Viral Infection and Airway Epithelial Immunity in Asthma
Abstract
1. Introduction
2. Role of Respiratory Viral Infection in Asthma Pathogenesis
2.1. Role of Viral Infection in the Development of Asthma
2.2. Pathophysiological Effects of Viral Infection on Asthma Exacerbations
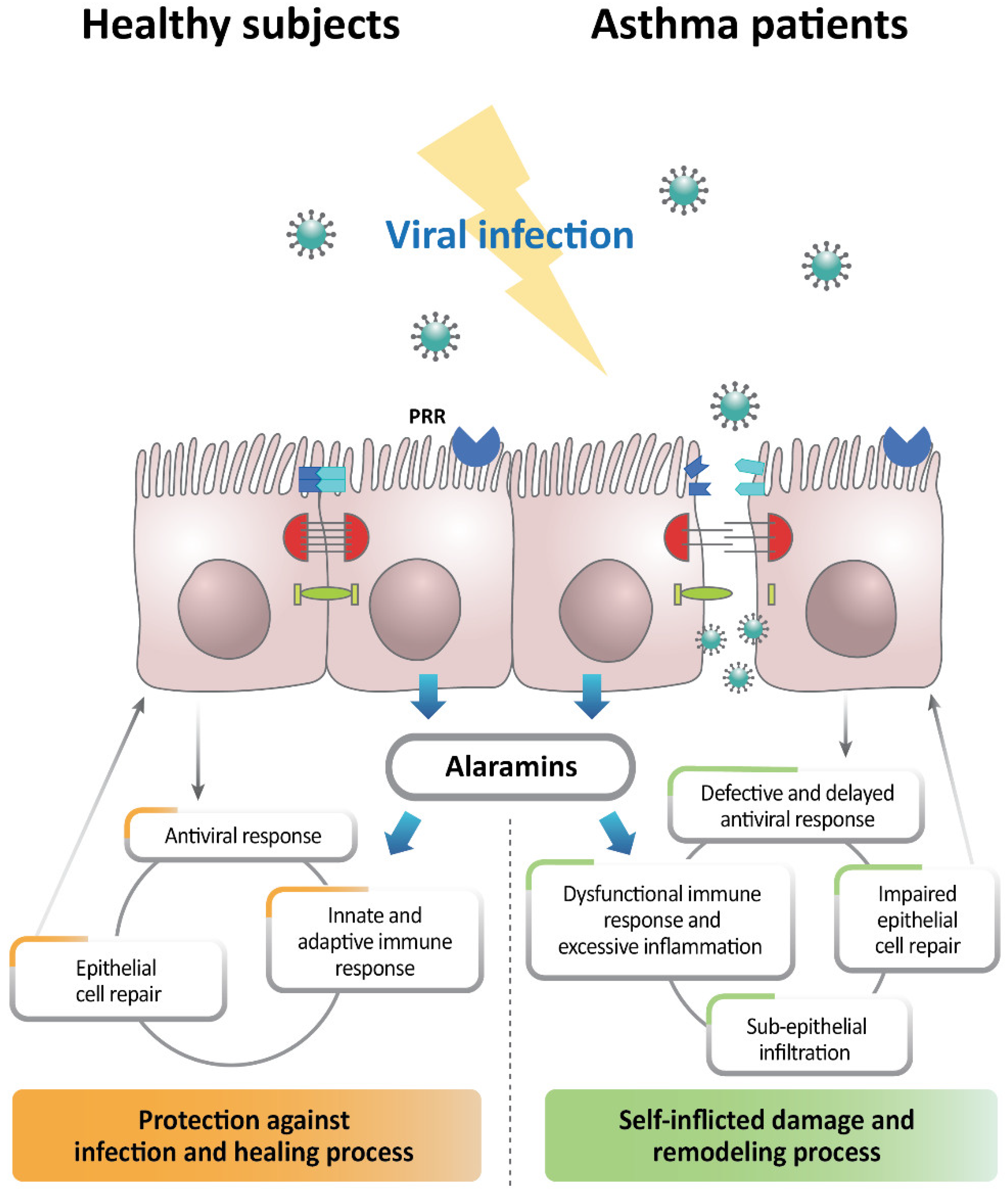
3. Alteration of Airway Epithelial Cells in Viral-Infection-Induced Asthma Exacerbation
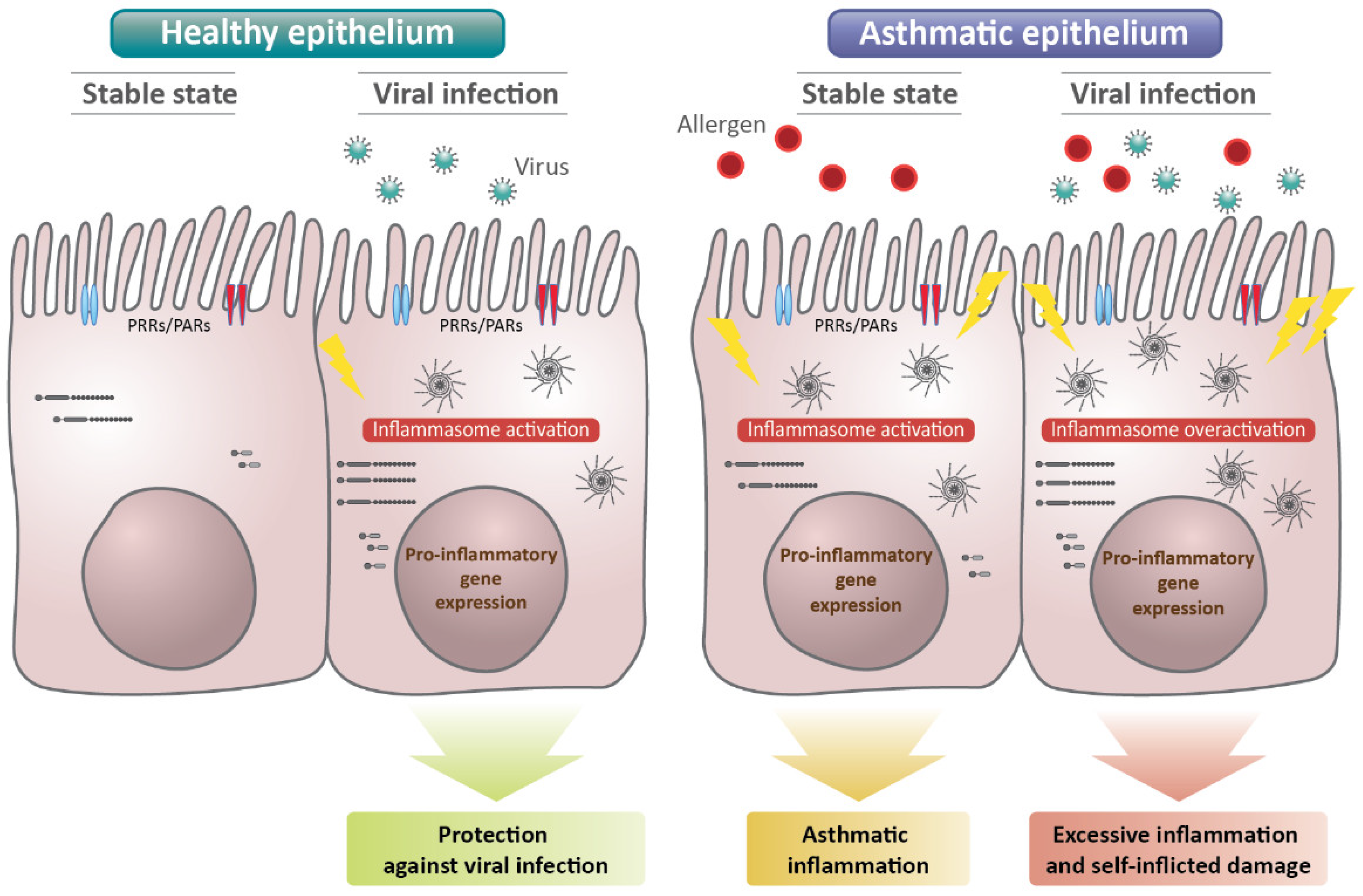
4. Role of the Epithelial Inflammasome in Viral Immunity in Asthma
5. Current and Potential Therapeutics for Viral Infection in Asthma
6. Conclusions
Funding
Conflicts of Interest
References
- Busse, W.W.; Lemanske, R.F., Jr.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Adeli, M.; El-Shareif, T.; Hendaus, M.A. Asthma exacerbation related to viral infections: An up to date summary. J. Fam. Med. Prim. Care 2019, 8, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Cabanillas, B. Viruses and asthma: The role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology 2020, 161, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Rubner, F.J.; Jackson, D.J.; Evans, M.D.; Gangnon, R.E.; Tisler, C.J.; Pappas, T.E.; Gern, J.E.; Lemanske, R.F., Jr. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J. Allergy Clin. Immunol. 2017, 139, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Soto-Quiros, M.; Avila, L.; Platts-Mills, T.A.; Hunt, J.F.; Erdman, D.D.; Carper, H.; Murphy, D.D.; Odio, S.; James, H.R.; Patrie, J.T.; et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J. Allergy Clin. Immunol. 2012, 129, 1499–1505.e5. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, P.I.; Keber, C.U.; Cohen, R.M.; Garn, H. The Hygiene Hypothesis—Learning from but Not Living in the Past. Front. Immunol. 2021, 12, 632. [Google Scholar] [CrossRef]
- Message, S.D.; Johnston, S.L. The immunology of virus infection in asthma. Eur. Respir. J. 2001, 18, 1013–1025. [Google Scholar] [CrossRef]
- Stier, M.T.; Peebles, R.S., Jr. Host and Viral Determinants of Respiratory Syncytial Virus-Induced Airway Mucus. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), S205–S209. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; Bont, L.; Bossios, A.; Bousquet, J.; et al. Viruses and bacteria in acute asthma exacerbations—A GA2 LEN-DARE systematic review. Allergy 2011, 66, 458–468. [Google Scholar] [CrossRef]
- Sigurs, N.; Bjarnason, R.; Sigurbergsson, F.; Kjellman, B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000, 161, 1501–1507. [Google Scholar] [CrossRef]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008, 178, 667–672. [Google Scholar] [CrossRef]
- Kusel, M.M.; de Klerk, N.H.; Kebadze, T.; Vohma, V.; Holt, P.G.; Johnston, S.L.; Sly, P.D. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007, 119, 1105–1110. [Google Scholar] [CrossRef]
- Makrinioti, H.; Hasegawa, K.; Lakoumentas, J.; Xepapadaki, P.; Tsolia, M.; Castro-Rodriguez, J.A.; Feleszko, W.; Jartti, T.; Johnston, S.L.; Bush, A.; et al. The role of respiratory syncytial virus- and rhinovirus-induced bronchiolitis in recurrent wheeze and asthma-A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2022, 33, e13741. [Google Scholar] [CrossRef]
- Loisel, D.A.; Du, G.; Ahluwalia, T.S.; Tisler, C.J.; Evans, M.D.; Myers, R.A.; Gangnon, R.E.; Kreiner-Møller, E.; Bønnelykke, K.; Bisgaard, H.; et al. Genetic associations with viral respiratory illnesses and asthma control in children. Clin. Exp. Allergy 2016, 46, 112–124. [Google Scholar] [CrossRef]
- Çalışkan, M.; Bochkov, Y.A.; Kreiner-Møller, E.; Bønnelykke, K.; Stein, M.M.; Du, G.; Bisgaard, H.; Jackson, D.J.; Gern, J.E.; Lemanske, R.F., Jr. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N. Engl. J. Med. 2013, 368, 1398–1407. [Google Scholar] [CrossRef]
- Loss, G.J.; Depner, M.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Roduit, C.; Kabesch, M.; Lauener, R.; Pfefferle, P.I.; et al. The Early Development of Wheeze. Environmental Determinants and Genetic Susceptibility at 17q21. Am. J. Respir. Crit. Care Med. 2016, 193, 889–897. [Google Scholar] [CrossRef]
- Stokholm, J.; Chawes, B.L.; Vissing, N.; Bønnelykke, K.; Bisgaard, H. Cat exposure in early life decreases asthma risk from the 17q21 high-risk variant. J. Allergy Clin. Immunol. 2018, 141, 1598–1606. [Google Scholar] [CrossRef]
- Lee, W.M.; Lemanske, R.F., Jr.; Evans, M.D.; Vang, F.; Pappas, T.; Gangnon, R.; Jackson, D.J.; Gern, J.E. Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care Med. 2012, 186, 886–891. [Google Scholar] [CrossRef]
- Bønnelykke, K.; Sleiman, P.; Nielsen, K.; Kreiner-Møller, E.; Mercader, J.M.; Belgrave, D.; den Dekker, H.T.; Husby, A.; Sevelsted, A.; Faura-Tellez, G.; et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 2014, 46, 51–55. [Google Scholar] [CrossRef]
