Effect of Different Molecular Weights of Polyacrylic Acid on Rat Lung Following Intratracheal Instillation
Abstract
:1. Introduction
2. Results
2.1. Characterization of PAA
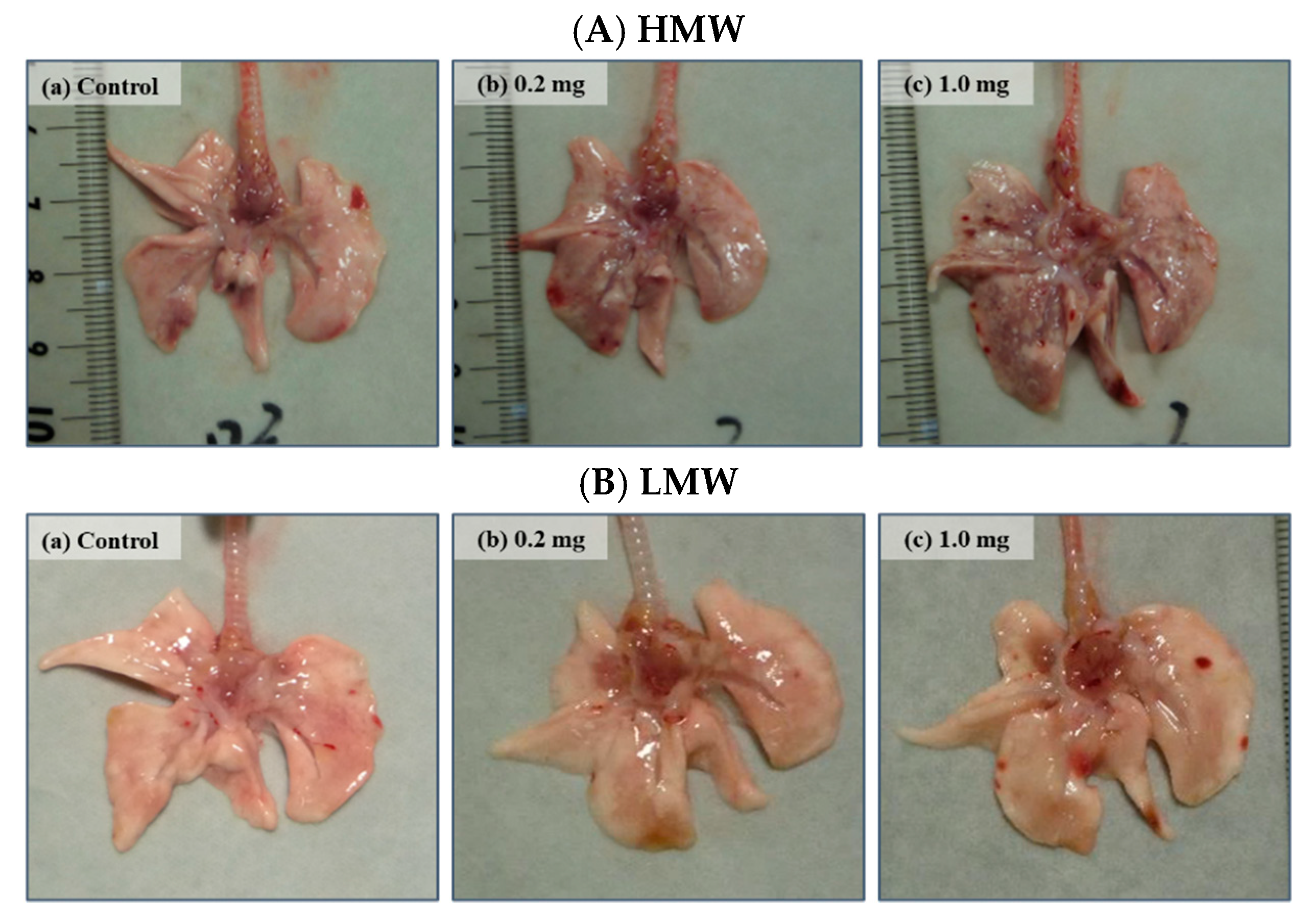
2.2. Relative Lung Weights
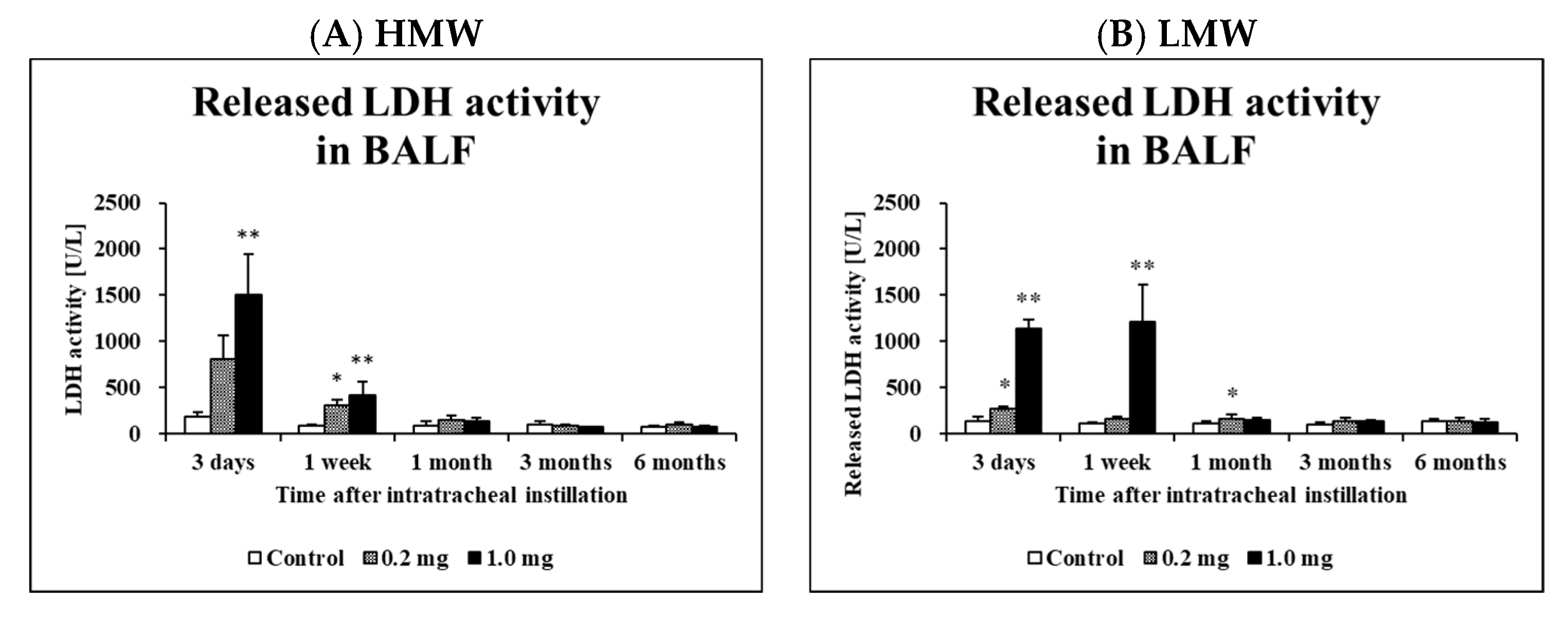
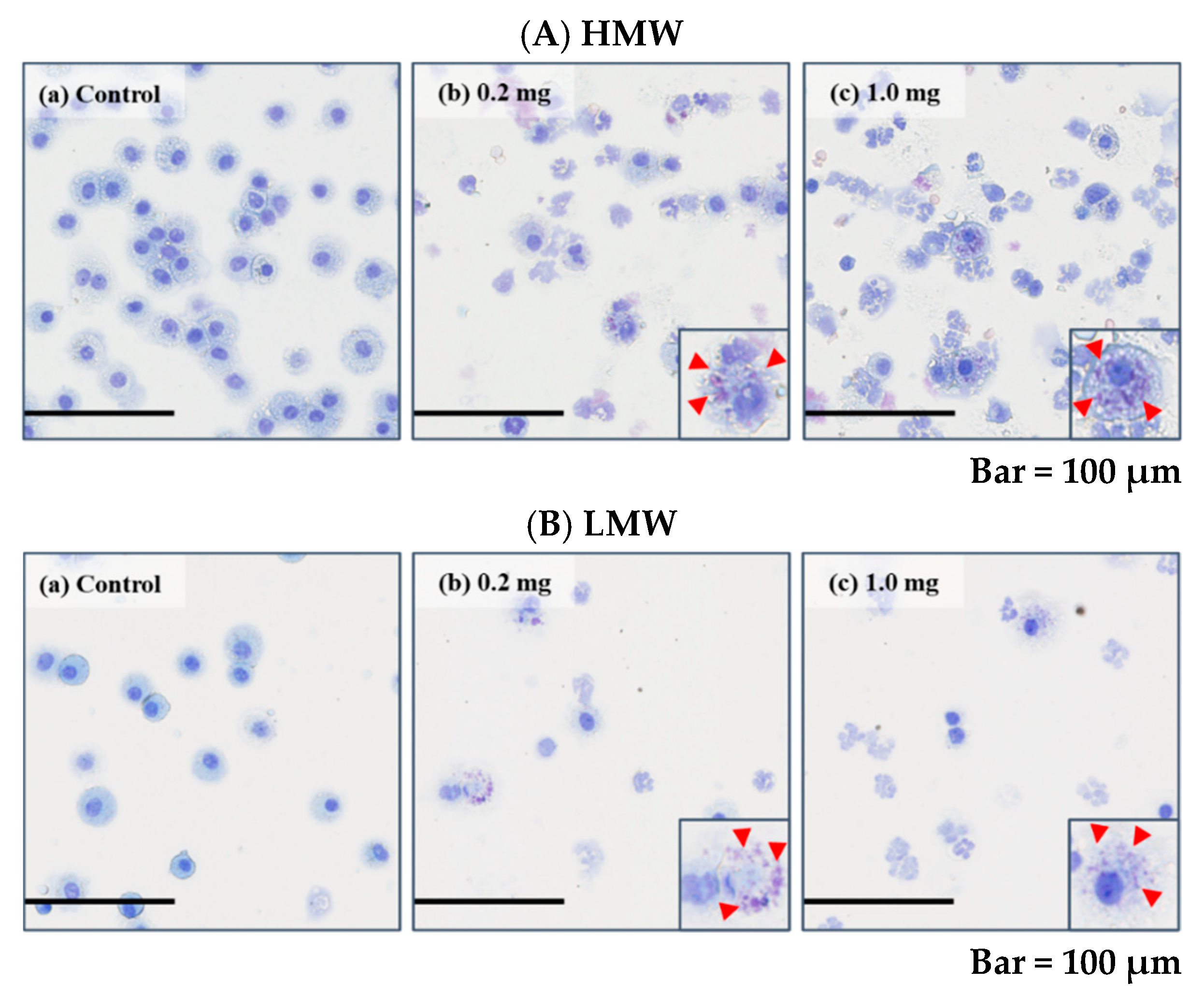
2.3. Cell Analysis and Lactate Dehydrogenase (LDH) Activity in Bronchoalveolar Lavage Fluid (BALF)
2.4. Concentration of Cytokine-Induced Neutrophil Chemoattractants (CINC) in BALF and Concentration of Heme Oxygenase (HO)-1 in Lung Tissue
2.5. Micro-CT Imaging
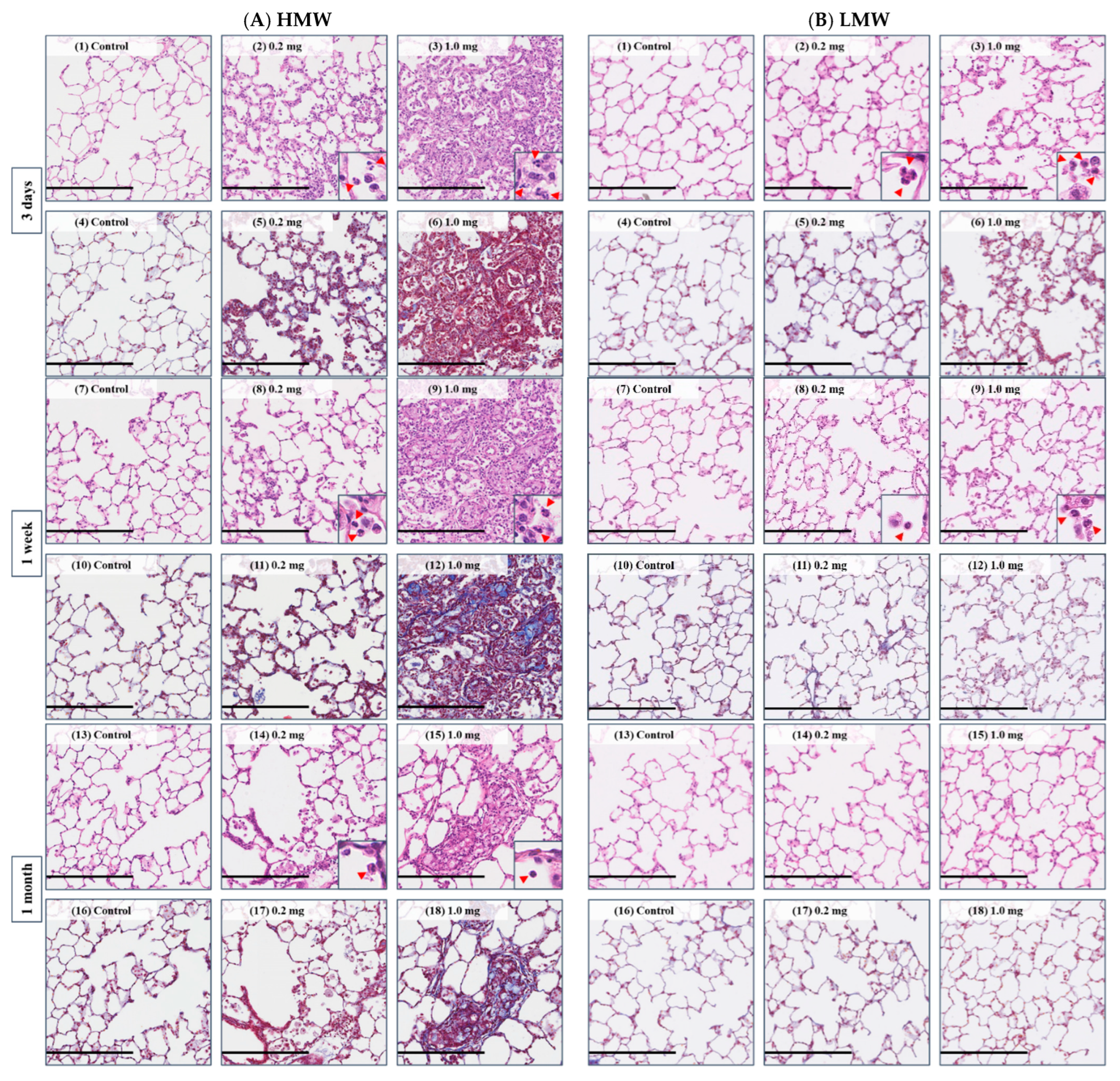
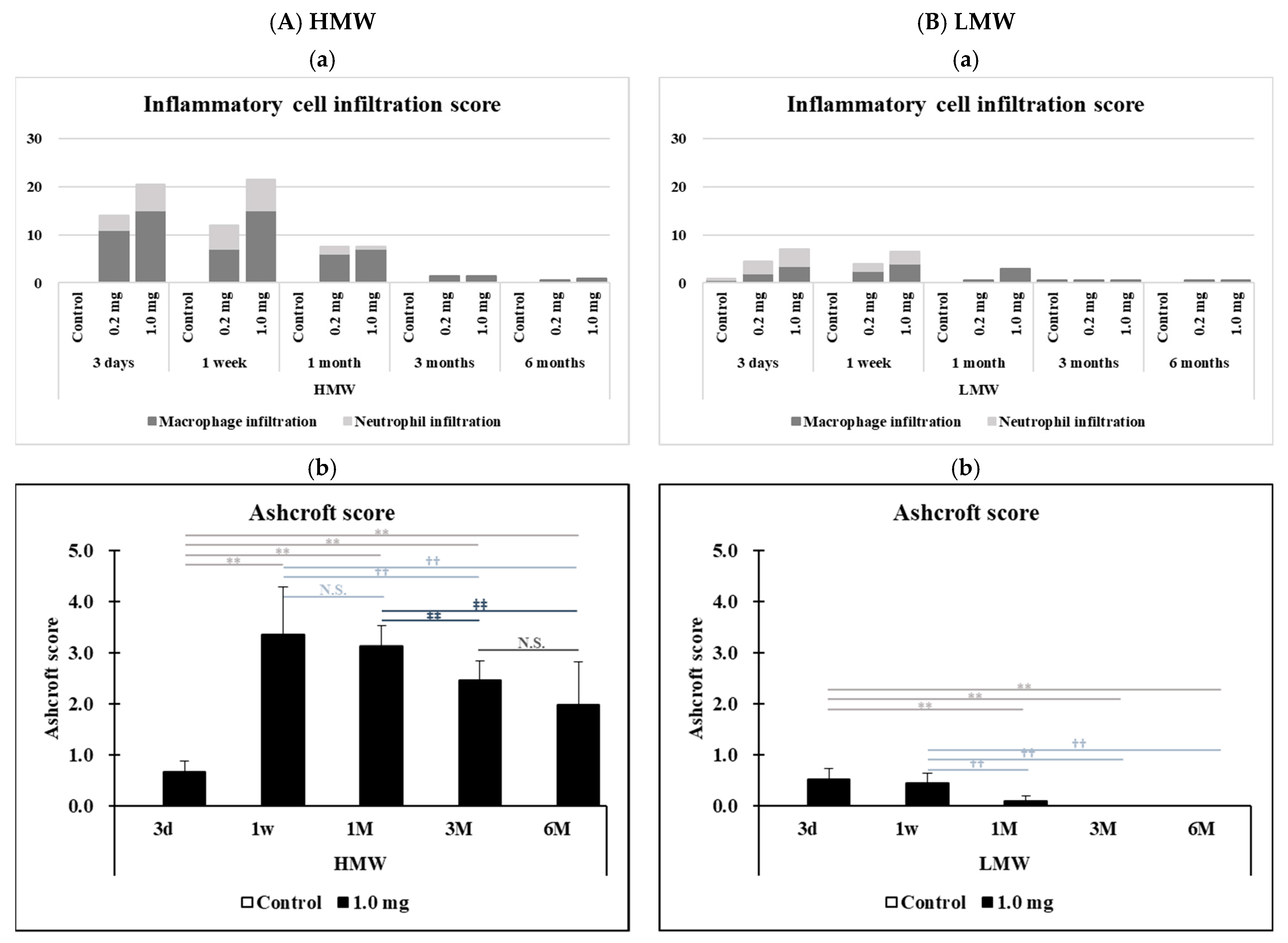
2.6. Histopathological Features in the Lung
3. Discussion
4. Materials and Methods
4.1. Sample Polymers
4.2. Animals
4.3. Intratracheal Instillation
4.4. Animals Following Intratracheal Instillation
4.5. Cytospin Analysis of Inflammatory Cells and Measurement of LDH in BALF
4.6. Measurement of Chemokines in BALF and HO-1 in Lung Tissue
4.7. 3D Micro-CT Imaging
4.8. Histopathology
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| (A) HMW | |||||||||||
| Observation Point | Group | Ashcroft Score | vs. 3 Days | vs. 1 Week | vs. 1 Month | ||||||
| Control | 0.2 mg | 1.0 mg | Control | 0.2 mg | 1.0 mg | Control | 0.2 mg | 1.0 mg | |||
| 3 days | Control | 0.0 ± 0.0 | - | N.S. | N.S. | N.S. | p < 0.01 | p < 0.01 | N.S. | N.S. | p < 0.01 |
| 0.2 mg | 0.5 ± 0.2 | N.S. | - | N.S. | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | |
| 1.0 mg | 0.7 ± 0.2 | N.S. | N.S. | - | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | |
| 1 week | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | - | p < 0.01 | p < 0.01 | N.S. | N.S. | p < 0.01 |
| 0.2 mg | 1.0 ± 0.5 | N.S. | N.S. | N.S. | N.S. | - | p < 0.01 | p < 0.01 | N.S. | p < 0.01 | |
| 1.0 mg | 3.4 ± 0.9 | N.S. | N.S. | N.S. | N.S. | N.S. | - | p < 0.01 | p < 0.01 | ||
| 1 month | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | - | N.S. | p < 0.01 |
| 0.2 mg | 0.6 ± 0.1 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | - | p < 0.01 | |
| 1.0 mg | 3.1 ± 0.4 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | - | |
| 3 months | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| 0.2 mg | 0.1 ± 0.1 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| 1.0 mg | 2.5 ± 0.4 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| 6 months | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| 0.2 mg | 0.1 ± 0.1 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| 1.0 mg | 2.0 ± 0.8 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| (A) HMW | |||||||||||
| Observation Point | Group | Ashcroft Score | vs. 3 Months | vs. 6 Months | |||||||
| Control | 0.2 mg | 1.0 mg | Control | 0.2 mg | 1.0 mg | ||||||
