foulk, R.A.; Krtolica, A.R.; Ilic, D.; Singer, M.S.; Yang, Z.Q.; Kiessling, L.L.; Rosen, S.D.; Fisher, S.J. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003, 299, 405–408. [Google Scholar] [CrossRef]
- Hambartsoumian, E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am. J. Reprod. Immunol. 1998, 39, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, M.P.; Beloni, L.; Livi, C.; Maggi, E.; Scarselli, G.; Romagnani, S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 1998, 4, 1020–1024. [Google Scholar] [CrossRef]
- Mestre-Citrinovitz, A.C.; Kleff, V.; Vallejo, G.; Winterhager, E.; Saragüeta, P. A Suppressive antagonism evidences progesterone and estrogen receptor pathway interaction with concomitant regulation of Hand2, Bmp2 and ERK during early decidualization. PLoS ONE 2015, 10, e0124756. [Google Scholar] [CrossRef]
- Daftary, G.S.; Taylor, H.S. Implantation in the human: The role of HOX genes. Semin. Reprod. Med. 2000, 18, 311–320. [Google Scholar] [CrossRef]
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The growth hormone receptor: Mechanism of receptor activation, cell signaling, and physiological aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef]
- Pawar, S.; Starosvetsky, E.; Orvis, G.D.; Behringer, R.R.; Bagchi, I.C.; Bagchi, M.K. STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Mol. Endocrinol 2013, 27, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Depenbusch, M.; Schultze-Mosgau, A.; Griesinger, G. Strong variation in progesterone production of the placenta in early pregnancy—What are the clinical implications? Reprod. Biomed. Online 2020, 41, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.G.; Sherkow, J.S.; Adashi, E.Y. Handle with care: The WHO report on human genome editing. Hastings Cent. Rep. 2022, 52, 10–14. [Google Scholar] [CrossRef]
- Rybouchkin, A.V.; Van der Straeten, F.; Quatacker, J.; De Sutter, P.; Dhont, M. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil. Steril. 1997, 68, 1144–1147. [Google Scholar] [CrossRef]
- Shan, Y.; Zhao, H.; Zhao, D.; Wang, J.; Cui, Y.; Bao, H. Assisted oocyte activation with calcium ionophore improves pregnancy outcomes and offspring safety in infertile patients: A systematic review and meta-analysis. Front. Physiol. 2022, 12, 751905. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Androl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.; Agarwal, A.; Majzoub, A.; Panner Selvam, M.K.; Baskaran, S.; Henkel, R.; Elbardisi, H. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants 2020, 9, 219. [Google Scholar] [CrossRef]
- Amor, H.; Shelko, N.; Mohammed, M.; Jankowski, P.M.; Hammadeh, M.E. Role of antioxidants supplementation in the treatment of male infertility. In Antioxidants-Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Agarwal, A.; Panner Selvam, M.K.; Arafa, M.; Okada, H.; Homa, S.; Killeen, A.; Balaban, B.; Saleh, R.; Armagan, A.; Roychoudhury, S.; et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J. Androl. 2019, 21, 565–569. [Google Scholar] [CrossRef]
- Henkel, R.; Morris, A.; Vogiatzi, P.; Saleh, R.; Sallam, H.; Boitrelle, F.; Garrido, N.; Arafa, M.; Gül, M.; Rambhatla, A.; et al. Predictive value of seminal oxidation-reduction potential (ORP) analysis for reproductive outcomes of intracytoplasmic sperm injection (ICSI) cycles. Reprod. Biomed. Online, 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Hasanen, E.; Elqusi, K.; ElTanbouly, S.; Hussin, A.E.; AlKhadr, H.; Zaki, H.; Henkel, R.; Agarwal, A. PICSI vs. MACS for abnormal sperm DNA fragmentation ICSI cases: A prospective randomized trial. J. Assist. Reprod. Genet. 2020, 37, 2605–2613. [Google Scholar] [CrossRef]
- Gosálvez, J.; Migueles, B.; López-Fernández, C.; Sánchez-Martín, F.; Sánchez-Martín, P. Single sperm selection and DNA fragmentation analysis: The case of MSOME/IMSI. Nat. Sci. 2013, 5, 7–14. [Google Scholar] [CrossRef]
