Unilateral Cervical Vagotomy Modulates Immune Cell Profiles and the Response to a Traumatic Brain Injury
Abstract
:1. Introduction
2. Results
2.1. Left and Right Vagotomy Alters the Immune Profile of Macrophages, B Cells, and T Cells
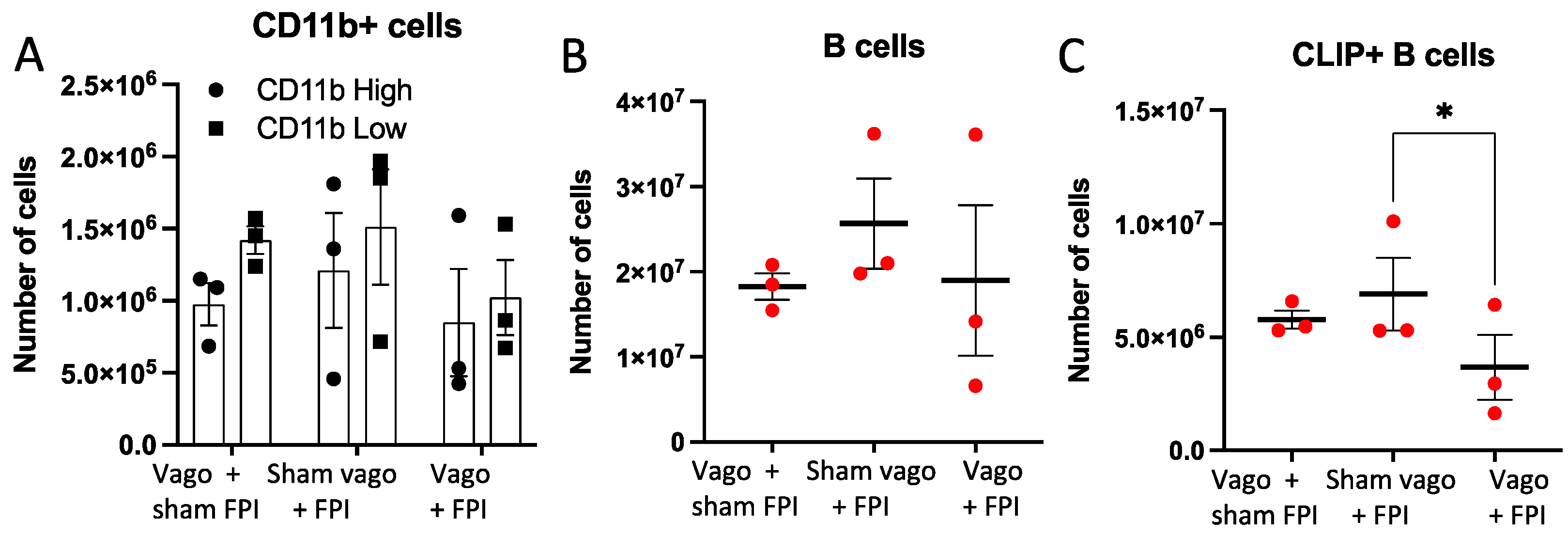
2.2. Left Vagotomy Prior to FPI Reduces Macrophages, B Cells, and CLIP+ B Cells
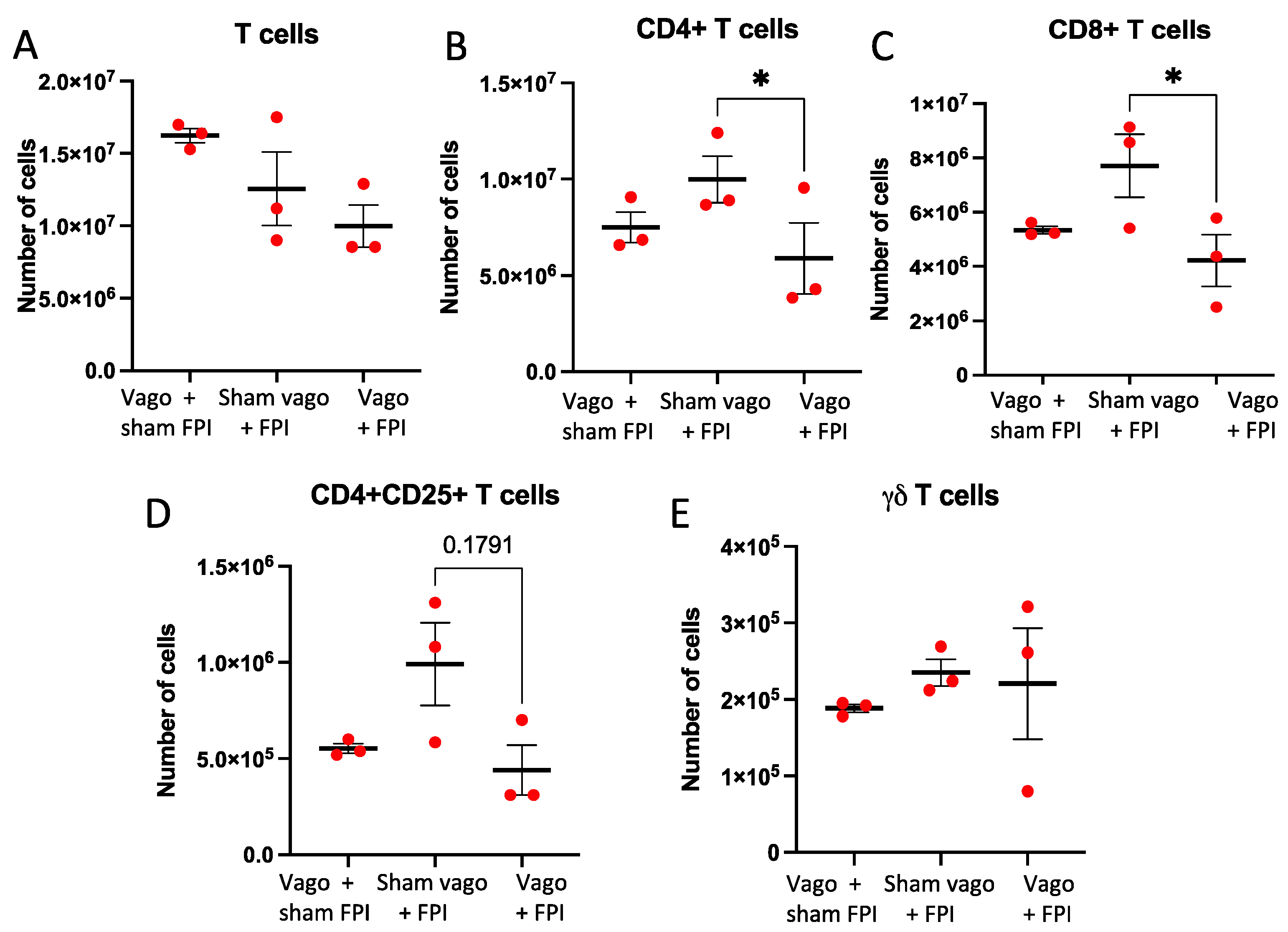
2.3. T Cell and T Cell Subsets Are Influenced by Vagotomy and FPI
3. Discussion
4. Materials and Methods
4.1. Cervical Vagotomy
4.2. Fluid Percussion Injury
4.3. Flow Cytometry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Leikin, J.B. Traumatic Brain Injury. Dis. Mon. 2019, 65, 100857. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Venigalla, H.; Mekala, H.M.; Dar, S.; Hassan, M.; Ayub, S. Traumatic Brain Injury and Neuropsychiatric Complications. Indian J. Psychol. Med. 2017, 39, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; Heaney, D.; Rugg-Gunn, F.; Friedland, D. Neuropsychological Outcomes Following Traumatic Brain Injury. Pract. Neurol. 2019, 19, 476–482. [Google Scholar] [CrossRef]
- Cannella, L.A.; McGary, H.; Ramirez, S.H. Brain Interrupted: Early Life Traumatic Brain Injury and Addiction Vulnerability. Exp. Neurol. 2019, 317, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Robinson, R.G.; Moser, D.; Tateno, A.; Crespo-Facorro, B.; Arndt, S. Major Depression Following Traumatic Brain Injury. Arch. Gen. Psychiatry 2004, 61, 42–50. [Google Scholar] [CrossRef]
- Piccenna, L.; Shears, G.; O’Brien, T.J. Management of Post-Traumatic Epilepsy: An Evidence Review Over the Last 5 Years and Future Directions. Epilepsia Open 2017, 2, 123–144. [Google Scholar] [CrossRef]
- Armstrong, R.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Marin, J.R.; Weaver, M.D.; Mannix, R.C. Burden of USA Hospital Charges for Traumatic Brain Injury. Brain Inj. 2017, 31, 24–31. [Google Scholar] [CrossRef]