- Bochkov, Y.A.; Watters, K.; Ashraf, S.; Griggs, T.F.; Devries, M.K.; Jackson, D.J.; Palmenberg, A.C.; Gern, J.E. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc. Natl. Acad. Sci. USA 2015, 112, 5485–5490. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Moore, M.L.; Rudd, B.D.; Berlin, A.A.; Collins, R.D.; Olson, S.J.; Ho, S.B.; Peebles, R.S. Differential Immune Responses and Pulmonary Pathophysiology Are Induced by Two Different Strains of Respiratory Syncytial Virus. Am. J. Pathol. 2006, 169, 977–986. [Google Scholar] [CrossRef]
- Moore, M.L.; Chi, M.H.; Luongo, C.; Lukacs, N.W.; Polosukhin, V.V.; Huckabee, M.M.; Newcomb, D.C.; Buchholz, U.J.; Crowe, J.E., Jr.; Goleniewska, K.; et al. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J. Virol. 2009, 83, 4185–4194. [Google Scholar] [CrossRef]
- Pech, M.; Weckmann, M.; König, I.R.; Franke, A.; Heinsen, F.A.; Oliver, B.; Ricklefs, I.; Fuchs, O.; Rabe, K.; Hansen, G.; et al. Rhinovirus infections change DNA methylation and mRNA expression in children with asthma. PLoS ONE 2018, 13, e0205275. [Google Scholar] [CrossRef]
- Lund, R.J.; Osmala, M.; Malonzo, M.; Lukkarinen, M.; Leino, A.; Salmi, J.; Vuorikoski, S.; Turunen, R.; Vuorinen, T.; Akdis, C.; et al. Atopic asthma after rhinovirus-induced wheezing is associated with DNA methylation change in the SMAD3 gene promoter. Allergy 2018, 73, 1735–1740. [Google Scholar] [CrossRef]
- Rupani, H.; Martinez-Nunez, R.T.; Dennison, P.; Lau, L.C.; Jayasekera, N.; Havelock, T.; Francisco-Garcia, A.S.; Grainge, C.; Howarth, P.H.; Sanchez-Elsner, T. Toll-like Receptor 7 Is Reduced in Severe Asthma and Linked to an Altered MicroRNA Profile. Am. J. Respir. Crit. Care Med. 2016, 194, 26–37. [Google Scholar] [CrossRef]
- Du, X.; Yang, Y.; Xiao, G.; Yang, M.; Yuan, L.; Qin, L.; He, R.; Wang, L.; Wu, M.; Wu, S.; et al. Respiratory syncytial virus infection-induced mucus secretion by down-regulation of miR-34b/c-5p expression in airway epithelial cells. J. Cell. Mol. Med. 2020, 24, 12694–12705. [Google Scholar] [CrossRef]
- Moheimani, F.; Koops, J.; Williams, T.; Reid, A.T.; Hansbro, P.M.; Wark, P.A.; Knight, D.A. Influenza A virus infection dysregulates the expression of microRNA-22 and its targets; CD147 and HDAC4, in epithelium of asthmatics. Respir. Res. 2018, 19, 145. [Google Scholar] [CrossRef]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef]
- Teo, S.M.; Tang, H.H.F.; Mok, D.; Judd, L.M.; Watts, S.C.; Pham, K.; Holt, B.J.; Kusel, M.; Serralha, M.; Troy, N.; et al. Airway Microbiota Dynamics Uncover a Critical Window for Interplay of Pathogenic Bacteria and Allergy in Childhood Respiratory Disease. Cell Host Microbe 2018, 24, 341–352.e5. [Google Scholar] [CrossRef]
- Anderson, G.P. Endotyping asthma: New insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008, 372, 1107–1119. [Google Scholar] [CrossRef]
- Hossain, F.M.A.; Choi, J.Y.; Uyangaa, E.; Park, S.O.; Eo, S.K. The Interplay between Host Immunity and Respiratory Viral Infection in Asthma Exacerbation. Immune Netw. 2019, 19, e31. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Gani, F.; Berti, A.; Comberiati, P.; Peroni, D.; Cottini, M. Asthma and COVID-19: A dangerous liaison? Asthma Res. Pract. 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Beurnier, A.; Jutant, E.M.; Jevnikar, M.; Boucly, A.; Pichon, J.; Preda, M.; Frank, M.; Laurent, J.; Richard, C.; Monnet, X.; et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur. Respir. J. 2020, 56, 2001875. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef]
- Grandbastien, M.; Piotin, A.; Godet, J.; Abessolo-Amougou, I.; Ederlé, C.; Enache, I.; Fraisse, P.; Hoang, T.C.T.; Kassegne, L.; Labani, A.; et al. SARS-CoV-2 Pneumonia in Hospitalized Asthmatic Patients Did Not Induce Severe Exacerbation. J. Allergy Clin. Immunol. Pract. 2020, 8, 2600–2607. [Google Scholar] [CrossRef]
- Jaswaney, R.; Foster, K.; Moore, D.; Andy-Nweye, A.; Mahdavinia, M. Allergic Asthma Patients Experience Lower Rates of Asthma Exacerbation Compared to Non-Allergic Asthma Patients Following COVID-19 Infection. J. Allergy Clin. Immunol. 2022, 149 (Suppl. S2), AB58. [Google Scholar] [CrossRef]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e8. [Google Scholar] [CrossRef]
- Sajuthi, S.P.; DeFord, P.; Li, Y.; Jackson, N.D.; Montgomery, M.T.; Everman, J.L.; Rios, C.L.; Pruesse, E.; Nolin, J.D.; Plender, E.G.; et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020, 11, 5139. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wark, P.A.; Johnston, S.L.; Moric, I.; Simpson, J.L.; Hensley, M.J.; Gibson, P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002, 19, 68–75. [Google Scholar] [CrossRef]
- Grissell, T.V.; Powell, H.; Shafren, D.R.; Boyle, M.J.; Hensley, M.J.; Jones, P.D.; Whitehead, B.F.; Gibson, P.G. Interleukin-10 gene expression in acute virus-induced asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 433–439. [Google Scholar] [CrossRef]
- Jackson, D.J.; Johnston, S.L. The role of viruses in acute exacerbations of asthma. J. Allergy Clin. Immunol. 2010, 125, 1178–1187. [Google Scholar] [CrossRef]
