Elevated Oxytocin Receptor Blood Concentrations Predict Higher Risk for, More, and Earlier 24-Month Hospital Readmissions after In-Patient Detoxification in Males with Alcohol Use Disorder
Abstract
1. Introduction
Aims of the Study
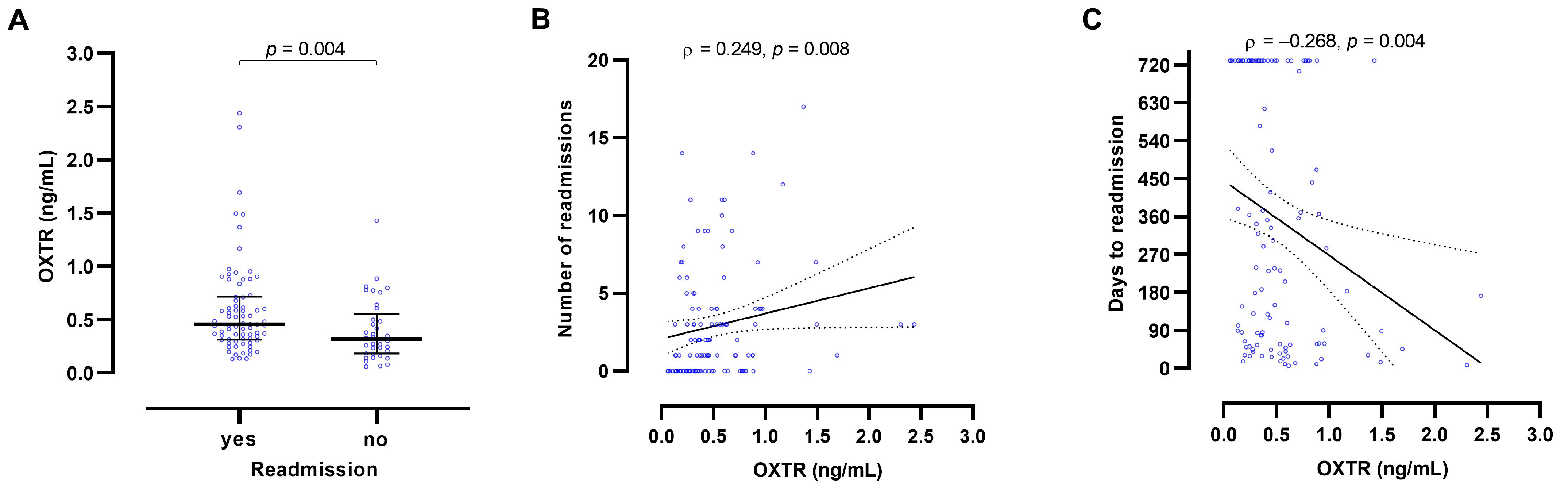
2. Results
2.1. Demographic Characteristics
2.2. Oxytocin Receptor Blood Concentrations
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Description
4.2. Determination of Oxytocin Receptor, Oxytocin, and Sex Hormone Blood Concentrations, and Routine Laboratory Parameters
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol use disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The oxytocin receptor: From intracellular signaling to behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Warfvinge, K.; Krause, D.; Edvinsson, L. The distribution of oxytocin and the oxytocin receptor in rat brain: Relation to regions active in migraine. J. Headache Pain 2020, 21, 10. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. Cholesterol as stabilizer of the oxytocin receptor. Biochim. Biophys. Acta 2002, 1564, 384–392. [Google Scholar] [CrossRef]
- Leong, K.C.; Cox, S.; King, C.; Becker, H.; Reichel, C.M. Oxytocin and rodent models of addiction. Int. Rev. Neurobiol. 2018, 140, 201–247. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.; Neumann, I.D. Brain oxytocin: How puzzle stones from animal studies translate into psychiatry. Mol. Psychiatry 2021, 26, 265–279. [Google Scholar] [CrossRef]
- King, C.E.; Gano, A.; Becker, H.C. The role of oxytocin in alcohol and drug abuse. Brain Res. 2020, 1736, 146761. [Google Scholar] [CrossRef]
- Jirikowski, G.F.; Ochs, S.D.; Caldwell, J.D. Oxytocin and steroid actions. Curr. Top Behav. Neurosci. 2018, 35, 77–95. [Google Scholar] [CrossRef]
- Breese, G.R.; Chu, K.; Dayas, C.V.; Funk, D.; Knapp, D.J.; Koob, G.F.; Lê, D.A.; O’Dell, L.E.; Overstreet, D.H.; Roberts, A.J.; et al. Stress enhancement of craving during sobriety: A risk for relapse. Alcohol. Clin. Exp. Res. 2005, 29, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S., 3rd. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Valdez, G.R.; Zorrilla, E.P.; Roberts, A.J.; Koob, G.F. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol 2003, 29, 55–60. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Windle, R.J.; Shanks, N.; Lightman, S.L.; Ingram, C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997, 138, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.A.; Smedley, K.L.; Leserman, J.; Jarskog, L.F.; Rau, S.W.; Kampov-Polevoi, A.; Casey, R.L.; Fender, T.; Garbutt, J.C. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol. Clin. Exp. Res. 2013, 37, 484–489. [Google Scholar] [CrossRef]
- Peters, S.T.; Bowen, M.T.; Bohrer, K.; McGregor, I.S.; Neumann, I.D. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict. Biol. 2017, 22, 702–711. [Google Scholar] [CrossRef]
- Peris, J.; Steck, M.R.; Krause, E.G. Oxytocin treatment for alcoholism: Potential neurocircuitry targets. Neuropharmacology 2020, 171, 108091. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Grinevich, V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014, 8, 31. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The brain on drugs: From reward to addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef]