- Cederberg, D.; Siesjo, P. What Has Inflammation to Do with Traumatic Brain Injury? Childs Nerv. Syst. 2010, 26, 221–226. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Sundman, M.H.; Chen, N.K.; Subbian, V.; Chou, Y.H. The Bidirectional Gut-Brain-Microbiota Axis as a Potential Nexus Between Traumatic Brain Injury, Inflammation, and Disease. Brain Behav. Immun. 2017, 66, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lu, X.J.; Wu, Q. Gut Microbiota and Acute Central Nervous System Injury: A New Target for Therapeutic Intervention. Front. Immunol. 2021, 12, 800796. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani, N. Relationship between Mild Traumatic Brain Injury and the Gut Microbiome: A Scoping Review. J. Neurosci. Res. 2022, 100, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Murugan, M.; Ravula, A.; Gandhi, A.; Vegunta, G.; Mukkamalla, S.; Mujib, W.; Chandra, N. Chemokine Signaling Mediated Monocyte Infiltration Affects Anxiety-Like Behavior Following Blast Injury. Brain Behav. Immun. 2020, 88, 340–352. [Google Scholar] [CrossRef]
- Wang, R.; He, M.; Ou, X.; Xie, X.; Kang, Y. CRP Albumin Ratio Is Positively Associated with Poor Outcome in Patients with Traumatic Brain Injury. Clin. Neurol. Neurosurg. 2020, 195, 106051. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; DeMorrow, S.; McMillin, M.; Kain, J.; Mukherjee, S.; Zeitouni, S.; Frampton, G.; Bricker, P.C.; Hurst, J.; Shapiro, L.A. Hepatic Alterations Are Accompanied by Changes to Bile Acid Transporter-Expressing Neurons in the Hypothalamus After Traumatic Brain Injury. Sci. Rep. 2017, 7, 40112. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-Phase Proteins: As Diagnostic Tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Tanaka, S.; Abe, C.; Abbott, S.B.G.; Zheng, S.; Yamaoka, Y.; Lipsey, J.E.; Skrypnyk, N.I.; Yao, J.; Inoue, T.; Nash, W.T.; et al. Vagus nerve Stimulation Activates Two Distinct Neuroimmune Circuits Converging in the Spleen to Protect Mice from Kidney Injury. Proc. Natl. Acad. Sci. USA 2021, 118, e2021758118. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The Vagus Nerve and the Inflammatory Reflex–Linking Immunity and Metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Liu, C.H.; Yang, M.H.; Zhang, G.Z.; Wang, X.X.; Li, B.; Li, M.; Woelfer, M.; Walter, M.; Wang, L. Neural Networks and the Anti-Inflammatory Effect of Transcutaneous Auricular Vagus Nerve Stimulation in Depression. J. Neuroinflammation 2020, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Kobrzycka, A.; Napora, P.; Pearson, B.L.; Pierzchala-Koziec, K.; Szewczyk, R.; Wieczorek, M. Peripheral and Central Compensatory Mechanisms for Impaired Vagus Nerve Function During Peripheral Immune Activation. J. Neuroinflammation 2019, 16, 150. