Cell Contact with Endothelial Cells Favors the In Vitro Maintenance of Human Chronic Myeloid Leukemia Stem and Progenitor Cells
Abstract
:1. Introduction
2. Results
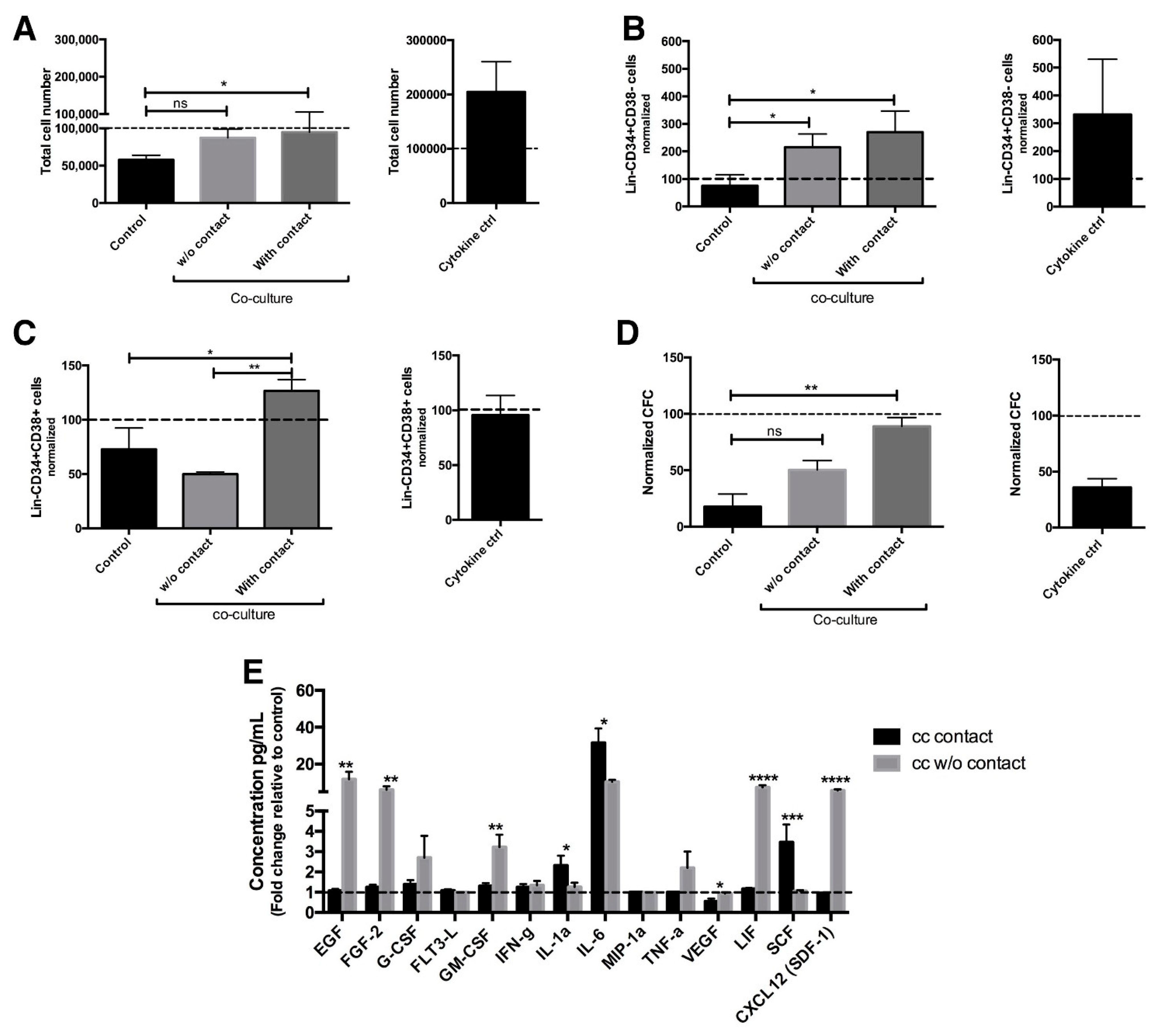
2.1. Co-Culture with Endothelial Cells Allows Maintenance of Primitive CML Cells
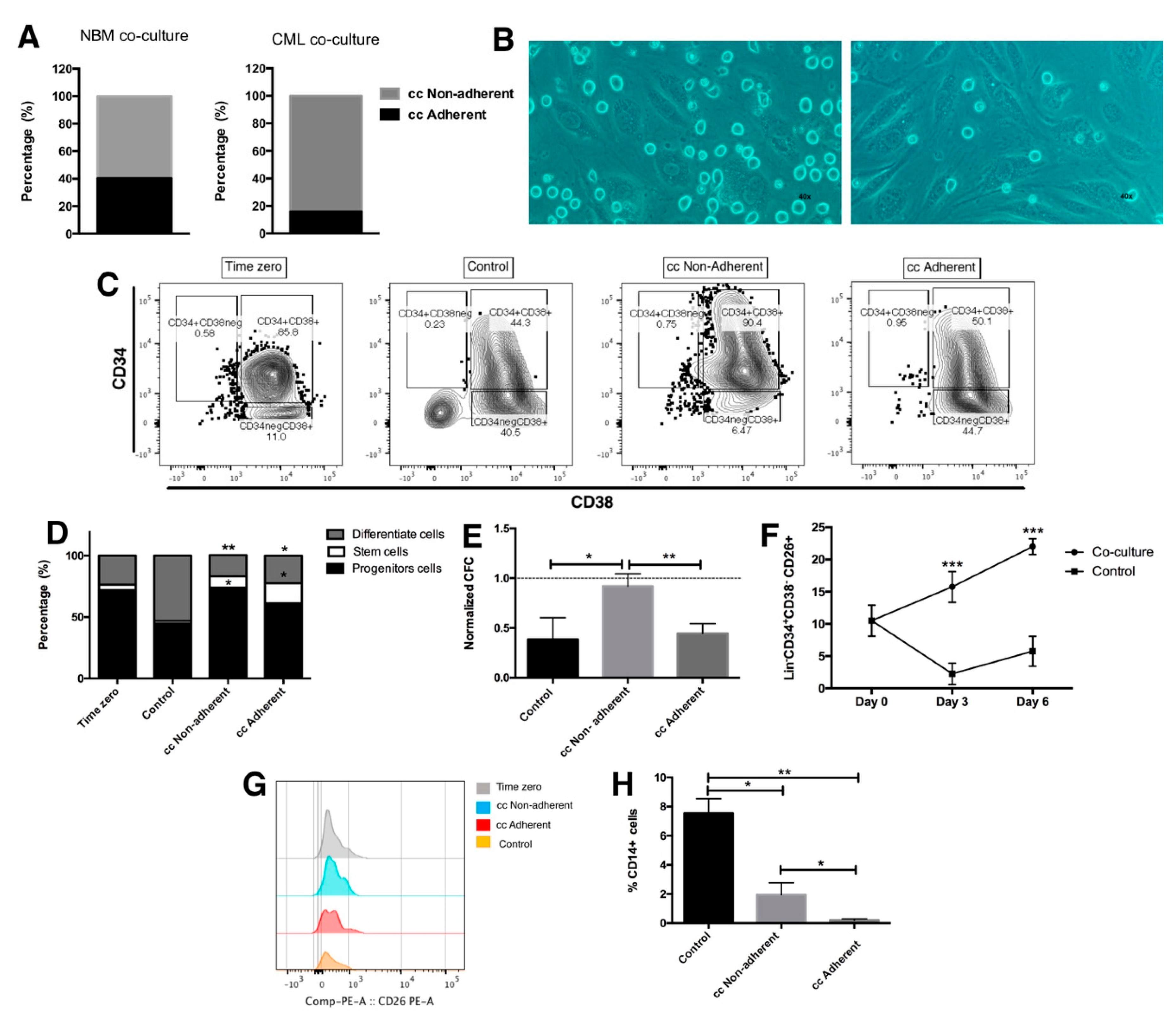
2.2. Leukemic Stem Cells Remain Attached to Endothelial Cells
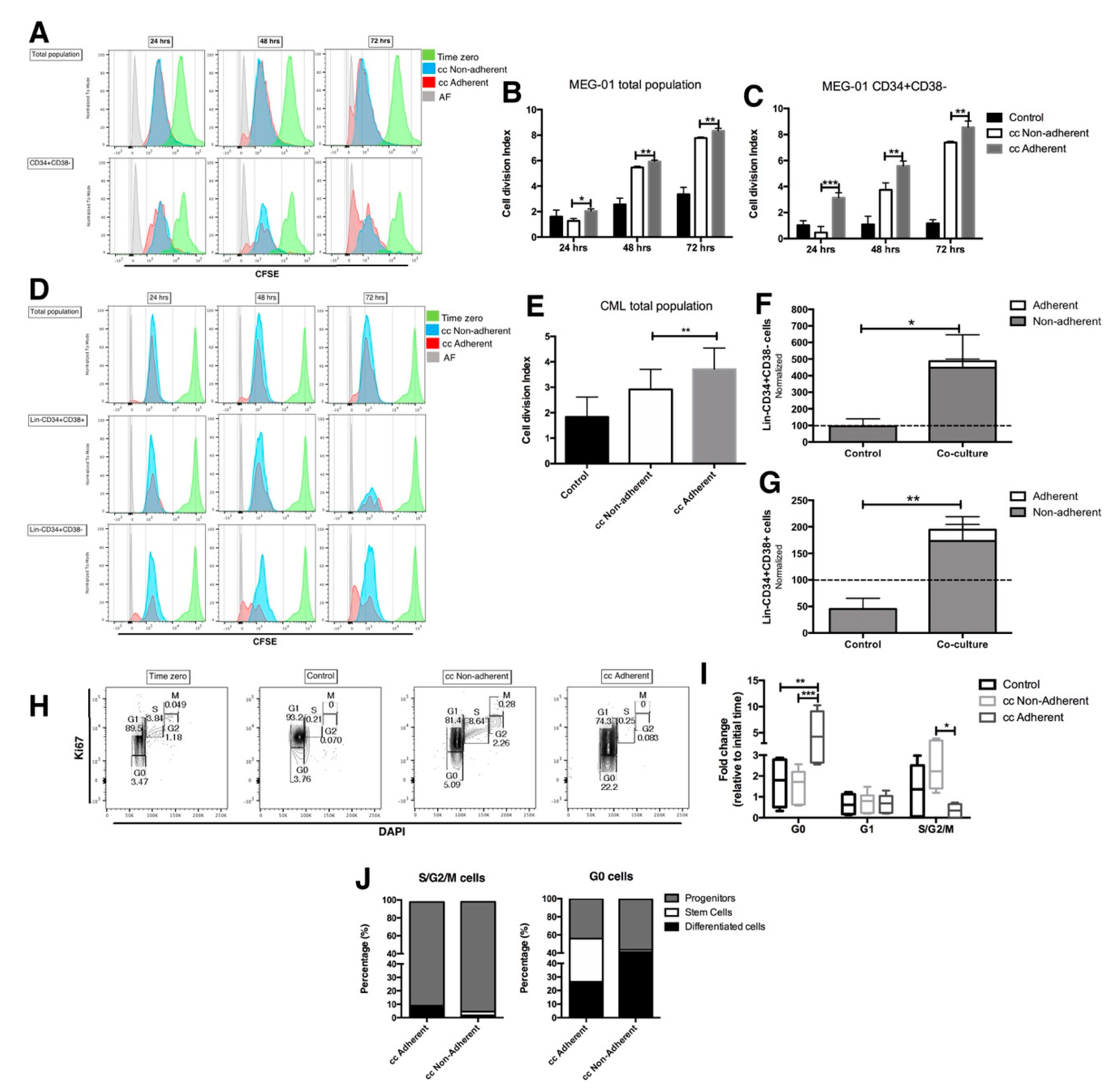
2.3. The Proliferation Status of CML LSC Is Regulated by the Co-Culture with EC
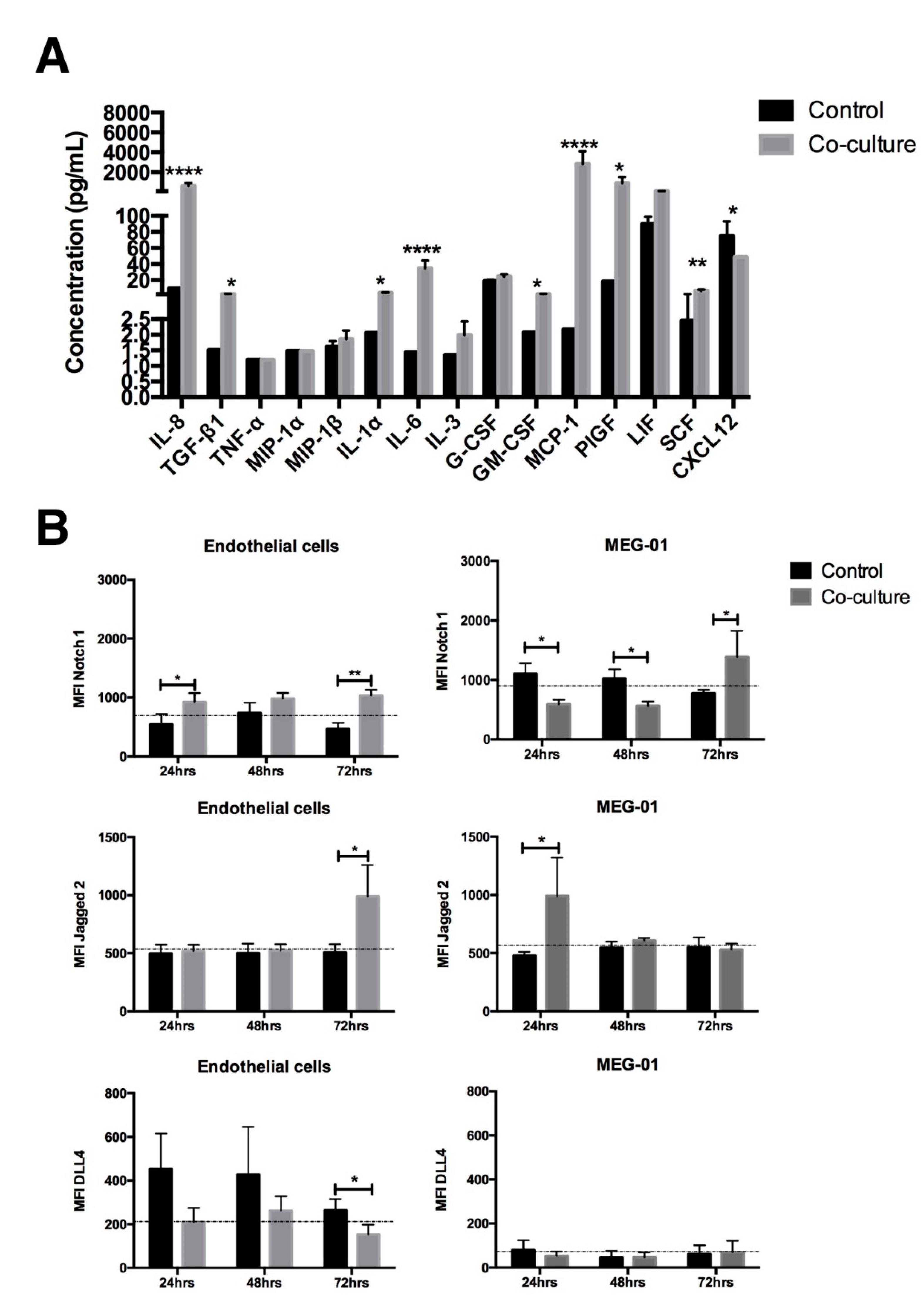
2.4. The Cytokine Profile and Notch Expression Are Modified after Co-Culture with EC
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Lin- CD34+ Cell Enrichment
4.3. Endothelial Cells
4.4. MEG-01 Cell Line
4.5. Co-Culture of Hematopoietic Cells with Endothelial Cells
4.6. Hematopoietic Cells’ Recovery
4.7. Immunophenotype and Adhesion Molecules
4.8. Colony Forming Cell Assays
4.9. Proliferation and Cell Cycle Assay
4.10. Cytokines’ Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Chávez-González, A.; Ayala-Sánchez, M.; Sánchez-Valle, E.; Ruiz-Sánchez, E.; Arana-Trejo, R.M.; Vela-Ojeda, J.; Mayani, H. Functional integrity in vitro of hematopoietic progenitor cells from patients with chronic myeloid leukemia that have achieved hematological remission after different therapeutic procedures. Leuk. Res. 2006, 30, 286–295. [Google Scholar] [CrossRef]