- Wang, X.; Gallegos, D.A.; Pogorelov, V.M.; O’Hare, J.K.; Calakos, N.; Wetsel, W.C.; West, A.E. Parvalbumin interneurons of the mouse nucleus accumbens are required for amphetamine-induced locomotor sensitization and conditioned place preference. Neuropsychopharmacology 2018, 43, 953–963. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Qi, J.; Han, W.Y.; Yang, J.Y.; Wang, L.H.; Dong, Y.X.; Wang, F.; Song, M.; Wu, C.F. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict. Biol. 2012, 17, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Soyka, M. Diagnosis and pharmacotherapy of alcohol use disorder: A review. JAMA 2018, 320, 815–824. [Google Scholar] [CrossRef]
- Torner, L.; Plotsky, P.M.; Neumann, I.D.; de Jong, T.R. Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology 2017, 77, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Krömer, S.A.; Toschi, N.; Ebner, K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regul. Pept. 2000, 96, 31–38. [Google Scholar] [CrossRef]
- Huber, S.E.; Zoicas, I.; Reichel, M.; Mühle, C.; Büttner, C.; Ekici, A.B.; Eulenburg, V.; Lenz, B.; Kornhuber, J.; Müller, C.P. Prenatal androgen receptor activation determines adult alcohol and water drinking in a sex-specific way. Addict. Biol. 2018, 23, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Lenz, B.; Frieling, H.; Jacob, C.; Heberlein, A.; Kornhuber, J.; Bleich, S.; Hillemacher, T. The modulating effect of the androgen receptor on craving in alcohol withdrawal of men is partially mediated by leptin. Pharmacogenom. J. 2010, 10, 226–231. [Google Scholar] [CrossRef]
- Lenz, B.; Jacob, C.; Frieling, H.; Jacobi, A.; Hillemacher, T.; Muschler, M.; Watson, K.; Kornhuber, J.; Bleich, S. Polymorphism of the long polyglutamine tract in the human androgen receptor influences craving of men in alcohol withdrawal. Psychoneuroendocrinology 2009, 34, 968–971. [Google Scholar] [CrossRef]
- Lenz, B.; Mühle, C.; Braun, B.; Weinland, C.; Bouna-Pyrrou, P.; Behrens, J.; Kubis, S.; Mikolaiczik, K.; Muschler, M.R.; Saigali, S.; et al. Prenatal and adult androgen activities in alcohol dependence. Acta Psychiatr. Scand. 2017, 136, 96–107. [Google Scholar] [CrossRef]
- Lenz, B.; Müller, C.P.; Stoessel, C.; Sperling, W.; Biermann, T.; Hillemacher, T.; Bleich, S.; Kornhuber, J. Sex hormone activity in alcohol addiction: Integrating organizational and activational effects. Prog. Neurobiol. 2012, 96, 136–163. [Google Scholar] [CrossRef]
- Mühle, C.; Barry, B.; Weinland, C.; Kornhuber, J.; Lenz, B. Estrogen receptor 1 gene variants and estradiol activities in alcohol dependence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 301–307. [Google Scholar] [CrossRef]
- Weinland, C.; Mühle, C.; Kornhuber, J.; Lenz, B. Progesterone serum levels correlate negatively with craving in female postmenopausal in-patients with alcohol use disorder: A sex- and menopausal status-separated study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110278. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L.; Dorsa, D.M. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology 1995, 136, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L.; Dorsa, D.M. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology 1995, 136, 27–32. [Google Scholar] [CrossRef]