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic Nervous System and Neurobiology of the Liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Cailotto, C.; Costes, L.M.; van der Vliet, J.; van Bree, S.H.; van Heerikhuize, J.J.; Buijs, R.M.; Boeckxstaens, G.E. Neuroanatomical Evidence Demonstrating the Existence of the Vagal Anti-Inflammatory Reflex in the Intestine. Neurogastroenterol. Motil. 2012, 24, 191-e93. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The Cholinergic Anti-Inflammatory Pathway. Brain Behav. Immun. 2005, 19, 493–499. [Google Scholar] [CrossRef]
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Powley, T.L. Characterization of Vagal Innervation to the Rat Celiac, Suprarenal and Mesenteric Ganglia. J. Auton. Nerv. Syst. 1993, 42, 153–169. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex Control of Immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Ochani, M.; Parrish, W.R.; Ochani, K.; Harris, Y.T.; Huston, J.M.; Chavan, S.; Tracey, K.J. Splenic Nerve Is Required for Cholinergic Antiinflammatory Pathway Control of TNF in Endotoxemia. Proc. Natl. Acad. Sci. USA 2008, 105, 11008–11013. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic Acetylcholine Receptor Alpha7 Subunit is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.H.; Hudson, L.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF Release by Choline Requires Alpha7 Subunit Nicotinic Acetylcholine Receptor-Mediated Signaling. Mol. Med. 2008, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Physiology and Immunology of the Cholinergic Anti-Inflammatory Pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; van der Vliet, J.; Garidou, M.L.; Huitinga, I.; Escobar, C. Spleen Vagal Denervation Inhibits the Production of Antibodies to Circulating Antigens. PLoS ONE 2008, 3, e3152. [Google Scholar] [CrossRef]