- Hayden, F.G. Rhinovirus and the lower respiratory tract. Rev. Med. Virol. 2004, 14, 17–31. [Google Scholar] [CrossRef]
- Schroth, M.K.; Grimm, E.; Frindt, P.; Galagan, D.M.; Konno, S.I.; Love, R.; Gern, J.E. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1220–1228. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Bates, P.J.; Bardin, P.G.; Papi, A.; Leir, S.H.; Fraenkel, D.J.; Meyer, J.; Lackie, P.M.; Sanderson, G.; Holgate, S.T.; et al. Rhinoviruses infect the lower airways. J. Infect. Dis. 2000, 181, 1875–1884. [Google Scholar] [CrossRef]
- Subauste, M.C.; Jacoby, D.B.; Richards, S.M.; Proud, D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J. Clin. Investig. 1995, 96, 549–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gern, J.E.; Galagan, D.M.; Jarjour, N.N.; Dick, E.C.; Busse, W.W. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am. J. Respir. Crit. Care Med. 1997, 155, 1159–1161. [Google Scholar] [CrossRef]
- Bartlett, N.W.; Walton, R.P.; Edwards, M.R.; Aniscenko, J.; Caramori, G.; Zhu, J.; Glanville, N.; Choy, K.J.; Jourdan, P.; Burnet, J.; et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 2008, 14, 199–204. [Google Scholar] [CrossRef]
- Yılmaz, İ. Is Asthma-COPD overlap an asthma phenotype or a COPD phenotype? Tuberk. Toraks 2018, 66, 78–79. [Google Scholar] [CrossRef]
- Muehling, L.M.; Heymann, P.W.; Wright, P.W.; Eccles, J.D.; Agrawal, R.; Carper, H.T.; Murphy, D.D.; Workman, L.J.; Word, C.R.; Ratcliffe, S.J.; et al. Human TH1 and TH2 cells targeting rhinovirus and allergen coordinately promote allergic asthma. J. Allergy Clin. Immunol. 2020, 146, 555–570. [Google Scholar] [CrossRef]
- Agache, I. Non-Eosinophilic Asthma Endotypes. Curr. Treat. Options Allergy 2015, 2, 257–267. [Google Scholar] [CrossRef]
- James, K.M.; Peebles, R.S., Jr.; Hartert, T.V. Response to infections in patients with asthma and atopic disease: An epiphenomenon or reflection of host susceptibility? J. Allergy Clin. Immunol. 2012, 130, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lebre, M.C.; van Capel, T.M.; Bos, J.D.; Knol, E.F.; Kapsenberg, M.L.; de Jong, E.C. Aberrant function of peripheral blood myeloid and plasmacytoid dendritic cells in atopic dermatitis patients. J. Allergy Clin. Immunol. 2008, 122, 969–976.e5. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.A.; Johnston, S.L.; Bucchieri, F.; Powell, R.; Puddicombe, S.; Laza-Stanca, V.; Holgate, S.T.; Davies, D.E. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005, 201, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Durrani, S.R.; Montville, D.J.; Pratt, A.S.; Sahu, S.; DeVries, M.K.; Rajamanickam, V.; Gangnon, R.E.; Gill, M.A.; Gern, J.E.; Lemanske, R.F., Jr.; et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J. Allergy Clin. Immunol. 2012, 130, 489–495. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Gaffin, J.M.; Castro, M.; Bacharier, L.B.; Fuhlbrigge, A.L. The Role of Comorbidities in Difficult-to-Control Asthma in Adults and Children. J. Allergy Clin. Immunol. Pract. 2022, 10, 397–408. [Google Scholar] [CrossRef]
- Rogliani, P.; Sforza, M.; Calzetta, L. The impact of comorbidities on severe asthma. Curr. Opin. Pulm. Med. 2020, 26, 47–55. [Google Scholar] [CrossRef]
- Gibson, P.G.; McDonald, V.M.; Granchelli, A.; Olin, J.T. Asthma and Comorbid Conditions-Pulmonary Comorbidity. J. Allergy Clin. Immunol. Pract. 2021, 9, 3868–3875. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Xiong, X.-F.; Wu, Z.-H.; Huang, T.-T.; Cheng, D.-Y. Clinical features of asthma with comorbid bronchiectasis: A systematic review and meta-analysis. Medicine 2021, 100, e23858. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Menzies-Gow, A. Co-morbidities in severe asthma: Clinical impact and management. Respirology 2017, 22, 651–661. [Google Scholar] [CrossRef]
- Polverino, E.; Dimakou, K.; Hurst, J.; Martinez-Garcia, M.A.; Miravitlles, M.; Paggiaro, P.; Shteinberg, M.; Aliberti, S.; Chalmers, J.D. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J. 2018, 52, 1800328. [Google Scholar] [CrossRef]
- Park, Y.E.; Sung, H.; Oh, Y.M. Respiratory Viruses in Acute Exacerbations of Bronchiectasis. J. Korean Med. Sci. 2021, 36, e217. [Google Scholar] [CrossRef]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A.; LeBeau, P.K.; Tran, H.T.; Fujimura, K.E.; LaMere, B.; et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef]
- McCauley, K.E.; Flynn, K.; Calatroni, A.; DiMassa, V.; LaMere, B.; Fadrosh, D.W.; Lynch, K.V.; Gill, M.A.; Pongracic, J.A.; Hershey, G.K.K.; et al. Seasonal airway microbiome and transcriptome interactions promote childhood asthma exacerbations. J. Allergy Clin. Immunol. 2022, 150, 204–213. [Google Scholar] [CrossRef]
- Watkinson, R.L.; Looi, K.; Laing, I.A.; Cianferoni, A.; Kicic, A. Viral Induced Effects on a Vulnerable Epithelium; Lessons Learned from Paediatric Asthma and Eosinophilic Oesophagitis. Front. Immunol. 2021, 12, 773600. [Google Scholar] [CrossRef]