- Viero, C.; Dayanithi, G. Neurosteroids are excitatory in supraoptic neurons but inhibitory in the peripheral nervous system: It is all about oxytocin and progesterone receptors. Prog. Brain Res. 2008, 170, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef]
- Taylor, A.H.; Whitley, G.S.; Nussey, S.S. The interaction of arginine vasopressin and oxytocin with bovine adrenal medulla cells. J. Endocrinol. 1989, 121, 133–139. [Google Scholar] [CrossRef]
- Ostrowski, N.L.; Young, W.S., 3rd; Lolait, S.J. Estrogen increases renal oxytocin receptor gene expression. Endocrinology 1995, 136, 1801–1804. [Google Scholar] [CrossRef]
- Gutkowska, J.; Jankowski, M. Oxytocin revisited: Its role in cardiovascular regulation. J. Neuroendocrinol. 2012, 24, 599–608. [Google Scholar] [CrossRef]
- Colaianni, G.; Tamma, R.; Di Benedetto, A.; Yuen, T.; Sun, L.; Zaidi, M.; Zallone, A. The oxytocin-bone axis. J. Neuroendocrinol. 2014, 26, 53–57. [Google Scholar] [CrossRef]
- Yi, K.J.; So, K.H.; Hata, Y.; Suzuki, Y.; Kato, D.; Watanabe, K.; Aso, H.; Kasahara, Y.; Nishimori, K.; Chen, C.; et al. The regulation of oxytocin receptor gene expression during adipogenesis. J. Neuroendocrinol. 2015, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yulia, A.; Johnson, M.R. Myometrial oxytocin receptor expression and intracellular pathways. Minerva Ginecol. 2014, 66, 267–280. [Google Scholar] [PubMed]
- Hofmann, J.; Huber, C.; Novak, B.; Schreckenbach, M.; Schubert, C.F.; Touma, C.; Rutten, B.P.F.; Schmidt, U. Oxytocin receptor is a potential biomarker of the hyporesponsive HPA axis subtype of PTSD and might be modulated by HPA axis reactivity traits in humans and mice. Psychoneuroendocrinology 2021, 129, 105242. [Google Scholar] [CrossRef]
- Krause, S.; Boeck, C.; Gumpp, A.M.; Rottler, E.; Schury, K.; Karabatsiakis, A.; Buchheim, A.; Gündel, H.; Kolassa, I.T.; Waller, C. Child maltreatment is associated with a reduction of the oxytocin receptor in peripheral blood mononuclear cells. Front. Psychol. 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Voinsky, I.; Bennuri, S.C.; Svigals, J.; Frye, R.E.; Rose, S.; Gurwitz, D. Peripheral blood mononuclear cell oxytocin and vasopressin receptor expression positively correlates with social and behavioral function in children with autism. Sci. Rep. 2019, 9, 13443. [Google Scholar] [CrossRef]
- Akdemir, N.; Cinemre, F.B.; Cinemre, H.; Sevinc, L.; Aydemir, B.; Coban, B.; Cevrioglu, A.S.; Ozden, S. Polymorphism of the oxytocin receptor (OXTR) gene affects the circulating oxytocin receptor levels in late-term pregnancy in a Turkish population. Gynecol. Obstet. Investig. 2020, 85, 343–351. [Google Scholar] [CrossRef]
- Camerino, C.; Conte, E.; Caloiero, R.; Fonzino, A.; Carratù, M.; Lograno, M.D.; Tricarico, D. Evaluation of short and long term cold stress challenge of nerve grow factor, brain-derived neurotrophic factor, osteocalcin and oxytocin mRNA expression in BAT, brain, bone and reproductive tissue of male mice using real-time PCR and linear correlation analysis. Front. Physiol. 2018, 8, 1101. [Google Scholar] [CrossRef]