- Lehner, K.R.; Silverman, H.A.; Addorisio, M.E.; Roy, A.; Al-Onaizi, M.A.; Levine, Y.; Olofsson, P.S.; Chavan, S.S.; Gros, R.; Nathanson, N.M.; et al. Forebrain Cholinergic Signaling Regulates Innate Immune Responses and Inflammation. Front. Immunol. 2019, 10, 585. [Google Scholar] [CrossRef]
- Chu, W.; Li, M.; Li, F.; Hu, R.; Chen, Z.; Lin, J.; Feng, H. Immediate Splenectomy Down-Regulates The MAPK-NF-Kappab Signaling Pathway in Rat Brain after Severe Traumatic Brain Injury. J. Trauma Acute Care Surg. 2013, 74, 1446–1453. [Google Scholar] [CrossRef]
- Li, M.; Li, F.; Luo, C.; Shan, Y.; Zhang, L.; Qian, Z.; Zhu, G.; Lin, J.; Feng, H. Immediate Splenectomy Decreases Mortality and Improves Cognitive Function of Rats after Severe Traumatic Brain Injury. J. Trauma Acute Care Surg. 2011, 71, 141–147. [Google Scholar] [CrossRef]
- Tobin, R.P.; Mukherjee, S.; Kain, J.M.; Rogers, S.K.; Henderson, S.K.; Motal, H.L.; Newell Rogers, M.K.; Shapiro, L.A. Traumatic Brain Injury Causes Selective, CD74-Dependent Peripheral Lymphocyte Activation That Exacerbates Neurodegeneration. Acta Neuropathol. Commun. 2014, 2, 143. [Google Scholar] [CrossRef]
- Rong, P.; Liu, A.; Zhang, J.; Wang, Y.; He, W.; Yang, A.; Li, L.; Ben, H.; Li, L.; Liu, H.; et al. Transcutaneous Vagus Nerve Stimulation for Refractory Epilepsy: A Randomized Controlled Trial. Clin. Sci. 2014, 16, 371. [Google Scholar] [CrossRef]
- Gaul, C.; Diener, H.C.; Silver, N.; Magis, D.; Reuter, U.; Andersson, A.; Liebler, E.J.; Straube, A.; PREVA Study Group. Non-Invasive Vagus Nerve Stimulation for Prevention and Acute Treatment of Chronic Cluster Headache (PREVA): A Randomised Controlled Study. Cephalalgia 2016, 36, 534–546. [Google Scholar] [CrossRef]
- Newell-Rogers, M.K.; Rogers, S.K.; Tobin, R.P.; Mukherjee, S.; Shapiro, L.A. Antagonism of Macrophage Migration Inhibitory Factory (MIF) after Traumatic Brain Injury Ameliorates Astrocytosis and Peripheral Lymphocyte Activation and Expansion. Int. J. Mol. Sci. 2020, 21, 7448. [Google Scholar] [CrossRef]