- Moreno-Lorenzana, D.; Avilés-Vazquez, S.; Sandoval Esquivel, M.A.; Alvarado-Moreno, A.; Ortiz-Navarrete, V.; Torres-Martínez, H.; Ayala-Sánchez, M.; Mayani, H.; Chavez-Gonzalez, A. CDKIs p18(INK4c) and p57(Kip2) are involved in quiescence of CML leukemic stem cells after treatment with TKI. Cell cycle 2016, 15, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, T.; Jiang, X.; Eaves, C.; Eaves, A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 1999, 94, 2056–2064, Epub 1999/09/09. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chu, S.; Agarwal, P.; Campbell, V.L.; Hopcroft, L.; Jørgensen, H.G.; Lin, A.; Gaal, K.; Holyoake, T.L.; Bhatia, R. Inhibition of interleukin-1 signaling enhances elimination of tyrosine kinase inhibitor-treated CML stem cells. Blood 2016, 128, 2671–2682. [Google Scholar] [CrossRef]
- Zhang, B.; Ho, Y.W.; Huang, Q.; Maeda, T.; Lin, A.; Lee, S.U.; Hair, A.; Holyoake, T.L.; Huettner, C.; Bhatia, R. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer cell 2012, 21, 577–592, Epub 2012/04/21. [Google Scholar] [CrossRef]
- Torres-Barrera, P.; Mayani, H.; Chávez-González, A. Understanding the hematopoietic microenvironment in chronic myeloid leukemia: A concise review. Curr. Res. Transl. Med. 2021, 69, 103295. [Google Scholar] [CrossRef] [PubMed]