- Hansson, A.C.; Koopmann, A.; Uhrig, S.; Bühler, S.; Domi, E.; Kiessling, E.; Ciccocioppo, R.; Froemke, R.C.; Grinevich, V.; Kiefer, F.; et al. Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology 2018, 43, 1235–1246. [Google Scholar] [CrossRef]
- Hansson, A.C.; Spanagel, R. No changes in the oxytocin system in alcohol-dependent female rodents and humans: Towards a sex-specific psychopharmacology in alcoholism. Addict. Biol. 2021, 26, e12945. [Google Scholar] [CrossRef]
- Bach, P.; Reinhard, I.; Bühler, S.; Vollstädt-Klein, S.; Kiefer, F.; Koopmann, A. Oxytocin modulates alcohol-cue induced functional connectivity in the nucleus accumbens of social drinkers. Psychoneuroendocrinology 2019, 109, 104385. [Google Scholar] [CrossRef]
- Bach, P.; Vollstädt-Klein, S.; Kirsch, M.; Hoffmann, S.; Jorde, A.; Frank, J.; Charlet, K.; Beck, A.; Heinz, A.; Walter, H.; et al. Increased mesolimbic cue-reactivity in carriers of the mu-opioid-receptor gene OPRM1 A118G polymorphism predicts drinking outcome: A functional imaging study in alcohol dependent subjects. Eur. Neuropsychopharmacol. 2015, 25, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Lenz, B.; Weinland, C.; Bach, P.; Kiefer, F.; Grinevich, V.; Zoicas, I.; Kornhuber, J.; Mühle, C. Oxytocin blood concentrations in alcohol use disorder: A cross-sectional, longitudinal, and sex-separated study. Eur. Neuropsychopharmacol. 2021, 51, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.; Havemann-Reinecke, U.; Kornhuber, J. Fagerström-Test für Nikotinabhängigkeit (FTNA) [Fagerström Test for Nicotine Dependence]; Hogrefe-Verlag: Göttingen, Germany, 2002. [Google Scholar]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerström, K.O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.W.; Poehlmann, M.L.; Li, S.; Ratnaseelan, A.M.; Bredewold, R.; Veenema, A.H. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: Focus on the social decision-making network. Brain Struct. Funct. 2017, 222, 981–1006. [Google Scholar] [CrossRef]
- Lee, M.R.; Schwandt, M.L.; Sankar, V.; Suchankova, P.; Sun, H.; Leggio, L. Effect of alcohol use disorder on oxytocin peptide and receptor mRNA expression in human brain: A post-mortem case-control study. Psychoneuroendocrinology 2017, 85, 14–19. [Google Scholar] [CrossRef]
- Badrick, E.; Kirschbaum, C.; Kumari, M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007, 92, 819–824. [Google Scholar] [CrossRef]
- Chuang, H.J.; Chang, C.Y.; Ho, H.P.; Chou, M.Y. Oxytocin signaling acts as a marker for environmental stressors in zebrafish. Int. J. Mol. Sci. 2021, 22, 7459. [Google Scholar] [CrossRef]
- Kanamori, C.; Yasuda, K.; Sumi, G.; Kimura, Y.; Tsuzuki, T.; Cho, H.; Okada, H.; Kanzaki, H. Effect of cigarette smoking on mRNA and protein levels of oxytocin receptor and on contractile sensitivity of uterine myometrium to oxytocin in pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 142–147. [Google Scholar] [CrossRef]