- Lyeth, B.G. Historical Review of the Fluid-Percussion TBI Model. Front. Neurol. 2016, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Todd, B.P.; Chimenti, M.S.; Luo, Z.; Ferguson, P.J.; Bassuk, A.G.; Newell, E.A. Traumatic Brain Injury Results in Unique Microglial and Astrocyte Transcriptomes Enriched for Type I Interferon Response. J. Neuroinflammation 2021, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Witcher, K.G.; Dziabis, J.E.; Bray, C.E.; Gordillo, A.J.; Kumar, J.E.; Eiferman, D.S.; Godbout, J.P.; Kokiko-Cochran, O.N. Comparison between Midline and Lateral Fluid Percussion Injury in Mice Reveals Prolonged but Divergent Cortical Neuroinflammation. Brain Res. 2020, 1746, 146987. [Google Scholar] [CrossRef]
- Bassi, G.S.; Kanashiro, A.; Coimbra, N.C.; Terrando, N.; Maixner, W.; Ulloa, L. Anatomical and Clinical Implications of Vagal Modulation of the Spleen. Neurosci. Biobehav. Rev. 2020, 112, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D.; Perrotta, M.; Pallante, F.; Fardella, V.; Iacobucci, R.; Fardella, S.; Carnevale, L.; Carnevale, R.; De Lucia, M.; Cifelli, G.; et al. A Cholinergic-Sympathetic Pathway Primes Immunity in Hypertension and Mediates Brain-To-Spleen Communication. Nat. Commun. 2016, 7, 13035. [Google Scholar] [CrossRef]
- Kenny, B.J.; Bordoni, B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). In StatPearls; Treasure Island: Florida, FL, USA, 2020. [Google Scholar]
- Johnson, R.L.; Wilson, C.G. A Review of Vagus Nerve Stimulation as a Therapeutic Intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef]
- Mertens, A.; Raedt, R.; Gadeyne, S.; Carrette, E.; Boon, P.; Vonck, K. Recent Advances in Devices for Vagus Nerve Stimulation. Expert Rev. Med. Devices 2018, 15, 527–539. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus Nerve Stimulation in Brain Diseases: Therapeutic Applications and Biological Mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef]
- Needham, E.J.; Helmy, A.; Zanier, E.R.; Jones, J.L.; Coles, A.J.; Menon, D.K. The Immunological Response to Traumatic Brain Injury. J. Neuroimmunol. 2019, 332, 112–125. [Google Scholar] [CrossRef]
- McKee, C.A.; Lukens, J.R. Emerging Roles for the Immune System in Traumatic Brain Injury. Front. Immunol. 2016, 7, 556. [Google Scholar] [CrossRef] [Green Version]
