Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection
Abstract
1. Introduction
2. Results
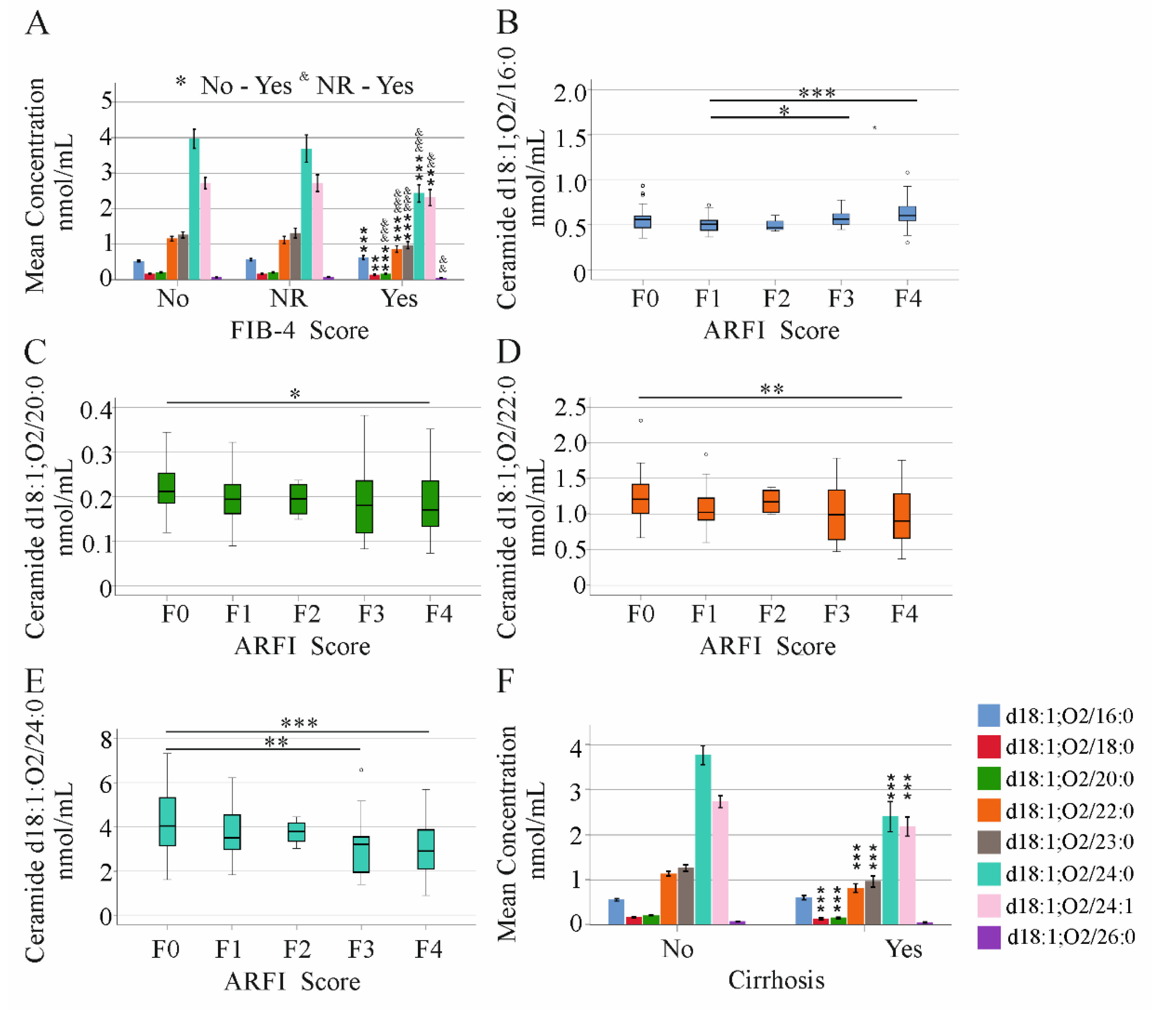
2.1. Association of Ceramide Species Levels with Gender, Fatty Liver, Diabetes, Age, and Body Mass Index in HCV Patients
2.2. Ceramide Species Levels and the Model for End-Stage Liver Disease Score in HCV Patients before DAA Therapy
2.3. Ceramide Species Levels and MELD Score in HCV Patients at 12 Weeks after the Start of Therapy
2.4. Ceramide Species Levels and Non-Invasive Scores of Liver Fibrosis/Cirrhosis in HCV Patients
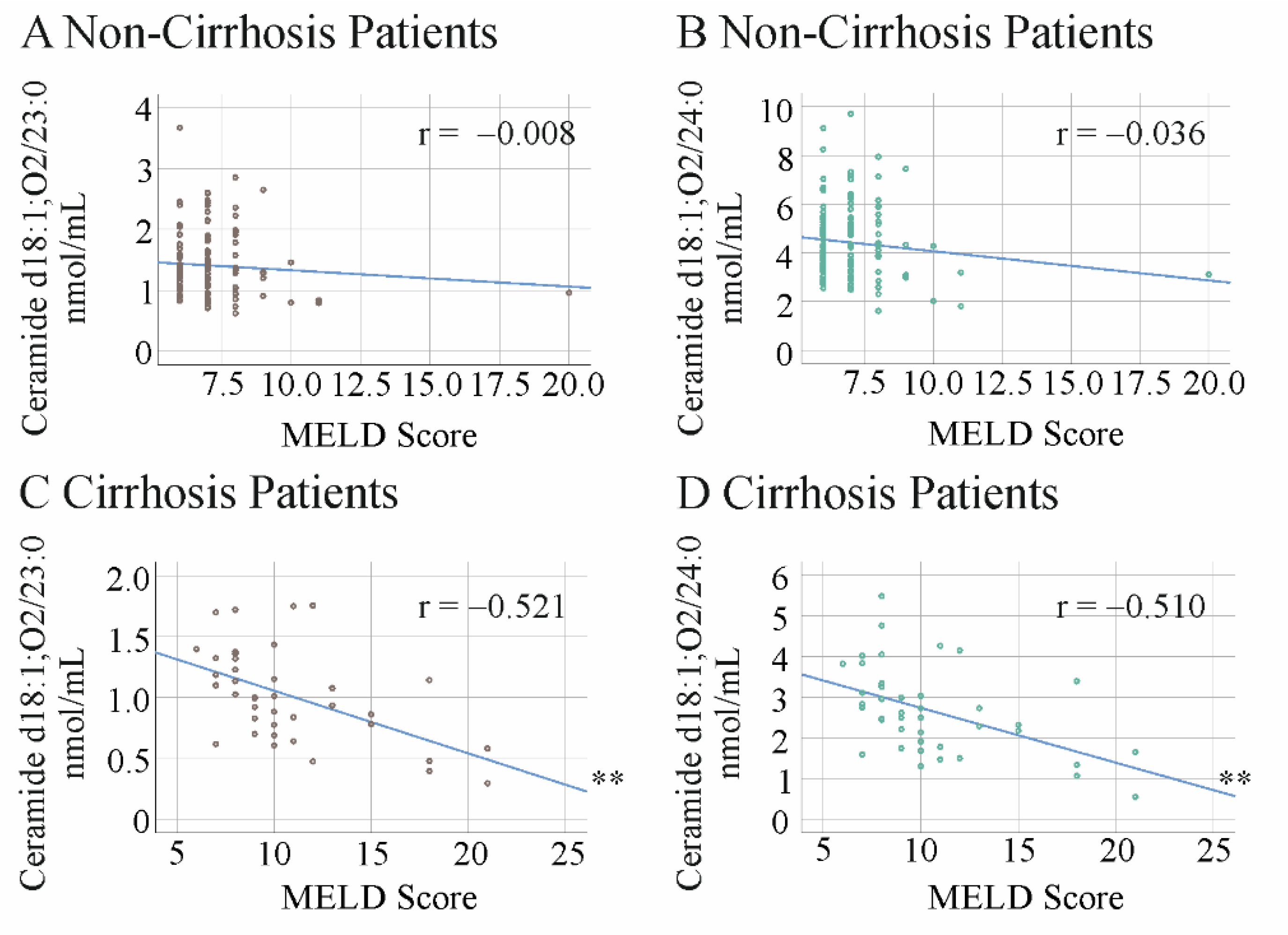
2.5. Ceramide Species Levels and MELD Score in HCV Patients with and without Liver Cirrhosis
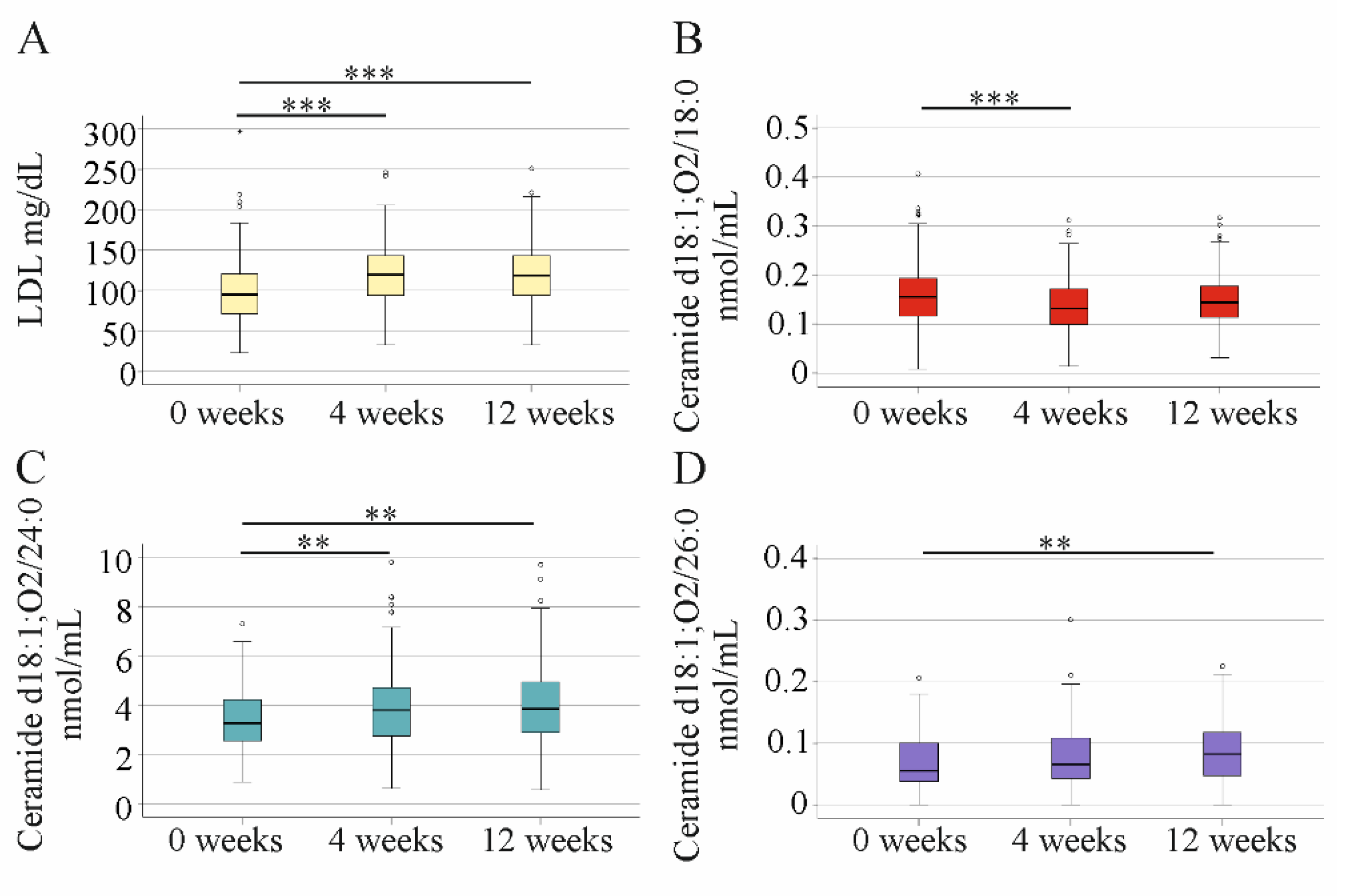
2.6. Impact of DAA Therapy on Serum LDL and Ceramide Levels
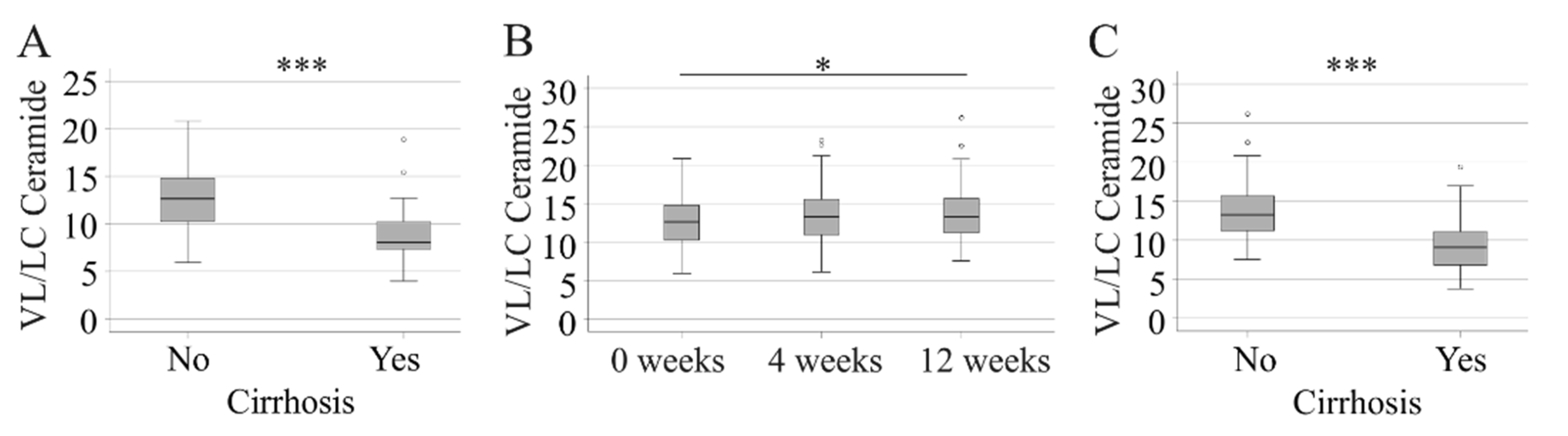
2.7. Very-Long-Chain to Long-Chain Ceramides in HCV Patients before Therapy and at 12 Weeks after Start of Treatment
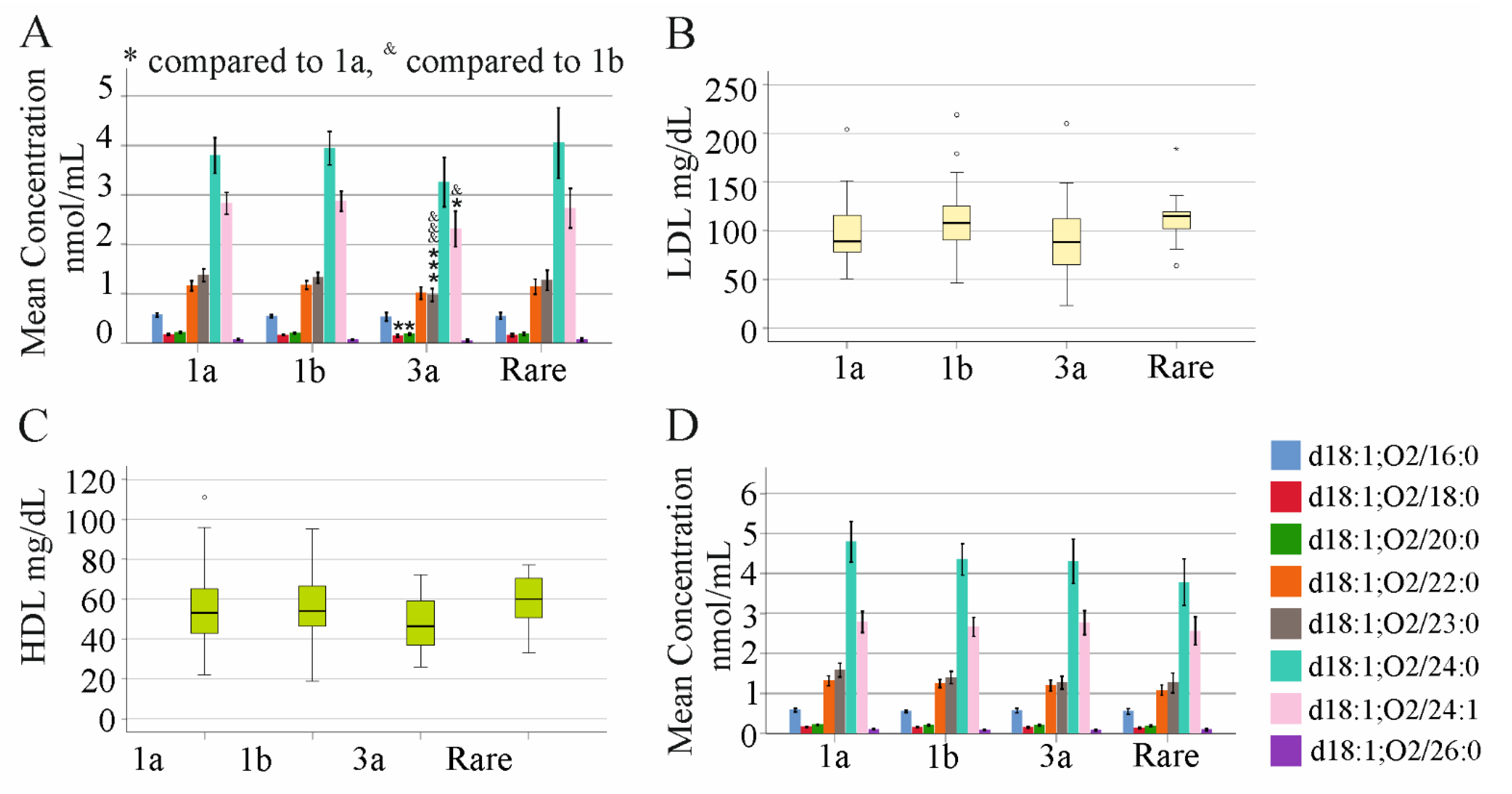
2.8. Association of Ceramide Species Levels with Viral Load and Genotype in Non-Cirrhosis HCV Patients
3. Discussion
4. Materials and Methods
4.1. Study Cohort
4.2. Lipid Extraction and Analysis of Ceramide Species