- Vierhapper, H.; Nowotny, P.; Maier, H.; Waldhäusl, W. Production rates of dihydrotestosterone in healthy men and women and in men with male pattern baldness: Determination by stable isotope/dilution and mass spectrometry. J. Clin. Endocrinol. Metab. 2001, 86, 5762–5764. [Google Scholar] [CrossRef][Green Version]
- Siegmann, E.M.; Bouna-Pyrrou, P.; Lenz, B.; Kornhuber, J. Digit ratio (2D:4D) in relation to substance and computer use: A meta-analysis. J. Neural. Transm 2019, 126, 623–636. [Google Scholar] [CrossRef]
- Aulinas, A.; Pulumo, R.L.; Asanza, E.; Mancuso, C.J.; Slattery, M.; Tolley, C.; Plessow, F.; Thomas, J.J.; Eddy, K.T.; Miller, K.K.; et al. Endogenous oxytocin levels in relation to food intake, menstrual phase, and age in females. J. Clin. Endocrinol. Metab. 2019, 104, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Nappi, R.E.; Pontillo, M.; Daverio, R.; Smeraldi, A.; Briganti, A.; Fabbri, F.; Zanni, G.; Rigatti, P.; Montorsi, F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm. Behav. 2005, 47, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.C.; Lin, T.; Muradoglu, M.; Weir, D.H.; Plasencia, G.M.; Lillard, T.S.; Pournajafi-Nazarloo, H.; Cohen, R.A.; Sue Carter, C.; Connelly, J.J. Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int. J. Psychophysiol. 2019, 136, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rung, J.M.; Kidder, Q.A.; Horta, M.; Nazarloo, H.P.; Carter, C.S.; Berry, M.S.; Ebner, N.C. Associations between alcohol use and peripheral, genetic, and epigenetic markers of oxytocin in a general sample of young and older adults. Brain Behav. 2022, 12, e2425. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.; Weinland, C.; Kornhuber, J.; Lenz, B. Religiosity, guilt, altruism and forgiveness in alcohol dependence: Results of a cross-sectional and prospective cohort study. Alcohol Alcohol. 2018, 53, 426–434. [Google Scholar] [CrossRef]
- Gegenhuber, B.; Weinland, C.; Kornhuber, J.; Mühle, C.; Lenz, B. OPRM1 A118G and serum beta-endorphin interact with sex and digit ratio (2D:4D) to influence risk and course of alcohol dependence. Eur. Neuropsychopharmacol. 2018, 28, 1418–1428. [Google Scholar] [CrossRef]
- Lenz, B.; Köllner, M.G.; Mühle, C.; Weinland, C.; Kornhuber, J. Basic human body dimensions relate to alcohol dependence and predict hospital readmission. J. Clin. Med. 2019, 8, 2076. [Google Scholar] [CrossRef]
- Mühle, C.; Weinland, C.; Gulbins, E.; Lenz, B.; Kornhuber, J. Peripheral acid sphingomyelinase activity is associated with biomarkers and phenotypes of alcohol use and dependence in patients and healthy controls. Int. J. Mol. Sci. 2018, 19, 4028. [Google Scholar] [CrossRef]
- Müller, C.P.; Mühle, C.; Kornhuber, J.; Lenz, B. Sex-dependent alcohol instrumentalization goals in non-addicted alcohol consumers versus patients with alcohol use disorder: Longitudinal change and outcome prediction. Alcohol. Clin. Exp. Res. 2021, 45, 577–586. [Google Scholar] [CrossRef]
- Weinland, C.; Braun, B.; Mühle, C.; Kornhuber, J.; Lenz, B. Cloninger type 2 score and Lesch typology predict hospital readmission of female and male alcohol-dependent inpatients during a 24-month follow-up. Alcohol. Clin. Exp. Res. 2017, 41, 1760–1767. [Google Scholar] [CrossRef]