- Korkolopoulou, P.; Viniou, N.; Kavantzas, N.; Patsouris, E.; Thymara, I.; Pavlopoulos, P.M.; Terpos, E.; Stamatopoulos, K.; Plata, E.; Anargyrou, K.; et al. Clinicopathologic correlations of bone marrow angiogenesis in chronic myeloid leukemia: A morphometric study. Leukemia 2003, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Kharabi Masouleh, B.; Loges, S.; Cauwenberghs, S.; Fraisl, P.; Maes, C.; Jonckx, B.; De Keersmaecker, K.; Kleppe, M.; Tjwa, M.; et al. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer cell 2011, 19, 740–753. [Google Scholar] [CrossRef]
- Zhang, B.; Nguyen, L.X.T.; Li, L.; Zhao, D.; Kumar, B.; Wu, H.; Lin, A.; Pellicano, F.; Hopcroft, L.; Su, Y.-L.; et al. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat. Med. 2018, 24, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Godavarthy, P.S.; Kumar, R.; Herkt, S.C.; Pereira, R.S.; Hayduk, N.; Weissenberger, E.S.; Aggoune, D.; Manavski, Y.; Lucas, T.; Pan, K.-T.; et al. The vascular bone marrow niche influences outcome in chronic myeloid leukemia via the E-selectin—SCL/TAL1—CD44 axis. Haematologica 2019, 105, 136–147. [Google Scholar] [CrossRef]
- Agarwal, P.; Isringhausen, S.; Li, H.; Paterson, A.J.; He, J.; Gomariz, A.; Nagasawa, T.; Nombela-Arrieta, C.; Bhatia, R. Mesenchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells. Cell Stem Cell 2019, 24, 769–784.e6. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Reynaud, D.; Pietras, E.; Barry-Holson, K.; Mir, A.; Binnewies, M.; Jeanne, M.; Sala-Torra, O.; Radich, J.P.; Passegué, E. IL-6 Controls Leukemic Multipotent Progenitor Cell Fate and Contributes to Chronic Myelogenous Leukemia Development. Cancer Cell 2011, 20, 661–673. [Google Scholar] [CrossRef]