- Greco, E.; Scarselli, F.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Franco, G.; Anniballo, N.; Mendoza, C.; Tesarik, J. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum. Reprod. 2005, 20, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Sánchez-Martín, F.; Sánchez-Martín, P.; Schneider, D.T.; Gosálvez, J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil. Steril. 2015, 104, 1398–1405. [Google Scholar] [CrossRef]
- Tesarik, J.; Galán-Lázaro, M. Clinical scenarios of unexplained sperm DNA fragmentation and their management. Transl. Androl. Urol. 2017, 6 (Suppl. 4), S566–S569. [Google Scholar] [CrossRef]
- Tesarik, J. Towards personalized antioxidant use in female infertility: Need for more molecular and clinical studies. Biomedicines 2021, 9, 1933. [Google Scholar] [CrossRef]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef]
- Vitale, S.G.; Palumbo, M.; Conde-López, C.; Mendoza, N.; Mendoza-Tesarik, R.; Tesarik, J. Effect of growth hormone administration on ICSI outcomes in patients with polycystic ovary syndrome and recurrent implantation failure: A retrospective cross-over study. Int. J. Gynaecol. Obstet. 2021, 153, 357–358. [Google Scholar] [CrossRef]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S.; Kamali, K.; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; et al. Role of vitamin E and D3 supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN 2017, 18, 23–30. [Google Scholar] [CrossRef]
- Panti, A.A.; Shehu, C.E.; Saidu, Y.; Tunau, K.A.; Nwobodo, E.I.; Jimoh, A.; Bilbis, L.S.; Umar, A.B.; Hassan, M. Oxidative stress and outcome of antioxidant supplementation in patients with polycystic ovarian syndrome (PCOS). Int. J. Reprod. Contracept. Obstet. Gynecol. 2018, 7, 1667–1672. [Google Scholar] [CrossRef]
- Baboo, K.D.; Chen, Z.Y.; Zhang, X.M. Role of oxidative stress and antioxidant therapies in endometriosis. Reprod. Dev. Med. 2019, 3, 170–176. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef] [PubMed]
- Gat, I.; Blanco Mejia, S.; Balakier, H.; Librach, C.L.; Claessens, A.; Ryan, E.A. The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecol. Endocrinol. 2016, 32, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Budani, M.C.; Tiboni, G.M. Effects of supplementation with natural antioxidants on oocytes and preimplantation embryos. Antioxidants 2020, 9, 612. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef]
- Tesarik, J.; Hazout, A.; Mendoza, C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum. Reprod. 2005, 20, 2536–2541. [Google Scholar] [CrossRef]
- Alviggi, C.; Humaidan, P.; Howles, C.M.; Tredway, D.; Hillier, S.G. Biological versus chronological ovarian age: Implications for assisted reproductive technology. Reprod. Biol. Endocrinol. 2009, 7, 101. [Google Scholar] [CrossRef]
- Keane, K.N.; Yovich, J.L.; Hamidi, A.; Hinchliffe, P.M.; Dhaliwal, S.S. Single-centre retrospective analysis of growth hormone supplementation in IVF patients classified as poor-prognosis. BMJ Open 2017, 7, e018107. [Google Scholar] [CrossRef]
- Keane, K.N.; Ye, Y.; Hinchliffe, P.M.; Regan, S.L.; Dhaliwal, S.S.; Yovich, J.L. Live birth outcomes of vitrified embryos generated under growth hormone stimulation are improved for women categorized as poor-prognosis. Clin. Exp. Reprod. Med. 2019, 46, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.H.; Gao, L.Z.; Liang, X.Y.; Fang, C.; Wu, Y.Q.; Yang, X. The effect of growth hormone on the clinical outcomes of poor ovarian reserve patients undergoing in vitro fertilization/intracytoplasmic sperm injection treatment: A retrospective study based on POSEIDON criteria. Front. Endocrinol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Yovich, J.L.; Ye, Y.; Regan, S.L.P.; Keane, K.N. The evolving concept of poor-prognosis for women undertaking IVF and the notion of growth hormone as an adjuvant: A single-center viewpoint. Front. Endocrinol. 2019, 10, 808. [Google Scholar] [CrossRef]