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Huang, M.H.; Jiang, J.D.; Peng, Z.G. Hepatitis C: From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J. Gastroenterol. 2018, 24, 5297–5311. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, M. Hepatitis C Virus Uses Host Lipids to Its Own Advantage. Metabolites 2021, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Vere, C.C.; Streba, C.T.; Streba, L.; Rogoveanu, I. Lipid serum profile in patients with viral liver cirrhosis. Med. Princ. Pract. 2012, 21, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Xiaoqiang, W.; Budoff, M.; Leaf, D.; Kuller, L.H.; Justice, A.C. Hepatitis C virus infection and the risk of coronary disease. Clin. Infect. Dis. 2009, 49, 225–232. [Google Scholar] [CrossRef]
- Corey, K.E.; Kane, E.; Munroe, C.; Barlow, L.L.; Zheng, H.; Chung, R.T. Hepatitis C virus infection and its clearance alter circulating lipids: Implications for long-term follow-up. Hepatology 2009, 50, 1030–1037. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Taura, N.; Motoyoshi, Y.; Akahoshi, H.; Nakamura, J.; Takahashi, Y.; Honda, T.; Yajima, H.; et al. Changes in serum LDL, PCSK9 and microRNA-122 in patients with chronic HCV infection receiving Daclatasvir/Asunaprevir. Biomed. Rep. 2019, 10, 156–164. [Google Scholar] [CrossRef]