- Herrmann, H.; Sadovnik, I.; Cerny-Reiterer, S.; Rülicke, T.; Stefanzl, G.; Willmann, M.; Hoermann, G.; Bilban, M.; Blatt, K.; Herndlhofer, S.; et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood 2014, 123, 3951–3962. [Google Scholar] [CrossRef]
- Mizuno, T.; Yamasaki, N.; Miyazaki, K.; Tazaki, T.; Koller, R.; Oda, H.; Honda, Z.-I.; Ochi, M.; Wolff, L.; Honda, H. Overexpression/enhanced kinase activity of BCR/ABL and altered expression of Notch1 induced acute leukemia in p210BCR/ABL transgenic mice. Oncogene 2008, 27, 3465–3474. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Barbier, V.; Nowlan, B.; Jacobsen, R.N.; Forristal, C.E.; Patton, J.T.; Magnani, J.L.; Lévesque, J.-P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012, 18, 1651–1657. [Google Scholar] [CrossRef]
- Verfaillie, C.M.; McCarthy, J.B.; McGlave, P.B. Mechanisms underlying abnormal trafficking of malignant progenitors in chronic myelogenous leukemia. Decreased adhesion to stroma and fibronectin but increased adhesion to the basement membrane components laminin and collagen type IV. J. Clin. Investig. 1992, 90, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Anand, T.; Bhattacharyya, J.; Sharma, A.; Jaganathan, B.G. K562 chronic myeloid leukemia cells modify osteogenic differentiation and gene expression of bone marrow stromal cells. J. Cell Commun. Signal. 