- Lan, K.C.; Lin, P.Y.; Chang, Y.C.; Chen, Y.J.; Tsai, Y.R.; Ismaeil Mohamed, I.S.; Kang, H.Y. Growth hormone supplementation may improve the pregnancy rate and endometrial receptivity among women aged more than 40 years undergoing in vitro fertilization. Biomed. J. 2019, 42, 411–416. [Google Scholar] [CrossRef]
- Yang, P.; Wu, R.; Zhang, H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: A meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2020, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza-Tesarik, R. New criteria for the use of growth hormone in the treatment of female infertility: Minireview and a case series. EC Gynaecol. 2020, 9.3, 1–4. [Google Scholar]
- Tesarik, J.; Galán-Lázaro, M.; Conde-López, C.; Chiara-Rapisarda, A.M.; Mendoza-Tesarik, R. The effect of GH administration on oocyte and zygote quality in young women with repeated implantation failure after IVF. Front. Endocrinol. 2020, 11, 519572. [Google Scholar] [CrossRef]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Arfuso, F.; Dharmarajan, A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertil. Steril. 2018, 110, 1298–1310. [Google Scholar] [CrossRef]
- Vitale, S.G.; Palumbo, M.; Rapisarda, A.M.C.; Carugno, J.; Conde-López, C.; Mendoza, N.; Mendoza-Tesarik, R.; Tesarik, J. Use of pentoxifylline during ovarian stimulation to improve oocyte and embryo quality: A retrospective study. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102398. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Li, Y.; Tian, J.; Sun, X.; Song, T.; Yan, G.; Ding, L.; Sun, H. Growth hormone ameliorates the age-associated depletion of ovarian reserve and decline of oocyte quality via inhibiting the activation of Fos and Jun signaling. Aging 2021, 13, 6765–6781. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [PubMed]
- Leem, J.; Bai, G.Y.; Kim, J.S.; Oh, J.S. Melatonin protects mouse oocytes from DNA damage by enhancing nonhomologous end-joining repair. J. Pineal Res. 2019, 67, e12603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hao, J.; Li, Y. Individualized luteal phase support after fresh embryo transfer: Unanswered questions, a review. Reprod. Health 2022, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J. Can miscarriage caused by delayed luteoplacental shift be avoided? Reprod. Biomed. Online 2020, 41, 747. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Kim, A.; Michaeli, T.; Lee, H.J.; Shohat-Tal, A.; Lazzaroni, E.; Barad, D.H. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum. Reprod. 2013, 28, 172–177. [Google Scholar] [CrossRef]
- Li, J.; Mo, S.; Chen, Y. The effect of G-CSF on infertile women undergoing IVF treatment: A meta-analysis. Syst. Biol. Reprod. Med. 2017, 63, 239–247. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.H.; Fu, X.H.; Huang, Q.X.; Guo, X.Y.; Zhang, L.; Li, S.S.; Zhu, J.; Shu, J. Therapeutic role of granulocyte colony-stimulating factor (G-CSF) for infertile women under in vitro fertilization and embryo transfer (IVF-ET) treatment: A meta-analysis. Arch. Gynecol. Obstet. 2018, 298, 861–871. [Google Scholar] [CrossRef]
- Hou, Z.; Jiang, F.; Yang, J.; Liu, Y.; Zha, H.; Yang, X.; Bie, J.; Meng, Y. What is the impact of granulocyte colony-stimulating factor (G-CSF) in subcutaneous injection or intrauterine infusion and during both the fresh and frozen embryo transfer cycles on recurrent implantation failure: A systematic review and meta-analysis? Reprod. Biol. Endocrinol. 2021, 19, 125. [Google Scholar] [CrossRef]
- Marino, V.J.; Roguin, L.P. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J. Cell. Biochem. 2008, 103, 1512–1523. [Google Scholar] [CrossRef]