- Akdis, C.A. The epithelial barrier hypothesis proposes a comprehensive understanding of the origins of allergic and other chronic noncommunicable diseases. J. Allergy Clin. Immunol. 2022, 149, 41–44. [Google Scholar] [CrossRef]
- Kumar, K.; Singanayagam, A.; Johnston, S.L. Respiratory Virus Infections in Asthma: Research Developments and Therapeutic Advances. Acta Med. Acad. 2020, 49, 130–143. [Google Scholar]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556.e12. [Google Scholar] [CrossRef]
- Looi, K.; Buckley, A.G.; Rigby, P.J.; Garratt, L.W.; Iosifidis, T.; Zosky, G.R.; Larcombe, A.N.; Lannigan, F.J.; Ling, K.M.; Martinovich, K.M.; et al. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin. Exp. Allergy 2018, 48, 513–524. [Google Scholar] [CrossRef]
- Kicic, A.; Stevens, P.T.; Sutanto, E.N.; Kicic-Starcevich, E.; Ling, K.M.; Looi, K.; Martinovich, K.M.; Garratt, L.W.; Iosifidis, T.; Shaw, N.C.; et al. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin. Exp. Allergy 2016, 46, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Terajima, M.; Yamaya, M.; Sekizawa, K.; Okinaga, S.; Suzuki, T.; Yamada, N.; Nakayama, K.; Ohrui, T.; Oshima, T.; Numazaki, Y.; et al. Rhinovirus infection of primary cultures of human tracheal epithelium: Role of ICAM-1 and IL-1beta. Am. J. Physiol. 1997, 273, L749–L759. [Google Scholar] [CrossRef] [PubMed]
- Heymann, P.W.; Carper, H.T.; Murphy, D.D.; Platts-Mills, T.A.; Patrie, J.; McLaughlin, A.P.; Erwin, E.A.; Shaker, M.S.; Hellems, M.; Peerzada, J.; et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 2004, 114, 239–247. [Google Scholar] [CrossRef]
- Oliver, A.; Quinn, D.; Goldfrad, C.; van Hecke, B.; Ayer, J.; Boyce, M. Combined fluticasone furoate/vilanterol reduces decline in lung function following inhaled allergen 23 h after dosing in adult asthma: A randomised, controlled trial. Clin. Transl. Allergy 2012, 2, 11. [Google Scholar] [CrossRef]
- Lee, K.S.; Min, K.H.; Kim, S.R.; Park, S.J.; Park, H.S.; Jin, G.Y.; Lee, Y.C. Vascular endothelial growth factor modulates matrix metalloproteinase-9 expression in asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 161–170. [Google Scholar] [CrossRef]
- Altman, M.C.; Gill, M.A.; Whalen, E.; Babineau, D.C.; Shao, B.; Liu, A.H.; Jepson, B.; Gruchalla, R.S.; O’Connor, G.T.; Pongracic, J.A.; et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat. Immunol. 2019, 20, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Iosifidis, T.; Sutanto, E.N.; Buckley, A.G.; Coleman, L.; Gill, E.E.; Lee, A.H.; Ling, K.M.; Hillas, J.; Looi, K.; Garratt, L.W.; et al. Aberrant cell migration contributes to defective airway epithelial repair in childhood wheeze. JCI Insight 2020, 5, e133125. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Locksley, R.M. Asthma and allergic inflammation. Cell 2010, 140, 777–783. [Google Scholar] [CrossRef]
- Scanlon, S.T.; McKenzie, A.N. Type 2 innate lymphoid cells: New players in asthma and allergy. Curr. Opin. Immunol. 2012, 24, 707–712. [Google Scholar] [CrossRef]
- Bando, J.K.; Nussbaum, J.C.; Liang, H.E.; Locksley, R.M. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J. Leukoc. Biol. 2013, 94, 877–884. [Google Scholar] [CrossRef]
- Barlow, J.L.; Peel, S.; Fox, J.; Panova, V.; Hardman, C.S.; Camelo, A.; Bucks, C.; Wu, X.; Kane, C.M.; Neill, D.R.; et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013, 132, 933–941. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Jackson, D.J.; Makrinioti, H.; Rana, B.M.; Shamji, B.W.; Trujillo-Torralbo, M.B.; Footitt, J.; Jerico, D.-R.; Telcian, A.G.; Nikonova, A.; Zhu, J.; et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014, 190, 1373–1382. [Google Scholar] [CrossRef]
- Le Goffic, R.; Arshad, M.I.; Rauch, M.; L’Helgoualc’h, A.; Delmas, B.; Piquet-Pellorce, C.; Samson, M. Infection with influenza virus induces IL-33 in murine lungs. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1125–1132. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Loh, Z.; Spann, K.; Lynch, J.P.; Lalwani, A.; Zheng, Z.; Davidson, S.; Uematsu, S.; Akira, S.; Hayball, J.; et al. Toll-like receptor 7 gene deficiency and early-life Pneumovirus infection interact to predispose toward the development of asthma-like pathology in mice. J. Allergy Clin. Immunol. 2013, 131, 1331–1339.e10. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chang, Y.J.; Subramanian, S.; Lee, H.H.; Albacker, L.A.; Matangkasombut, P.; Savage, P.B.; McKenzie, A.N.; Smith, D.E.; Rottman, J.B.; et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 2012, 129, 216–227.e6. [Google Scholar] [CrossRef]
- Ravanetti, L.; Dijkhuis, A.; Dekker, T.; Pineros, Y.S.S.; Ravi, A.; Dierdorp, B.S.; Erjefält, J.S.; Mori, M.; Pavlidis, S.; Adcock, I.M.; et al. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. J. Allergy Clin. Immunol. 2019, 143, 1355–1370.e16. [Google Scholar] [CrossRef]