2017, 12, 441–450. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; McDonald, T.; Holyoake, T.; Moon, R.; Campana, D.; Shultz, L.; Bhatia, R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt–β-catenin signaling. Blood 2013, 121, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.Y.; Dowding, C.R.; Riley, G.; Goldman, J.M.; Greaves, M.F. Altered adhesive interactions with marrow stroma of haematopoietic progenitor cells in chronic myeloid leukaemia. Nature 1987, 328, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Eaves, A.C.; Cashman, J.D.; Gaboury, L.A.; Kalousek, D.K.; Eaves, C.J. Unregulated proliferation of primitive chronic myeloid leukemia progenitors in the presence of normal marrow adherent cells. Proc. Natl. Acad. Sci. USA 1986, 83, 5306–5310. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Raimondo, S.; Saieva, L.; Flugy, A.M.; De Leo, G.; Alessandro, R. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an Interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014, 348, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K. Tumour promoting functions of TGF- in CML-initiating cells. J. Biochem. 2012, 152, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.D.; Eaves, C.J.; Sarris, A.H.; Eaves, A.C. MCP-1, not MIP-1alpha, is the endogenous chemokine that cooperates with TGF-beta to inhibit the cycling of primitive normal but not leukemic (CML) progenitors in long-term human marrow cultures. Blood 1998, 92, 2338–2344. [Google Scholar] [CrossRef]