| 3 days | Control | 0.0 ± 0.0 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | |||
| 0.2 mg | 0.5 ± 0.2 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | ||||
| 1.0 mg | 0.7 ± 0.2 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | ||||
| 1 week | Control | 0.0 ± 0.0 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | |||
| 0.2 mg | 1.0 ± 0.5 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | ||||
| 1.0 mg | 3.4 ± 0.9 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | ||||
| 1 month | Control | 0.0 ± 0.0 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | |||
| 0.2 mg | 0.6 ± 0.1 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | ||||
| 1.0 mg | 3.1 ± 0.4 | p < 0.01 | p < 0.01 | N.S. | p < 0.01 | p < 0.01 | p < 0.01 | ||||
| 3 months | Control | 0.0 ± 0.0 | - | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | |||
| 0.2 mg | 0.1 ± 0.1 | N.S. | - | p < 0.01 | N.S. | N.S. | p < 0.01 | ||||
| 1.0 mg | 2.5 ± 0.4 | N.S. | N.S. | - | p < 0.01 | p < 0.01 | N.S. | ||||
| 6 months | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | - | N.S. | p < 0.01 | |||
| 0.2 mg | 0.1 ± 0.1 | N.S. | N.S. | N.S. | N.S. | - | p < 0.01 | ||||
| 1.0 mg | 2.0 ± 0.8 | N.S. | N.S. | N.S. | N.S. | N.S. | - | ||||
| (B) LMW | |||||||||||
| Observation Point | Group | Ashcroft Score | vs. 3 Days | vs. 1 Week | vs. 1 Month | ||||||
| Control | 0.2 mg | 1.0 mg | Control | 0.2 mg | 1.0 mg | Control | 0.2 mg | 1.0 mg | |||
| 3 days | Control | 0.0 ± 0.0 | - | N.S. | p < 0.01 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | N.S. |
| 0.2 mg | 0.0 ± 0.1 | N.S. | - | p < 0.01 | N.S. | N.S. | p < 0.01 | N.S. | N.S. | N.S. | |
| 1.0 mg | 0.5 ± 0.2 | N.S. | N.S. | - | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |
| 1 week | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | - | N.S. | p < 0.01 | N.S. | N.S. | N.S. |
| 0.2 mg | 0.1 ± 0.1 | N.S. | N.S. | N.S. | N.S. | - | p < 0.01 | N.S. | p < 0.01 | N.S. | |
| 1.0 mg | 0.4 ± 0.2 | N.S. | N.S. | N.S. | N.S. | N.S. | - | p < 0.01 | p < 0.01 | p < 0.01 | |
| 1 month | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | - | N.S. | N.S. |
| 0.2 mg | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | - | N.S. | |
| 1.0 mg | 0.1 ± 0.1 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | - | |
| 3 months | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| 0.2 mg | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| 1.0 mg | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| 6 months | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| 0.2 mg | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| 1.0 mg | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| (B) LMW | |||||||||||