- Jurak, L.M.; Xi, Y.; Landgraf, M.; Carroll, M.L.; Murray, L.; Upham, J.W. Interleukin 33 Selectively Augments Rhinovirus-Induced Type 2 Immune Responses in Asthmatic but Not Healthy People. Front. Immunol. 2018, 9, 1895. [Google Scholar] [CrossRef]
- Beale, J.; Jayaraman, A.; Jackson, D.J.; Macintyre, J.D.R.; Edwards, M.R.; Walton, R.P.; Zhu, J.; Ching, Y.M.; Shamji, B.; Edwards, M.; et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl. Med. 2014, 6, 256ra134. [Google Scholar] [CrossRef]
- Williams, T.C.; Loo, S.L.; Nichol, K.S.; Reid, A.T.; Veerati, P.C.; Esneau, C.; Wark, P.A.B.; Grainge, C.L.; Knight, D.A.; Vincent, T.; et al. IL-25 blockade augments antiviral immunity during respiratory virus infection. Commun. Biol. 2022, 5, 415. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Headley, M.B.; Loo, Y.M.; Berlin, A.; Gale, M., Jr.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5. [Google Scholar] [CrossRef]
- Mahmutovic-Persson, I.; Akbarshahi, H.; Bartlett, N.W.; Glanville, N.; Johnston, S.L.; Brandelius, A.; Uller, L. Inhaled dsRNA and rhinovirus evoke neutrophilic exacerbation and lung expression of thymic stromal lymphopoietin in allergic mice with established experimental asthma. Allergy 2014, 69, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Calvén, J.; Yudina, Y.; Hallgren, O.; Westergren-Thorsson, G.; Davies, D.E.; Brandelius, A.; Uller, L. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: Role of endosomal TLR3 and cytosolic RIG-I-like helicases. J. Innate Immun. 2012, 4, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Veerati, P.C.; Troy, N.M.; Reid, A.T.; Li, N.F.; Nichol, K.S.; Kaur, P.; Maltby, S.; Wark, P.A.B.; Knight, D.A.; Bosco, A.; et al. Airway Epithelial Cell Immunity Is Delayed During Rhinovirus Infection in Asthma and COPD. Front. Immunol. 2020, 11, 974. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, Y.; Xiong, L.; Zhang, W.; Li, Y.; Gibson, P.G.; Simpson, J.L.; Zhang, C.; Lu, J.; Sai, J.; et al. The Expression of IL-6, TNF-α, and MCP-1 in Respiratory Viral Infection in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. J. Immunol. Res. 2017, 2017, 8539294. [Google Scholar] [CrossRef]
- Johnston, S.L.; Papi, A.; Bates, P.J.; Mastronarde, J.G.; Monick, M.M.; Hunninghake, G.W. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J. Immunol. 1998, 160, 6172–6181. [Google Scholar]
- Papadopoulos, N.G.; Papi, A.; Meyer, J.; Stanciu, L.A.; Salvi, S.; Holgate, S.T.; Johnston, S.L. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin. Exp. Allergy 2001, 31, 1060–1066. [Google Scholar] [CrossRef]
- Jamieson, K.C.; Wiehler, S.; Michi, A.N.; Proud, D. Rhinovirus Induces Basolateral Release of IL-17C in Highly Differentiated Airway Epithelial Cells. Front. Cell Infect. Microbiol. 2020, 10, 103. [Google Scholar] [CrossRef]
- Sikazwe, C.T.; Laing, I.A.; Imrie, A.; Smith, D.W. Nasal Cytokine Profiles of Patients Hospitalised with Respiratory Wheeze Associated with Rhinovirus C. Viruses 2019, 11, 1038. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Farne, H.A.; Singanayagam, A.; Jackson, D.J.; Mallia, P.; Johnston, S.L. Pathogenesis of Viral Infection in Exacerbations of Airway Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S115–S132. [Google Scholar] [CrossRef]
- Hewitt, R.; Farne, H.; Ritchie, A.; Luke, E.; Johnston, S.L.; Mallia, P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther. Adv. Respir. Dis. 2016, 10, 158–174. [Google Scholar] [CrossRef]
- Contoli, M.; Message, S.D.; Laza-Stanca, V.; Edwards, M.R.; Wark, P.A.; Bartlett, N.W.; Kebadze, T.; Mallia, P.; Stanciu, L.A.; Parker, H.L.; et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 2006, 12, 1023–1026. [Google Scholar] [CrossRef]
- Parsons, K.S.; Hsu, A.C.; Wark, P.A. TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin. Exp. Allergy 2014, 44, 91–101. [Google Scholar] [CrossRef]
- Zhu, J.; Message, S.D.; Mallia, P.; Kebadze, T.; Contoli, M.; Ward, C.K.; Barnathan, E.S.; Mascelli, M.A.; Kon, O.M.; Papi, A.; et al. Bronchial mucosal IFN-α/β and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J. Allergy Clin. Immunol. 2019, 143, 114–125.e4. [Google Scholar] [CrossRef]
- Edwards, M.R.; Regamey, N.; Vareille, M.; Kieninger, E.; Gupta, A.; Shoemark, A.; Saglani, S.; Sykes, A.; Macintyre, J.; Davies, J.; et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013, 6, 797–806. [Google Scholar] [CrossRef]
- Holt, P.G.; Mok, D.; Panda, D.; Renn, L.; Fabozzi, G.; deKlerk, N.H.; Kusel, M.M.H.; Serralha, M.; Hollams, E.M.; Holt, B.J.; et al. Developmental regulation of type 1 and type 3 interferon production and risk for infant infections and asthma development. J. Allergy Clin. Immunol. 2019, 143, 1176–1182.e5. [Google Scholar] [CrossRef]
- Esteves, P.; Allard, B.; Celle, A.; Dupin, I.; Maurat, E.; Ousova, O.; Thumerel, M.; Dupuy, J.W.; Leste-Lasserre, T.; Marthan, R.; et al. Asthmatic bronchial smooth muscle increases rhinovirus replication within the bronchial epithelium. Cell Rep. 2022, 38, 110571. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, Y.C.; Kim, D.I.; Park, H.J. Effects of PKR inhibitor on poly (I:C)-induced exacerbation of severe asthma. Eur. Respir. J. 2016, 48 (Suppl. S60), PA1099. [Google Scholar]