- Kelso, M.L.; Gendelman, H.E. Bridge between Neuroimmunity and Traumatic Brain Injury. Curr. Pharm. Des. 2014, 20, 4284–4298. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.M.; Lefering, R.; Westphal, M.; Maegele, M.; Czorlich, P. Traumatic Brain Injury with Concomitant Injury to the Spleen: Characteristics and Mortality of a High-Risk Trauma Cohort from the Traumaregister DGU(R). Eur. J. Trauma Emerg. Surg. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Ajmo, C.T., Jr.; Vernon, D.O.; Collier, L.; Hall, A.A.; Garbuzova-Davis, S.; Willing, A.; Pennypacker, K.R. The Spleen Contributes to Stroke-Induced Neurodegeneration. J. Neurosci. Res. 2008, 86, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, R.P.; Schulte, R.W.; Nie, Y.; Ling, T.; Lee, T.; Manaenko, A.; Gridley, D.S.; Zhang, J.H. Acute Splenic Irradiation Reduces Brain Injury in the Rat Focal Ischemic Stroke Model. Transl. Stroke Res. 2012, 3, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.A.; Leonardo, C.C.; Hall, A.A.; Rowe, D.D.; Collier, L.A.; Benkovic, S.A.; Willing, A.E.; Pennypacker, K.R. The Spleen Contributes to Stroke Induced Neurodegeneration Through Interferon Gamma Signaling. Metab. Brain Dis. 2012, 27, 131–141. [Google Scholar] [CrossRef]
- Borgers, J.S.W.; Tobin, R.P.; Vorwald, V.M.; Smith, J.M.; Davis, D.M.; Kimball, A.K.; Clambey, E.T.; Couts, K.L.; McWilliams, J.A.; Jordan, K.R.; et al. High-Dimensional Analysis of Postsplenectomy Peripheral Immune Cell Changes. Immunohorizons 2020, 4, 82–92. [Google Scholar] [CrossRef]
- Pruitt, D.T.; Danaphongse, T.T.; Lutchman, M.; Patel, N.; Reddy, P.; Wang, V.; Parashar, A.; Rennaker, R.L., 2nd; Kilgard, M.P.; Hays, S.A. Optimizing Dosing of Vagus Nerve Stimulation for Stroke Recovery. Transl. Stroke Res. 2021, 12, 65–71. [Google Scholar] [CrossRef]
- Carreno, F.R.; Frazer, A. Vagal Nerve Stimulation for Treatment-Resistant Depression. Neurotherapeutics 2017, 14, 716–727. [Google Scholar] [CrossRef]
- Daglas, M.; Draxler, D.F.; Ho, H.; McCutcheon, F.; Galle, A.; Au, A.E.; Larsson, P.; Gregory, J.; Alderuccio, F.; Sashindranath, M.; et al. Activated CD8(+) T Cells Cause Long-Term Neurological Impairment after Traumatic Brain Injury in Mice. Cell Rep. 2019, 29, 1178–1191.E6. [Google Scholar] [CrossRef]
- Wu, L.; Ji, N.N.; Wang, H.; Hua, J.Y.; Sun, G.L.; Chen, P.P.; Hua, R.; Zhang, Y.M. Domino Effect of Interleukin-15 and CD8 T-Cell-Mediated Neuronal Apoptosis in Experimental Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1450–1463. [Google Scholar] [CrossRef]