| Observation Point | Group | Ashcroft Score | vs. 3 Months | vs. 6 Months | |||||||
| Control | 0.2 mg | 1.0 mg | Control | 0.2 mg | 1.0 mg | ||||||
| 3 days | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |||
| 0.2 mg | 0.5 ± 0.2 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||||
| 1.0 mg | 0.7 ± 0.2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | ||||
| 1 week | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |||
| 0.2 mg | 1.0 ± 0.5 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||||
| 1.0 mg | 3.4 ± 0.9 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | ||||
| 1 month | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |||
| 0.2 mg | 0.6 ± 0.1 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||||
| 1.0 mg | 3.1 ± 0.4 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||||
| 3 months | Control | 0.0 ± 0.0 | - | N.S. | N.S. | N.S. | N.S. | N.S. | |||
| 0.2 mg | 0.1 ± 0.1 | N.S. | - | N.S. | N.S. | N.S. | N.S. | ||||
| 1.0 mg | 2.5 ± 0.4 | N.S. | N.S. | - | N.S. | N.S. | N.S. | ||||
| 6 months | Control | 0.0 ± 0.0 | N.S. | N.S. | N.S. | - | N.S. | N.S. | |||
| 0.2 mg | 0.1 ± 0.1 | N.S. | N.S. | N.S. | N.S. | - | N.S. | ||||
| 1.0 mg | 2.0 ± 0.8 | N.S. | N.S. | N.S. | N.S. | N.S. | - | ||||
References
- Park, D.U.; Ryu, S.H.; Lim, H.K.; Kim, S.K.; Choi, Y.Y.; Ahn, J.J.; Lee, E.; Hong, S.B.; Do, K.H.; Cho, J.L.; et al. Types of Household Humidifier Disinfectant and Associated Risk of Lung Injury (HDLI) in South Korea. Sci. Total Environ. 2017, 596–597, 53–60. [Google Scholar] [CrossRef]
- Ryu, S.; Park, D.; Lee, E.; Park, S.; Lee, S.; Jung, S.; Hong, S.; Park, J.; Hong, S. Humidifier Disinfectant and Use Characteristics Associated with Lung Injury in Korea. Indoor Air 2019, 29, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Nishida, C.; Tomonaga, T.; Izumi, H.; Yatera, K.; Sakurai, K.; Kim, Y. Lung disorders induced by respirable organic chemicals. J. Occup. Health 2021, 63, e12240. [Google Scholar] [CrossRef]
- Nishida, C.; Tomonaga, T.; Izumi, H.; Wang, K.Y.; Higashi, H.; Ishidao, T.; Takeshita, J.I.; Ono, R.; Sumiya, K.; Fujii, S.; et al. Inflammogenic effect of polyacrylic acid in rat lung following intratracheal instillation. Part. Fibre Toxicol. 2022, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T. Physico-Chemical Properties of Water Soluble Polymers in Aqueous Solution. J. Surf. Finish. Soc. 2009, 60, 746. [Google Scholar] [CrossRef]
- Chen, T.M.; Dulfano, M.J. Mucus viscoelasticity and mucociliary transport rate. J. Lab. Clin. Med. 1978, 91, 423–431. [Google Scholar] [PubMed]
- Borm, P.J.; Driscoll, K. Particles, Inflammation and Respiratory Tract Carcinogenesis. Toxicol. Lett. 1996, 88, 109–113. [Google Scholar] [CrossRef]