- Fernandez, L.; Rodriguez, S.; Huang, H.; Chora, A.; Fernandes, J.; Mumaw, C.; Cruz, E.; Pollok, K.; Cristina, F.; Price, J.E.; et al. Tumor necrosis factor-α and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp. Hematol. 2008, 36, 545–558.e1. [Google Scholar] [CrossRef]
- Aljedai, A.; Buckle, A.-M.; Hiwarkar, P.; Syed, F. Potential Role of Notch Signalling in CD34+ Chronic Myeloid Leukaemia Cells: Cross-Talk between Notch and BCR-ABL. PLoS ONE 2015, 10, e0123016. [Google Scholar] [CrossRef]
- Paschalaki, K.E.; Randi, A.M. Recent Advances in Endothelial Colony Forming Cells Toward Their Use in Clinical Translation. Front. Med. 2018, 5, 295. [Google Scholar] [CrossRef]
- Alvarado-Moreno, J.A.; Hernandez-Lopez, R.; Chavez-Gonzalez, A.; Yoder, M.C.; Rangel-Corona, R.; Isordia-Salas, I.; Hernandez-Juarez, J.; Cerbulo-Vazquez, A.; Gonzalez-Jimenez, M.A.; Majluf-Cruz, A. Endothelial colony-forming cells: Biological and functional abnormalities in patients with recurrent, unprovoked venous thromboembolic disease. Thromb. Res. 2015, 137, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barrera, P.; Ramírez-Florencio, M.; Chávez-Gonzávez-Gonzá, A. Assessment of Cell Cycle in Primitive Chronic Myeloid Leukemia Cells by Flow Cytometry After Coculture with Endothelial Cells. Methods Mol. Biol. 2020, 2174, 