- Peschel, G.; Grimm, J.; Gulow, K.; Muller, M.; Buechler, C.; Weigand, K. Chemerin Is a Valuable Biomarker in Patients with HCV Infection and Correlates with Liver Injury. Diagnostics 2020, 10, 974. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Patel, A.; Backstedt, D.; Choi, M.; Seetharam, A.B. Genotype specific peripheral lipid profile changes with hepatitis C therapy. World J. Gastroenterol. 2016, 22, 10226–10231. [Google Scholar] [CrossRef]
- Lonardo, A.; Adinolfi, L.E.; Loria, P.; Carulli, N.; Ruggiero, G.; Day, C.P. Steatosis and hepatitis C virus: Mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology 2004, 126, 586–597. [Google Scholar] [CrossRef]
- Cespiati, A.; Petta, S.; Lombardi, R.; Di Marco, V.; Calvaruso, V.; Bertelli, C.; Pisano, G.; Fatta, E.; Sigon, G.; Iuculano, F.; et al. Metabolic comorbidities and male sex influence steatosis in chronic hepatitis C after viral eradication by direct-acting antiviral therapy (DAAs): Evaluation by the controlled attenuation parameter (CAP). Dig. Liver Dis. 2021, 53, 1301–1307. [Google Scholar] [CrossRef]
- Sung, J.C.; Wyatt, B.E.; Perumalswami, P.V.; Branch, A.D. Response to ‘hepatitis C cure improved patient-reported outcomes in patients with and without liver fibrosis in a prospective study at a large urban medical center’. J. Viral Hepat. 2020, 27, 1502–1503. [Google Scholar] [CrossRef]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef]