- Shacter, E.; Weitzman, S.A. Chronic Inflammation and Cancer. Oncology 2002, 16, 217–226. [Google Scholar]
- Bellmann, B.; Muhle, H.; Creutzenberg, O.; Ernst, H.; Müller, M.; Bernstein, D.M.; Riego Sintes, J.M. Calibration Study on Subchronic Inhalation Toxicity of Man-Made Vitreous Fibers in Rats. Inhal. Toxicol. 2003, 15, 1147–1177. [Google Scholar] [CrossRef]
- Kim, H.; Morimoto, Y.; Ogami, A.; Nagatomo, H.; Hirohashi, M.; Oyabu, T.; Kawanami, Y.; Kuroda, E.; Higashi, T.; Tanaka, I. Differential Expression of EC-SOD, Mn-SOD and CuZn-SOD in Rat Lung Exposed to Crystalline Silica. J. Occup. Health 2007, 49, 242–248. [Google Scholar] [CrossRef]
- Nishi, K.; Morimoto, Y.; Ogami, A.; Murakami, M.; Myojo, T.; Oyabu, T.; Kadoya, C.; Yamamoto, M.; Todoroki, M.; Hirohashi, M.; et al. Expression of Cytokine-Induced Neutrophil Chemoattractant in Rat Lungs by Intratracheal Instillation of Nickel Oxide Nanoparticles. Inhal. Toxicol. 2009, 21, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Pott, F.; Ziem, U.; Reiffer, F.J.; Huth, F.; Ernst, H.; Mohr, U. Carcinogenicity Studies on Fibres, Metal Compounds, and Some Other Dusts in Rats. Exp. Pathol. 1987, 32, 129–152. [Google Scholar] [CrossRef]
- Muhle, H.; Bellmann, B.; Creutzenberg, O.; Dasenbrock, C.; Ernst, H.; Kilpper, R.; MacKenzie, J.C.; Morrow, P.; Mohr, U.; Takenaka, S.; et al. Pulmonary Response to Toner upon Chronic Inhalation Exposure in Rats. Fundam. Appl. Toxicol. 1991, 17, 280–299. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.W.; Okada, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; et al. Comparison of Pulmonary Inflammatory Responses Following Intratracheal Instillation and Inhalation of Nanoparticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Pulmonary Toxicity of Well-Dispersed Cerium Oxide Nanoparticles Following Intratracheal Instillation and Inhalation. J. Nanopart. Res. 2015, 17, 442. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Todoroki, M.; Yamamoto, M.; Hashiba, M.; Mizuguchi, Y.; Lee, B.W.; et al. Pulmonary Toxicity of Well-Dispersed Multi-Wall Carbon Nanotubes Following Inhalation and Intratracheal Instillation. Nanotoxicology 2012, 6, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, S.H.; Jeon, D.; Kim, H.Y.; Lee, K. Changes in expression of cytokines in polyhexamethylene guanidine-induced lung fibrosis in mice: Comparison of bleomycin-induced lung fibrosis. Toxicology 2018, 393, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Lee, E.J.; Lee, S.Y.; In, K.H.; Kim, H.K.; Kang, M.S. Humidifier disinfectant-associated interstitial lung disease in an animal model induced by polyhexamethylene guanidine aerosol. Am. J. Respir. Crit. Care Med. 2014, 190, 706–708. [Google Scholar] [CrossRef]