- Denney, L.; Ho, L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018, 41, 218–233. [Google Scholar] [CrossRef]
- Slater, L.; Bartlett, N.W.; Haas, J.J.; Zhu, J.; Message, S.D.; Walton, R.P.; Sykes, A.; Dahdaleh, S.; Clarke, D.L.; Belvisi, M.G.; et al. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010, 6, e1001178. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schröder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Poeck, H.; Bscheider, M.; Gross, O.; Finger, K.; Roth, S.; Rebsamen, M.; Hannesschläger, N.; Schlee, M.; Rothenfusser, S.; Barchet, W.; et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 2010, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Douglas, T.; Saleh, M. Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev. 2014, 25, 681–697. [Google Scholar] [CrossRef]
- Rintahaka, J.; Wiik, D.; Kovanen, P.E.; Alenius, H.; Matikainen, S. Cytosolic antiviral RNA recognition pathway activates caspases 1 and 3. J. Immunol. 2008, 180, 1749–1757. [Google Scholar] [CrossRef]
- Chen, I.Y.; Ichinohe, T. Response of host inflammasomes to viral infection. Trends Microbiol. 2015, 23, 55–63. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Núñez, G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 2009, 183, 792–796. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Ozkurede, U.; Kim, Y.G.; Arindam, C.; Gale, M., Jr.; Silverman, R.H.; Colonna, M.; Akira, S.; et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J. Immunol. 2014, 193, 4214–4222. [Google Scholar] [CrossRef]
- De Nardo, D.; De Nardo, C.M.; Latz, E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am. J. Pathol. 2014, 184, 42–54. [Google Scholar] [CrossRef]
- Han, M.; Bentley, J.K.; Rajput, C.; Lei, J.; Ishikawa, T.; Jarman, C.R.; Lee, J.; Goldsmith, A.M.; Jackson, W.T.; Hoenerhoff, M.J.; et al. Inflammasome activation is required for human rhinovirus-induced airway inflammation in naive and allergen-sensitized mice. Mucosal Immunol. 2019, 12, 958–968. [Google Scholar] [CrossRef]
- Jeong, J.S.; Lee, K.B.; Kim, S.R.; Kim, D.I.; Park, H.J.; Lee, H.K.; Kim, H.J.; Cho, S.H.; Kolliputi, N.; Kim, S.H.; et al. Airway epithelial phosphoinositide 3-kinase-δ contributes to the modulation of fungi-induced innate immune response. Thorax 2018, 73, 758–768. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, D.I.; Kim, S.H.; Lee, H.; Lee, K.S.; Cho, S.H.; Lee, Y.C. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014, 5, e1498. [Google Scholar] [CrossRef]
- Kim, S.R.; Park, H.J.; Lee, K.B.; Kim, H.J.; Jeong, J.S.; Cho, S.H.; Lee, Y.C. Epithelial PI3K-δ Promotes House Dust Mite-Induced Allergic Asthma in NLRP3 Inflammasome-Dependent and -Independent Manners. Allergy Asthma Immunol. Res. 2020, 12, 338–358. [Google Scholar] [CrossRef]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef]
- Mitoma, H.; Hanabuchi, S.; Kim, T.; Bao, M.; Zhang, Z.; Sugimoto, N.; Liu, Y.J. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity 2013, 39, 123–135. [Google Scholar] [CrossRef]
- Delaloye, J.; Roger, T.; Steiner-Tardivel, Q.G.; Le Roy, D.; Knaup Reymond, M.; Akira, S.; Petrilli, V.; Gomez, C.E.; Perdiguero, B.; Tschopp, J.; et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009, 5, e1000480. [Google Scholar] [CrossRef]
- Pothlichet, J.; Meunier, I.; Davis, B.K.; Ting, J.P.; Skamene, E.; von Messling, V.; Vidal, S.M. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 2013, 9, e1003256. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Iwasaki, A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 2010, 11, 404–410. [Google Scholar] [CrossRef]
- Siu, K.L.; Yuen, K.S.; Castaño-Rodriguez, C.; Ye, Z.W.; Yeung, M.L.; Fung, S.Y.; Yuan, S.; Chan, C.P.; Yuen, K.Y.; Enjuanes, L.; et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019, 33, 8865–8877. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.Y.; Moriyama, M.; Chang, M.F.; Ichinohe, T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; Ingber, J.; Parry, B.; Ravid, S.; de Lacerda, L.B.; Lewandrowski, M.; Clark, S.; Ho, F.; et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv 2021. [Google Scholar] [CrossRef]
- Campbell, G.R.; To, R.K.; Hanna, J.; Spector, S.A. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience 2021, 24, 102295. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, K.; Kar, S.; van Kuppeveld, F.J.; Triantafilou, M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am. J. Respir. Cell Mol. Biol. 2013, 49, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Min, C.; Park, B.; Choi, H.G.; Mo, J.H. Bidirectional association between asthma and chronic rhinosinusitis: Two longitudinal follow-up studies using a national sample cohort. Sci. Rep. 2020, 10, 9589. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, Y.T.; Wang, L.Q.; Li, L.Y.; Bao, Q.; Tian, S.; Chen, M.X.; Chen, H.X.; Cui, J.; Li, C.W. NOD-like receptor family, pyrin domain containing 3 (NLRP3) contributes to inflammation, pyroptosis, and mucin production in human airway epithelium on rhinovirus infection. J. Allergy Clin. Immunol. 2019, 144, 777–787.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xing, J.; Xia, T.; Zhang, H.; Fang, M.; Li, S.; Du, Y.; Li, X.C.; Zhang, Z.; Zeng, M.S. EphA2 phosphorylates NLRP3 and inhibits inflammasomes in airway epithelial cells. EMBO Rep. 2020, 21, e49666. [Google Scholar] [CrossRef]