- Buechler, C.; Aslanidis, C. Role of lipids in pathophysiology, diagnosis and therapy of hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158658. [Google Scholar] [CrossRef]
- Hajduch, E.; Lachkar, F.; Ferre, P.; Foufelle, F. Roles of Ceramides in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 792. [Google Scholar] [CrossRef]
- Park, W.J.; Song, J.H.; Kim, G.T.; Park, T.S. Ceramide and Sphingosine 1-Phosphate in Liver Diseases. Mol. Cells 2020, 43, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, G.; Ferreiros, N.; Bon, D.; Schwalm, S.; Dietz, J.; Berkowski, C.; Fitting, D.; Herrmann, E.; Zeuzem, S.; Sarrazin, C.; et al. Variations in serum sphingolipid levels associate with liver fibrosis progression and poor treatment outcome in hepatitis C virus but not hepatitis B virus infection. Hepatology 2015, 61, 812–822. [Google Scholar] [CrossRef]
- Grammatikos, G.; Muhle, C.; Ferreiros, N.; Schroeter, S.; Bogdanou, D.; Schwalm, S.; Hintereder, G.; Kornhuber, J.; Zeuzem, S.; Sarrazin, C.; et al. Serum acid sphingomyelinase is upregulated in chronic hepatitis C infection and non alcoholic fatty liver disease. Biochim. Biophys. Acta 2014, 1841, 1012–1020. [Google Scholar] [CrossRef]