- Hong, S.B.; Kim, H.J.; Huh, J.W.; Do, K.H.; Jang, S.J.; Song, J.S.; Choi, S.J.; Heo, Y.; Kim, Y.B.; Lim, C.M.; et al. A cluster of lung injury associated with home humidifier use: Clinical, radiological and pathological description of a new syndrome. Thorax 2014, 69, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Leem, J.; Lee, K.; Lim, H.; Choi, Y.; Ahn, J.J.; Lim, S.; Park, J.; Choi, K.; Lee, N.; et al. Exposure characteristics of familial cases of lung injury associated with the use of humidifier disinfectants. Environ. Health 2014, 13, 70. [Google Scholar] [CrossRef]
- Meca, O.; Cruz, M.J.; Sánchez-Ortiz, M.; González-Barcala, F.J.; Ojanguren, I.; Munoz, X. Do Low Molecular Weight Agents Cause More Severe Asthma than High Molecular Weight Agents? PLoS ONE 2016, 11, e0156141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, O.; Godet, J.; Hurdubaea, L.; Rifflart, C.; Suojalehto, H.; Wiszniewska, M.; Munoz, X.; Sastre, J.; Klusackova, P.; Moore, V.; et al. Are high- and low-molecular-weight sensitizing agents associated with different clinical phenotypes of occupational asthma? Allergy 2019, 74, 261–272. [Google Scholar] [CrossRef]
- Maestrelli, P.; Boschetto, P.; Fabbri, L.M.; Mapp, C.E. Mechanisms of occupational asthma. J. Allergy. Clin. Immunol. 2009, 123, 531–542. [Google Scholar] [CrossRef]
- Berth, P.; Jakobi, G.; Schmadel, E.; Schwuger, M.J.; Krauch, C.H. The Replacement of Phosphates in Detergents—Possibilities and Limits. Angew. Chem. 1975, 14, 94–102. [Google Scholar] [CrossRef]
- Packham, R.F. Some studies of the coagulation of dispersed clays with hydrolyzing salts. J. Colloid Sci. 1965, 20, 81–92. [Google Scholar] [CrossRef]
- Suen, T.J.; Senior, A.; Sanson, D.L.; Jen, Y. Polycondensation of alkylene dichlorides with alkylene polyamines. J. Polym. Sci. 1960, 45, 289–303. [Google Scholar] [CrossRef]
- Michaels, A.S.; Molelos, O. Polyelectrolyte Adsorption by Kaolinite. Ind. Eng. Chem. 1955, 47, 1801–1809. [Google Scholar] [CrossRef]
- Ransom, T.C.; Roy, D.; Puskas, J.E.; Kaszas, G.; Roland, C.M. Molecular Weight Dependence of the Viscosity of Highly Entangled Polyisobutylene. Macromolecules 2019, 52, 5177–5182. [Google Scholar] [CrossRef]
- Colby, R.H.; Fetters, L.J.; Graessley, W.W. The melt viscosity-molecular weight relationship for linear polymers. Macromolecules 1987, 20, 2226–2237. [Google Scholar] [CrossRef]
- Zhong, L.; Xiong, Y.; Zheng, Z.; Liu, N.; Hu, J.; Yang, F.; Chen, R. Effect of short-term inhalation of warm saline atomised gas on patients with non-cystic fibrosis bronchiectasis. ERJ Open Res. 2020, 6, 00130–02019. [Google Scholar] [CrossRef]
- Andersen, D.H. Cystic Fibrosis of the Pancreas and Its Relation to Celiac Disease: A Clinical and Pathologic Study. Am. J. Dis. Child. 1938, 56, 344–399. [Google Scholar] [CrossRef]