- Radzikowska, U.; Eljaszewicz, A.; Tan, G.; Stocker, N.; Heider, A.; Westermann, P.; Steiner, S.; Dreher, A.; Wawrzyniak, P.; Rückert, B.; et al. Rhinovirus-induced epithelial RIG-I inflammasome activation suppresses antiviral immunity and promotes inflammatory responses in virus-induced asthma exacerbations and COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome—A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211. [Google Scholar] [CrossRef]
- Suruki, R.Y.; Daugherty, J.B.; Boudiaf, N.; Albers, F.C. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm. Med. 2017, 17, 74. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (accessed on 27 June 2022).
- Kew, K.M.; Quinn, M.; Quon, B.S.; Ducharme, F.M. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst. Rev. 2016, 2016, Cd007524. [Google Scholar] [CrossRef]
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J., Jr.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485. [Google Scholar] [CrossRef]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Nair, P.; Pizzichini, M.M.; Kjarsgaard, M.; Inman, M.D.; Efthimiadis, A.; Pizzichini, E.; Hargreave, F.E.; O’Byrne, P.M. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J. Med. 2009, 360, 985–993. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Esquivel, A.; Busse, W.W.; Calatroni, A.; Togias, A.G.; Grindle, K.G.; Bochkov, Y.A.; Gruchalla, R.S.; Kattan, M.; Kercsmar, C.M.; Khurana Hershey, G.; et al. Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 985–992. [Google Scholar] [CrossRef]
- Kantor, D.B.; McDonald, M.C.; Stenquist, N.; Schultz, B.J.; Smallwood, C.D.; Nelson, K.A.; Phipatanakul, W.; Hirschhorn, J.N. Omalizumab Is Associated with Reduced Acute Severity of Rhinovirus-triggered Asthma Exacerbation. Am. J. Respir. Crit. Care Med. 2016, 194, 1552–1555. [Google Scholar] [CrossRef]
- Edwards, M.R.; Strong, K.; Cameron, A.; Walton, R.P.; Jackson, D.J.; Johnston, S.L. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J. Allergy Clin. Immunol. 2017, 140, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.A.; Bajwa, G.; George, T.A.; Dong, C.C.; Dougherty, I.I.; Jiang, N.; Gan, V.N.; Gruchalla, R.S. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J. Immunol. 2010, 184, 5999–6006. [Google Scholar] [CrossRef]
- Contoli, M.; Ito, K.; Padovani, A.; Poletti, D.; Marku, B.; Edwards, M.R.; Stanciu, L.A.; Gnesini, G.; Pastore, A.; Spanevello, A.; et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 2015, 70, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Sabogal Piñeros, Y.S.; Bal, S.M.; van de Pol, M.A.; Dierdorp, B.S.; Dekker, T.; Dijkhuis, A.; Brinkman, P.; van der Sluijs, K.F.; Zwinderman, A.H.; Majoor, C.J.; et al. Anti-IL-5 in Mild Asthma Alters Rhinovirus-induced Macrophage, B-Cell, and Neutrophil Responses (MATERIAL). A Placebo-controlled, Double-Blind Study. Am. J. Respir. Crit. Care Med. 2019, 199, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Eger, K.; Hashimoto, S.; Braunstahl, G.J.; Ten Brinke, A.; Patberg, K.W.; Beukert, A.; Smeenk, F.; van der Sar–van der Brugge, S.; Weersink, E.J.M.; Bel, E.H. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir. Med. 2021, 177, 106287. [Google Scholar] [CrossRef]
- Tuncay, G.; Cakmak, M.E.; Can Bostan, O.; Kaya, S.B.; Damadoglu, E.; Karakaya, G.; Kalyoncu, A.F. The course of COVID-19 in patients with severe asthma receiving biological treatment. J. Asthma 2021, 2021, 1996599. [Google Scholar] [CrossRef]
- Djukanović, R.; Harrison, T.; Johnston, S.L.; Gabbay, F.; Wark, P.; Thomson, N.C.; Niven, R.; Singh, D.; Reddel, H.K.; Davies, D.E.; et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am. J. Respir. Crit. Care Med. 2014, 190, 145–154. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
- Silkoff, P.E.; Flavin, S.; Gordon, R.; Loza, M.J.; Sterk, P.J.; Lutter, R.; Diamant, Z.; Turner, R.B.; Lipworth, B.J.; Proud, D.; et al. Toll-like receptor 3 blockade in rhinovirus-induced experimental asthma exacerbations: A randomized controlled study. J. Allergy Clin. Immunol. 2018, 141, 1220–1230. [Google Scholar] [CrossRef]
- Simoes, E.A.; Groothuis, J.R.; Carbonell-Estrany, X.; Rieger, C.H.; Mitchell, I.; Fredrick, L.M.; Kimpen, J.L. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J. Pediatr. 2007, 151, 34–42.e1. [Google Scholar] [CrossRef]