- Weinland, C.; Mühle, C.; Kornhuber, J.; Lenz, B. Crossed eye/hand laterality and left-eyedness predict a positive 24-month outcome in alcohol-dependent patients. Alcohol. Clin. Exp. Res. 2019, 43, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Weinland, C.; Mühle, C.; Kornhuber, J.; Lenz, B. Body mass index and craving predict 24-month hospital readmissions of alcohol-dependent in-patients following withdrawal. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Weinland, C.; Mühle, C.; von Zimmermann, C.; Kornhuber, J.; Lenz, B. Sulphated dehydroepiandrosterone serum levels are reduced in women with alcohol use disorder and correlate negatively with craving: A sex-separated cross-sectional and longitudinal study. Addict. Biol. 2022, 27, e13135. [Google Scholar] [CrossRef] [PubMed]
- Weinland, C.; Tanovska, P.; Kornhuber, J.; Mühle, C.; Lenz, B. Serum lipids, leptin, and soluble leptin receptor in alcohol dependence: A cross-sectional and longitudinal study. Drug Alcohol Depend. 2020, 209, 107898. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association (APA): Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10); WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Skinner, H.A.; Sheu, W.J. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982, 43, 1157–1170. [Google Scholar] [CrossRef]
- Rumpf, H.J.; Meyer, C.; Hapke, U.; John, U. Deutsche Version des Alcohol Use Disorders Identification Test (AUDIT-G-L). In Elektronisches Handbuch zu Erhebungsinstrumenten im Suchtbereich (EHES). Version 3.00; Glöckner-Rist, A., Rist, F., Küfner, H., Eds.; Zentrum für Umfragen, Methoden und Analysen: Mannheim, Germany, 2003. [Google Scholar]
- Tannenbaum, C.; Ellis, R.P.; Eyssel, F.; Zou, J.; Schiebinger, L. Sex and gender analysis improves science and engineering. Nature 2019, 575, 137–146. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Heinz, A.; Kiefer, F.; Smolka, M.N.; Endrass, T.; Beste, C.; Beck, A.; Liu, S.; Genauck, A.; Romund, L.; Banaschewski, T.; et al. Addiction Research Consortium: Losing and regaining control over drug intake (ReCoDe)—From trajectories to mechanisms and interventions. Addict. Biol. 2020, 25, e12866. [Google Scholar] [CrossRef]
| AUD Group | Control Group | AUD Group vs. Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M/F | IQR | N | M/F | IQR | U or χ2 | p | |||
| Men | ||||||||||
| Age (years) | 113 | 48 | 40 | 53 | 133 | 48 | 38 | 56 | 7369 | 0.794 # |
| Fasting (%) | 103 | 16 | 127 | 24 | 2.8 | 0.097 + | ||||
| Alcohol concentration at admission (‰) | 108 | 1.7 | 0.5 | 2.4 | - | |||||
| Number of previous withdrawal treatments | 89 | 6 | 2 | 12 | - | |||||
| CDT (nephelometry, %) | 113 | 2.8 | 1.9 | 4.0 | 132 | 1.5 | 1.3 | 1.7 | 1636 | <0.001# |
| AUDIT score | - | 125 | 4 | 3 | 6 | |||||
| Smokers (%) | 104 | 78 | 133 | 22 | 73.8 | <0.001+ | ||||
| FTND score | 99 | 5.0 | 3.0 | 7.0 | 130 | 0.0 | 0.0 | 3.0 | 2556 | <0.001# |
| 24-month alcohol-related readmissions | ||||||||||
| Risk | 113 | 0.67 | ||||||||
| Total number | 113 | 2 | 0 | 4 | - | |||||