- Knowles, M.R.; Durie, P.R. What is cystic fibrosis? N. Engl. J. Med. 2002, 347, 439–442. [Google Scholar] [CrossRef]
- Slattery, D.M.; Waltz, D.A.; Denham, B.; O’Mahony, M.; Greally, P. Bronchoscopically administered recombinant human DNase for lobar atelectasis in cystic fibrosis. Pediatr. Pulmonol. 2001, 31, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Thornby, K.A.; Johnson, A.; Axtell, S. Dornase Alfa for Non-Cystic Fibrosis Pediatric Pulmonary Atelectasis. Ann. Pharmacother. 2014, 48, 1040–1049. [Google Scholar] [CrossRef]
- Kuehl, C.; Zhang, T.; Kaminskas, L.M.; Porter, C.J.; Davies, N.M.; Forrest, L.; Berkland, C. Hyaluronic Acid Molecular Weight Determines Lung Clearance and Biodistribution after Instillation. Mol. Pharm. 2016, 13, 1904–1914. [Google Scholar] [CrossRef]
- Tomonaga, T.; Izumi, H.; Oyabu, T.; Lee, B.W.; Kubo, M.; Shimada, M.; Noguchi, S.; Nishida, C.; Yatera, K.; Morimoto, Y. Assessment of Cytokine-Induced Neutrophil Chemoattractants as Biomarkers for Prediction of Pulmonary Toxicity of Nanomaterials. Nanomaterials 2020, 10, 1563. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seo, D.S.; Lee, M.J.; Cha, H.G. Immunohistochemical characterization of oxidative stress in the lungs of rats exposed to the humidifier disinfectant polyhexamethylene guanidine hydrochloride. J. Toxicol. Pathol. 2019, 32, 311–317. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sakashita, N.; Okuma, T.; Terasaki, Y.; Tsujita, K.; Suzuki, H.; Kodama, T.; Nomori, H.; Kawasuji, M.; Takeya, M. Class A scavenger receptor (CD204) attenuates hyperoxia-induced lung injury by reducing oxidative stress. J. Pathol. 2007, 212, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, K.; Matsunaga, T.; Tanaka, M.; Mochizuki, S.; Sakurai, Kazuo. Oligo-DNA Stoichiometrically Binds β-1,3-Glucan with the Best Fit Length. Biomacromolecules 2020, 21, 4823–4834. [Google Scholar] [CrossRef]
- Doan, V.T.H.; Lee, J.H.; Takahashi, R.; Nguyen, P.; Nguyen, V.A.T.; Pham, H.T.; Fujii, S.; Sakurai, K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: Potential carriers of α-mangostin for cancer therapy. Polym. J. 2020, 52, 457–466. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.D. Micromeritics: The Technology of Fine Particles; Pitman Publishing: New York, NY, USA; Chicago, IL, USA, 1948; Available online: https://babel.hathitrust.org/cgi/pt?id=mdp.39015068252751&view=1up&seq=5 (accessed on 23 July 2022).
- ISO 19749; Nanotechnologies—Measurements of Particle Size and Shape Distributions by Scanning Electron Microscopy. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/obp/ui/fr/#iso:std:iso:19749:ed-1:v1:en (accessed on 23 July 2022).