- Theyab, A.; Algahtani, M.; Alsharif, K.F.; Hawsawi, Y.M.; Alghamdi, A.; Alghamdi, A.; Akinwale, J. New insight into the mechanism of granulocyte colony-stimulating factor (G-CSF) that induces the mobilization of neutrophils. Hematology 2021, 26, 628–636. [Google Scholar] [CrossRef]
- Pinto, P.B.; Espinosa-Vázquez, J.M.; Rivas, M.L.; Hombría, J.C.-G. JAK/STAT and Hox dynamic interactions in an organogenetic gene cascade. PLoS Genet. 2015, 11, e1005412. [Google Scholar] [CrossRef] [PubMed]
- Barash, A.; Dekel, N.; Fieldust, S.; Segal, I.; Schechtman, E.; Granot, I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 2003, 79, 1317–1322. [Google Scholar] [CrossRef]
- Lensen, S.; Osavlyuk, D.; Armstrong, S.; Stadelmann, C.; Hennes, A.; Napier, E.; Wilkinson, J.; Sadler, L.; Gupta, D.; Strandell, A.; et al. A randomized trial of endometrial scratching before in vitro fertilization. N. Engl. J. Med. 2019, 380, 325–334. [Google Scholar] [CrossRef]
- Metwally, M.; Chatters, R.; White, D.; Hall, J.; Walters, S. Endometrial scratch in women undergoing first-time IVF treatment: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biomed. Online 2022, 44, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286–1290. [Google Scholar] [PubMed]
- Lin, Y.; Qi, J.; Sun, Y. Platelet-rich plasma as a potential new strategy in the endometrium treatment in assisted reproductive technology. Front. Endocrinol. 2021, 12, 707584. [Google Scholar] [CrossRef]
- Tesarik, J. Endometrial scratching: Less invasive methods should be tried first. Reprod. Biomed. Online, 2022; in press. [Google Scholar] [CrossRef]
- Moffett, A.; Loke, C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 2006, 6, 584–594. [Google Scholar] [CrossRef]
- Hiby, S.E.; Walker, J.J.; O’shaughnessy, K.M.; Redman, C.W.; Carrington, M.; Trowsdale, J.; Moffett, A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 2004, 200, 957–965. [Google Scholar] [CrossRef]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008, 23, 972–976. [Google Scholar] [CrossRef]
- Feyaerts, D.; Kuret, T.; van Cranenbroek, B.; van der Zeeuw-Hingrez, S.; van der Heijden, O.W.H.; van der Meer, A.; Joosten, I.; van der Molen, R.G. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum. Reprod. 2018, 33, 441–451. [Google Scholar] [CrossRef]
- Jerzak, M.; Kniotek, M.; Mrozek, J.; Górski, A.; Baranowski, W. Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil. Steril. 2008, 90, 1848–1853. [Google Scholar] [CrossRef]
- Kniotek, M.; Zych, M.; Roszczyk, A.; Szafarowska, M.; Jerzak, M.M. Decreased production of TNF-α and IL-6 inflammatory cytokines in non-pregnant idiopathic RPL women immunomodulatory effect of sildenafil citrate on the cellular response of idiopathic RPL women. J. Clin. Med. 2021, 10, 3115. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Mendoza-Tesarik, R. Molecular Clues to Understanding Causes of Human-Assisted Reproduction Treatment Failures and Possible Treatment Options. Int. J. Mol. Sci. 2022, 23, 10357. https://doi.org/10.3390/ijms231810357
Tesarik J, Mendoza-Tesarik R. Molecular Clues to Understanding Causes of Human-Assisted Reproduction Treatment Failures and Possible Treatment Options. International Journal of Molecular Sciences. 2022; 23(18):10357. https://doi.org/10.3390/ijms231810357
Chicago/Turabian StyleTesarik, Jan, and Raquel Mendoza-Tesarik. 2022. "Molecular Clues to Understanding Causes of Human-Assisted Reproduction Treatment Failures and Possible Treatment Options" International Journal of Molecular Sciences 23, no. 18: 10357. https://doi.org/10.3390/ijms231810357
APA StyleTesarik, J., & Mendoza-Tesarik, R. (2022). Molecular Clues to Understanding Causes of Human-Assisted Reproduction Treatment Failures and Possible Treatment Options. International Journal of Molecular Sciences, 23(18), 10357. https://doi.org/10.3390/ijms231810357