| Latency (days) | 113 | 285 | 57 | ≥730 | - | |||||
| Women | ||||||||||
| Age (years) | 87 | 48 | 42 | 55 | 107 | 49 | 39 | 55 | 4542 | 0.772 # |
| Fasting (%) | 80 | 18 | 101 | 26 | 1.8 | 0.184 + | ||||
| Postmenopausal status (%) | 73 | 51 | 100 | 44 | 0.8 | 0.384 + | ||||
| Alcohol concentration at admission (‰) | 85 | 1.2 | 0.1 | 1.8 | - | |||||
| Number of previous withdrawal treatments | 58 | 5 | 2 | 11 | - | |||||
| CDT (nephelometry, %) | 87 | 1.9 | 1.6 | 2.5 | 107 | 1.5 | 1.3 | 1.6 | 1415 | <0.001# |
| AUDIT score | - | 96 | 3 | 2 | 4 | |||||
| Smokers (%) | 78 | 77 | 107 | 19 | 62.3 | <0.001+ | ||||
| FTND score | 75 | 5.0 | 0.5 | 7.0 | 103 | 0.0 | 0.0 | 2.0 | 1757 | <0.001# |
| 24-month alcohol-related readmissions | ||||||||||
| Risk | 87 | 0.53 | ||||||||
| Total number | 87 | 1 | 0 | 3 | - | |||||
| Latency (days) | 87 | 625 | 90 | ≥730 | - | |||||
| AUD Group | Control Group | AUD vs. Control Group | T0 vs. T1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M | IQR | N | M | IQR | U | p # | z | p § | |||
| Men | ||||||||||||
| OXTR T0 | 113 | 0.417 | 0.273 | 0.642 | 133 | 0.437 | 0.288 | 0.678 | 7351 | 0.769 | −0.62 | 0.533 |
| OXTR T1 | 94 | 0.479 | 0.261 | 0.635 | 6239 | 0.980 | ||||||
| Women | ||||||||||||
| OXTR T0 | 87 | 0.470 | 0.317 | 0.749 | 107 | 0.428 | 0.230 | 0.758 | 4175 | 0.218 | −1.37 | 0.172 |
| OXTR T1 | 69 | 0.465 | 0.281 | 0.664 | 3469 | 0.500 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mühle, C.; Mazza, M.; Weinland, C.; von Zimmermann, C.; Bach, P.; Kiefer, F.; Grinevich, V.; Zoicas, I.; Kornhuber, J.; Lenz, B. Elevated Oxytocin Receptor Blood Concentrations Predict Higher Risk for, More, and Earlier 24-Month Hospital Readmissions after In-Patient Detoxification in Males with Alcohol Use Disorder. Int. J. Mol. Sci. 2022, 23, 9940. https://doi.org/10.3390/ijms23179940
Mühle C, Mazza M, Weinland C, von Zimmermann C, Bach P, Kiefer F, Grinevich V, Zoicas I, Kornhuber J, Lenz B. Elevated Oxytocin Receptor Blood Concentrations Predict Higher Risk for, More, and Earlier 24-Month Hospital Readmissions after In-Patient Detoxification in Males with Alcohol Use Disorder. International Journal of Molecular Sciences. 2022; 23(17):9940. https://doi.org/10.3390/ijms23179940
Chicago/Turabian StyleMühle, Christiane, Massimiliano Mazza, Christian Weinland, Claudia von Zimmermann, Patrick Bach, Falk Kiefer, Valery Grinevich, Iulia Zoicas, Johannes Kornhuber, and Bernd Lenz. 2022. "Elevated Oxytocin Receptor Blood Concentrations Predict Higher Risk for, More, and Earlier 24-Month Hospital Readmissions after In-Patient Detoxification in Males with Alcohol Use Disorder" International Journal of Molecular Sciences 23, no. 17: 9940. https://doi.org/10.3390/ijms23179940
APA StyleMühle, C., Mazza, M., Weinland, C., von Zimmermann, C., Bach, P., Kiefer, F., Grinevich, V., Zoicas, I., Kornhuber, J., & Lenz, B. (2022). Elevated Oxytocin Receptor Blood Concentrations Predict Higher Risk for, More, and Earlier 24-Month Hospital Readmissions after In-Patient Detoxification in Males with Alcohol Use Disorder. International Journal of Molecular Sciences, 23(17), 9940. https://doi.org/10.3390/ijms23179940