207–216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Barrera, P.; Moreno-Lorenzana, D.; Alvarado-Moreno, J.A.; García-Ruiz, E.; Lagunas, C.; Mayani, H.; Chávez-González, A. Cell Contact with Endothelial Cells Favors the In Vitro Maintenance of Human Chronic Myeloid Leukemia Stem and Progenitor Cells. Int. J. Mol. Sci. 2022, 23, 10326. https://doi.org/10.3390/ijms231810326
Torres-Barrera P, Moreno-Lorenzana D, Alvarado-Moreno JA, García-Ruiz E, Lagunas C, Mayani H, Chávez-González A. Cell Contact with Endothelial Cells Favors the In Vitro Maintenance of Human Chronic Myeloid Leukemia Stem and Progenitor Cells. International Journal of Molecular Sciences. 2022; 23(18):10326. https://doi.org/10.3390/ijms231810326
Chicago/Turabian StyleTorres-Barrera, Patricia, Dafne Moreno-Lorenzana, José Antonio Alvarado-Moreno, Elena García-Ruiz, Cesar Lagunas, Hector Mayani, and Antonieta Chávez-González. 2022. "Cell Contact with Endothelial Cells Favors the In Vitro Maintenance of Human Chronic Myeloid Leukemia Stem and Progenitor Cells" International Journal of Molecular Sciences 23, no. 18: 10326. https://doi.org/10.3390/ijms231810326
APA StyleTorres-Barrera, P., Moreno-Lorenzana, D., Alvarado-Moreno, J. A., García-Ruiz, E., Lagunas, C., Mayani, H., & Chávez-González, A. (2022). Cell Contact with Endothelial Cells Favors the In Vitro Maintenance of Human Chronic Myeloid Leukemia Stem and Progenitor Cells. International Journal of Molecular Sciences, 23(18), 10326. https://doi.org/10.3390/ijms231810326