- Turner, R.B.; Wecker, M.T.; Pohl, G.; Witek, T.J.; McNally, E.; St. George, R.; Winther, B.; Hayden, F.G. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: A randomized clinical trial. JAMA 1999, 281, 1797–1804. [Google Scholar] [CrossRef]
- Mirabelli, C.; Scheers, E.; Neyts, J. Novel therapeutic approaches to simultaneously target rhinovirus infection and asthma/COPD pathogenesis. F1000Research 2017, 6, 1860. [Google Scholar] [CrossRef]
- Vasileiou, E.; Sheikh, A.; Butler, C.; El Ferkh, K.; von Wissmann, B.; McMenamin, J.; Ritchie, L.; Schwarze, J.; Papadopoulos, N.G.; Johnston, S.L.; et al. Effectiveness of Influenza Vaccines in Asthma: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017, 65, 1388–1395. [Google Scholar] [CrossRef]
- Cates, C.J.; Rowe, B.H. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst. Rev. 2013, 2013, CD000364. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Podrazil, M.; Taborska, P.; Stakheev, D.; Rataj, M.; Lastovicka, J.; Vlachova, A.; Pohunek, P.; Bartunkova, J.; Smrz, D. Effectiveness and durability of the mRNA vaccine-induced SARS-CoV-2-specific humoral and cellular immunity in severe asthma patients on biological therapy. medRxiv 2022, 13, 892277. [Google Scholar] [CrossRef]
- Caminati, M.; Guarnieri, G.; Batani, V.; Scarpieri, E.; Finocchiaro, A.; Chieco-Bianchi, F.; Senna, G.; Vianello, A. COVID-19 Vaccination in Patients with Severe Asthma on Biologic Treatment: Safety, Tolerability, and Impact on Disease Control. Vaccines 2021, 9, 853. [Google Scholar] [CrossRef]
- Nieto, A.; Mazón, A.; Nieto, M.; Calderón, R.; Calaforra, S.; Selva, B.; Uixera, S.; Palao, M.J.; Brandi, P.; Conejero, L.; et al. Bacterial Mucosal Immunotherapy with MV130 Prevents Recurrent Wheezing in Children: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 462–472. [Google Scholar] [CrossRef]
- Troy, N.M.; Strickland, D.; Serralha, M.; de Jong, E.; Jones, A.C.; Read, J.; Galbraith, S.; Islam, Z.; Kaur, P.; Mincham, K.T.; et al. Protection against severe infant lower respiratory tract infections by immune training: Mechanistic studies. J. Allergy Clin. Immunol. 2022, 150, 93–103. [Google Scholar] [CrossRef]
- Pivniouk, V.; Pivniouk, O.; DeVries, A.; Uhrlaub, J.L.; Michael, A.; Pivniouk, D.; VanLinden, S.R.; Conway, M.Y.; Hahn, S.; Malone, S.P.; et al. The OM-85 bacterial lysate inhibits SARS-CoV-2 infection of epithelial cells by downregulating SARS-CoV-2 receptor expression. J. Allergy Clin. Immunol. 2022, 149, 923–933.e6. [Google Scholar] [CrossRef] [PubMed]
- Del Fresno, C.; García-Arriaza, J.; Martínez-Cano, S.; Heras-Murillo, I.; Jarit-Cabanillas, A.; Amores-Iniesta, J.; Brandi, P.; Dunphy, G.; Suay-Corredera, C.; Pricolo, M.R.; et al. The Bacterial Mucosal Immunotherapy MV130 Protects against SARS-CoV-2 Infection and Improves COVID-19 Vaccines Immunogenicity. Front. Immunol. 2021, 12, 748103. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef]
- Wong, E.H.; Porter, J.D.; Edwards, M.R.; Johnston, S.L. The role of macrolides in asthma: Current evidence and future directions. Lancet Respir. Med. 2014, 2, 657–670. [Google Scholar] [CrossRef]
- Johnston, S.L.; Blasi, F.; Black, P.N.; Martin, R.J.; Farrell, D.J.; Nieman, R.B. The effect of telithromycin in acute exacerbations of asthma. N. Engl. J. Med. 2006, 354, 1589–1600. [Google Scholar] [CrossRef]
- Hiles, S.A.; McDonald, V.M.; Guilhermino, M.; Brusselle, G.G.; Gibson, P.G. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur. Respir. J. 2019, 54, 1901381. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Vanderstichele, C.; Jordens, P.; Deman, R.; Slabbynck, H.; Ringoet, V.; Verleden, G.; Demedts, I.K.; Verhamme, K.; Delporte, A.; et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): A multicentre randomised double-blind placebo-controlled trial. Thorax 2013, 68, 322–329. [Google Scholar] [CrossRef]
- Gielen, V.; Johnston, S.L.; Edwards, M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010, 36, 646–654. [Google Scholar] [CrossRef]
- Porter, J.D.; Watson, J.; Roberts, L.R.; Gill, S.K.; Groves, H.; Dhariwal, J.; Almond, M.H.; Wong, E.; Walton, R.P.; Jones, L.H.; et al. Identification of novel macrolides with antibacterial, anti-inflammatory and type I and III IFN-augmenting activity in airway epithelium. J. Antimicrob. Chemother. 2016, 71, 2767–2781. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.R. Viral Infection and Airway Epithelial Immunity in Asthma. Int. J. Mol. Sci. 2022, 23, 9914. https://doi.org/10.3390/ijms23179914
Kim SR. Viral Infection and Airway Epithelial Immunity in Asthma. International Journal of Molecular Sciences. 2022; 23(17):9914. https://doi.org/10.3390/ijms23179914
Chicago/Turabian StyleKim, So Ri. 2022. "Viral Infection and Airway Epithelial Immunity in Asthma" International Journal of Molecular Sciences 23, no. 17: 9914. https://doi.org/10.3390/ijms23179914
APA StyleKim, S. R. (2022). Viral Infection and Airway Epithelial Immunity in Asthma. International Journal of Molecular Sciences, 23(17), 9914. https://doi.org/10.3390/ijms23179914