- Nishida, C.; Izumi, H.; Tomonaga, T.; Takeshita, J.I.; Wang, K.Y.; Yamasaki, K.; Yatera, K.; Morimoto, Y. Predictive Biomarkers for the Ranking of Pulmonary Toxicity of Nanomaterials. Nanomaterials 2020, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
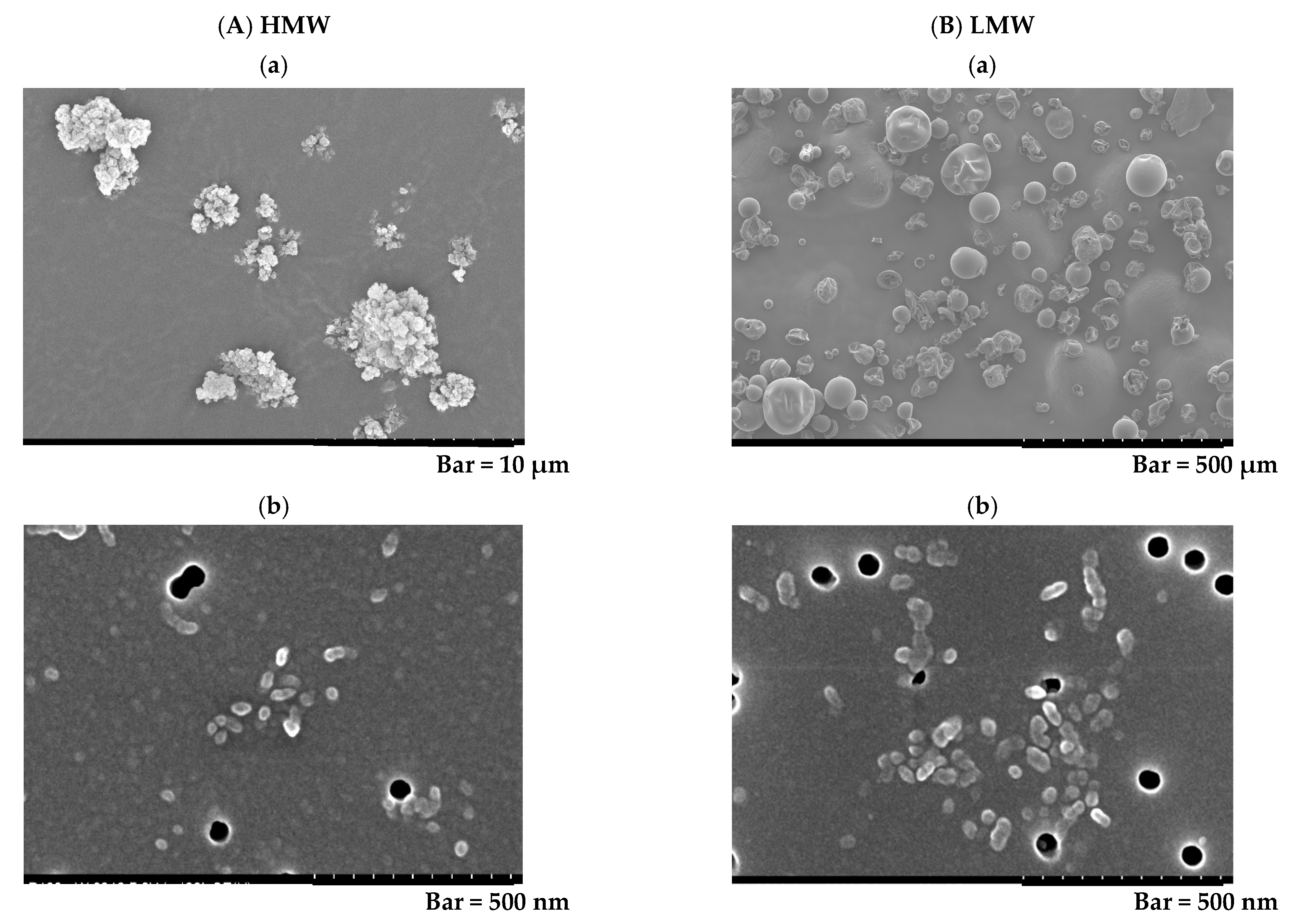




| Name | Polyacrylic Acid | Structural Formula |  | |
|---|---|---|---|---|
| CAS number | 9003-01-4 | |||
| High molecular weight | Low molecular weight | |||
| Bulk | Purity | ≦100% | ≦100% | |
| Weight average molecular weight (MW) | 598,000 g/mol | 30,900 g/mol | ||
| Number average molecular weight (Mn) | 451,000 g/mol | 25,800 g/mol | ||
| Poly dispersity index (PDI) | 1.33 | 1.20 | ||
| Cross-linking | None | None | ||
| Appearance | Solid, white powdered | Solid, white powdered | ||
| Odor | None | None | ||
| Secondary diameter | 2.83 µm | 269 nm | ||
| Suspension | Radius of gyration (Rg) | 57.5 nm | 13.5 nm | |
| Hydrodynamic radius (Rh) | 39.7 nm (Molecular dispersion) | 2.78 nm (Molecular dispersion) | ||
| 217 nm (H2O dispersion: Suspension for instillation) | 1.11 nm (H2O dispersion: Suspension for instillation) | |||
| Secondary diameter | 39.3 nm | 53.2 nm | ||
| 3 Days | 1 Week | 1 Month | 3 Months | 6 Months | ||
|---|---|---|---|---|---|---|
| Relative lung weight (% ± SD) | ||||||
| Control (distilled water) | 0.36 ± 0.03 | 0.34 ± 0.01 | 0.32 ± 0.01 | 0.32 ± 0.01 | 0.32 ± 0.01 | |
| HMW | 0.2 mg | 0.50 ± 0.03 ** | 0.48 ± 0.02 ** | 0.38 ± 0.01 ** | 0.33 ± 0.01 | 0.32 ± 0.01 |
| HMW | 1.0 mg | 0.73 ± 0.03 ** | 0.70 ± 0.05 ** | 0.50 ± 0.01 ** | 0.43 ± 0.02 ** | 0.39 ± 0.02 ** |
| Control (distilled water) | 0.37 ± 0.01 | 0.35 ± 0.03 | 0.33 ± 0.02 | 0.30 ± 0.01 | 0.30 ± 0.03 | |
| LMW | 0.2 mg | 0.42 ± 0.02 ** | 0.38 ± 0.02 | 0.34 ± 0.01 | 0.31 ± 0.01 | 0.30 ± 0.01 |
| LMW | 1.0 mg | 0.54 ± 0.02 ** | 0.48 ± 0.02 ** | 0.39 ± 0.02 ** | 0.31 ± 0.01 * | 0.30 ± 0.01 |
| 3 Days | 1 Week | 1 Month | 3 Months | 6 Months | ||
|---|---|---|---|---|---|---|
| Total cell count (×1000 cells/mL ± SD) | ||||||
| Control (distilled water) | 122.4 ± 36.3 | 137.5 ± 21.8 | 98.4 ± 34.5 | 185.0 ± 64.5 | 174.8 ± 59.0 | |
| HMW | 0.2 mg | 580.0 ± 228.9 ** | 449.4 ± 42.5 * | 403.7 ± 42.3 ** | 191.7 ± 29.6 | 212.3 ± 74.3 |
| HMW | 1.0 mg | 1043.6 ± 165.3 ** | 592.3 ± 189.0 ** | 407.3 ± 154.6 ** | 166.0 ± 53.2 | 186.5 ± 34.2 |
| Control (distilled water) | 94.5 ± 9.9 | 69.3 ± 14.5 | 80.4 ± 28.4 | 75.2 ± 19.6 | 89.4 ± 28.2 | |
| LMW | 0.2 mg | 342.2 ± 98.5 ** | 147.1 ± 27.9 | 88.8 ± 39.0 | 91.2 ± 15.3 | 102.9 ± 30.1 |
| LMW | 1.0 mg | 1021.1 ± 116.9 ** | 606.0 ± 235.8 ** | 61.4 ± 15.3 | 59.4 ± 17.7 | 96.8 ± 21.4 |
| Macrophage count (×1000 cells/mL ± SD) | ||||||
| Control (distilled water) | 121.6 ± 34.8 | 137.2 ± 21.8 | 97.4 ± 34.1 | 183.8 ± 63.5 | 172.4 ± 57.6 | |
| HMW | 0.2 mg | 215.5 ± 92.9 | 272.2 ± 41.9 * | 359.9 ± 37.7 ** | 190.4 ± 29.4 | 210.6 ± 72.3 |
| HMW | 1.0 mg | 411.7 ± 73.1 ** | 379.3 ± 114.5 ** | 395.0 ± 158.0 ** | 164.9 ± 53.3 | 182.6 ± 32.2 |
| Control (distilled water) | 93.0 ± 9.1 | 69.1 ± 14.6 | 79.6 ± 27.8 | 73.9 ± 19.4 | 87.9 ± 28.3 | |
| LMW | 0.2 mg | 168.1 ± 29.2 ** | 128.4 ± 22.1 | 87.9 ± 38.6 | 90.2 ± 15.3 | 102.2 ± 29.2 |
| LMW | 1.0 mg | 255.5 ± 28.5 ** | 323.0 ± 137.8 ** | 60.6 ± 15.2 | 57.8 ± 16.7 | 95.5 ± 21.3 |