- Li, M.; Lin, Y.P.; Chen, J.L.; Li, H.; Jiang, R.C.; Zhang, J.N. Role of Regulatory T Cell in Clinical Outcome of Traumatic Brain Injury. Chin. Med. J. 2015, 128, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.K. Cranial Nerve XII: The Hypoglossal Nerve. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Mukherjee, S.; Zeitouni, S.; Cavarsan, C.F.; Shapiro, L.A. Increased Seizure Susceptibility in Mice 30 Days After Fluid Percussion Injury. Front. Neurol. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
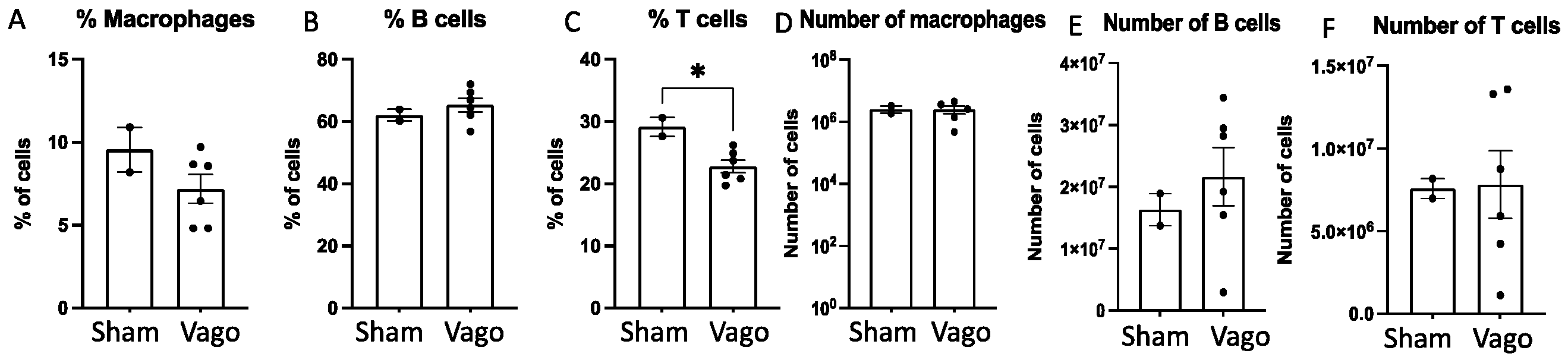
| Sham | L-Vagotomy | R-Vagotomy | ||
|---|---|---|---|---|
| B Cells | Mean | 62.05 | 65.27 | 65.33 |
| SD | 2.62 | 7.75 | 3.72 | |
| Macrophages | Mean | 9.55 | 7.90 | 6.45 |
| SD | 1.91 | 1.25 | 2.83 | |
| T Cells | Mean | 29.10 | 22.23 | 23.33 |
| SD | 2.12 | 3.48 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newell-Rogers, M.K.; Duong, A.; Nazarali, R.; Tobin, R.P.; Rogers, S.K.; Shapiro, L.A. Unilateral Cervical Vagotomy Modulates Immune Cell Profiles and the Response to a Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 9851. https://doi.org/10.3390/ijms23179851
Newell-Rogers MK, Duong A, Nazarali R, Tobin RP, Rogers SK, Shapiro LA. Unilateral Cervical Vagotomy Modulates Immune Cell Profiles and the Response to a Traumatic Brain Injury. International Journal of Molecular Sciences. 2022; 23(17):9851. https://doi.org/10.3390/ijms23179851
Chicago/Turabian StyleNewell-Rogers, M. Karen, Amanda Duong, Rizwan Nazarali, Richard P. Tobin, Susannah K. Rogers, and Lee A. Shapiro. 2022. "Unilateral Cervical Vagotomy Modulates Immune Cell Profiles and the Response to a Traumatic Brain Injury" International Journal of Molecular Sciences 23, no. 17: 9851. https://doi.org/10.3390/ijms23179851
APA StyleNewell-Rogers, M. K., Duong, A., Nazarali, R., Tobin, R. P., Rogers, S. K., & Shapiro, L. A. (2022). Unilateral Cervical Vagotomy Modulates Immune Cell Profiles and the Response to a Traumatic Brain Injury. International Journal of Molecular Sciences, 23(17), 9851. https://doi.org/10.3390/ijms23179851