| Neutrophil count (×1000 cells/mL ± SD) | ||||||
| Control (distilled water) | 1.7 ± 2.1 | 0.3 ± 0.4 | 0.4 ± 0.6 | 1.1 ± 1.3 | 1.9 ± 2.0 | |
| HMW | 0.2 mg | 352.2 ± 137.1 ** | 169.2 ± 27.2 ** | 33.5 ± 18.7 ** | 1.1 ± 0.8 | 1.4 ± 1.9 |
| HMW | 1.0 mg | 606.1 ± 141.4 ** | 197.4 ± 83.0 ** | 5.1 ± 3.3 | 0.8 ± 0.6 | 3.9 ± 2.6 |
| Control (distilled water) | 0.7 ± 0.6 | 0.1 ± 0.1 | 0.5 ± 0.6 | 0.8 ± 0.8 | 0.9 ± 1.2 | |
| LMW | 0.2 mg | 171.1 ± 76.3 ** | 17.0 ± 9.2 | 0.4 ± 0.6 | 0.6 ± 0.8 | 0.4 ± 0.7 |
| LMW | 1.0 mg | 739.7 ± 81.4 ** | 251.5 ± 93.4 ** | 0.7 ± 0.3 | 1.5 ± 1.2 | 0.2 ± 0.4 |
| Percentage of neutrophil (% ± SD) | ||||||
| Control (distilled water) | 1.1 ± 1.3 | 0.2 ± 0.3 | 0.5 ± 0.7 | 0.5 ± 0.5 | 1.0 ± 0.7 | |
| HMW | 0.2 mg | 60.8 ± 4.4 ** | 37.8 ± 5.9 ** | 8.3 ± 4.5 ** | 0.6 ± 0.4 | 0.5 ± 0.7 |
| HMW | 1.0 mg | 57.8 ± 6.3 ** | 32.8 ± 7.1 ** | 1.5 ± 1.3 | 0.5 ± 0.4 | 2.0 ± 1.2 |
| Control (distilled water) | 0.7 ± 0.6 | 0.1 ± 0.2 | 0.5 ± 0.5 | 1.0 ± 0.9 | 1.1 ± 1.4 | |
| LMW | 0.2 mg | 48.2 ± 8.7 ** | 11.1 ± 5.5 ** | 0.4 ± 0.4 | 0.7 ± 0.8 | 0.3 ± 0.4 |
| LMW | 1.0 mg | 72.5 ± 2.2 ** | 41.6 ± 6.7 ** | 1.1 ± 0.5 | 2.2 ± 1.3 | 0.2 ± 0.4 |
| Lymphocyte count (×1000 cells/mL ± SD) | ||||||
| Control (distilled water) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.9 | 0.1 ± 0.3 | 0.4 ± 0.8 | |
| HMW | 0.2 mg | 1.6 ± 3.7 | 0.4 ± 0.8 | 9.9 ± 6.7 * | 0.0 ± 0.0 | 0.3 ± 0.7 |
| HMW | 1.0 mg | 7.0 ± 7.6 | 3.1 ± 2.6 * | 7.1 ± 5.0 | 0.3 ± 0.4 | 0.0 ± 0.0 |
| Control (distilled water) | 0.7 ± 0.8 | 0.1 ± 0.2 | 0.3 ± 0.4 | 0.5 ± 0.5 | 0.5 ± 0.2 | |
| LMW | 0.2 mg | 2.3 ± 2.0 | 0.5 ± 0.7 | 0.4 ± 0.4 | 0.4 ± 0.4 | 0.3 ± 0.3 |
| LMW | 1.0 mg | 10.8 ± 11.2 | 14.6 ± 12.2 * | 0.1 ± 0.1 | 0.1 ± 0.1 | 1.0 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, C.; Izumi, H.; Tomonaga, T.; Wang, K.-Y.; Higashi, H.; Takeshita, J.-I.; Ono, R.; Sumiya, K.; Fujii, S.; Hata, Y.; et al. Effect of Different Molecular Weights of Polyacrylic Acid on Rat Lung Following Intratracheal Instillation. Int. J. Mol. Sci. 2022, 23, 10345. https://doi.org/10.3390/ijms231810345
Nishida C, Izumi H, Tomonaga T, Wang K-Y, Higashi H, Takeshita J-I, Ono R, Sumiya K, Fujii S, Hata Y, et al. Effect of Different Molecular Weights of Polyacrylic Acid on Rat Lung Following Intratracheal Instillation. International Journal of Molecular Sciences. 2022; 23(18):10345. https://doi.org/10.3390/ijms231810345
Chicago/Turabian StyleNishida, Chinatsu, Hiroto Izumi, Taisuke Tomonaga, Ke-Yong Wang, Hidenori Higashi, Jun-Ichi Takeshita, Ryohei Ono, Kazuki Sumiya, Shota Fujii, Yuki Hata, and et al. 2022. "Effect of Different Molecular Weights of Polyacrylic Acid on Rat Lung Following Intratracheal Instillation" International Journal of Molecular Sciences 23, no. 18: 10345. https://doi.org/10.3390/ijms231810345
APA StyleNishida, C., Izumi, H., Tomonaga, T., Wang, K.-Y., Higashi, H., Takeshita, J.-I., Ono, R., Sumiya, K., Fujii, S., Hata, Y., Sakurai, K., Higashi, Y., Yamasaki, K., Yatera, K., & Morimoto, Y. (2022). Effect of Different Molecular Weights of Polyacrylic Acid on Rat Lung Following Intratracheal Instillation. International Journal of Molecular Sciences, 23(18), 10345. https://doi.org/10.3390/ijms231810345