- Grammatikos, G.; Ferreiros, N.; Waidmann, O.; Bon, D.; Schroeter, S.; Koch, A.; Herrmann, E.; Zeuzem, S.; Kronenberger, B.; Pfeilschifter, J. Serum Sphingolipid Variations Associate with Hepatic Decompensation and Survival in Patients with Cirrhosis. PLoS ONE 2015, 10, e0138130. [Google Scholar] [CrossRef]
- Ghadir, M.R.; Riahin, A.A.; Havaspour, A.; Nooranipour, M.; Habibinejad, A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010, 10, 285–288. [Google Scholar]
- Bassani, L.; Fernandes, S.A.; Raimundo, F.V.; Harter, D.L.; Gonzalez, M.C.; Marroni, C.A. Lipid profile of cirrhotic patients and its association with prognostic scores: A cross-sectional study. Arq. Gastroenterol. 2015, 52, 210–215. [Google Scholar] [CrossRef]
- Ballester, M.P.; Lluch, P.; Tosca, J.; Capilla, M.; Gomez, C.; Moreno, O.; Jordan-Iborra, C.; Sunsundegui, P.; Argemi, J.; Guijarro, J.; et al. Serum cholesterol predicts transplant-free survival in cirrhotic patients undergoing transjugular intrahepatic portosystemic shunt. Dig. Liver Dis. 2021, 53, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, F.; Xiong, W.J.; Zhong, L.; Xu, W.; Xu, F.; Liu, Y.B. Combined MELD and blood lipid level in evaluating the prognosis of decompensated cirrhosis. World J. Gastroenterol. 2010, 16, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Brown, S.H.; Lim, X.Y.; Fiveash, C.E.; Osborne, B.; Bentley, N.L.; Braude, J.P.; Mitchell, T.W.; Coster, A.C.; Don, A.S.; et al. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: A beneficial role for very long-chain sphingolipid species. Biochim. Biophys. Acta 2016, 1861, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, W.J.; Futerman, A.H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 2014, 1841, 671–681. [Google Scholar] [CrossRef]
- Mah, M.; Febbraio, M.; Turpin-Nolan, S. Circulating Ceramides-Are Origins Important for Sphingolipid Biomarkers and Treatments? Front. Endocrinol. 2021, 12, 684448. [Google Scholar] [CrossRef]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Volzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J. Am. Heart Assoc. 2018, 7, e007931. [Google Scholar] [CrossRef]
- Zeng, H.; Li, L.; Hou, Z.; Zhang, Y.; Tang, Z.; Liu, S. Direct-acting Antiviral in the Treatment of Chronic Hepatitis C: Bonuses and Challenges. Int. J. Med. Sci. 2020, 17, 892–902. [Google Scholar] [CrossRef]
- Graf, C.; Welzel, T.; Bogdanou, D.; Vermehren, J.; Beckel, A.; Bojunga, J.; Friedrich-Rust, M.; Dietz, J.; Kubesch, A.; Mondorf, A.; et al. Hepatitis C Clearance by Direct-Acting Antivirals Impacts Glucose and Lipid Homeostasis. J. Clin. Med. 2020, 9, 2702. [Google Scholar] [CrossRef]
- Mucke, V.T.; Thomas, D.; Mucke, M.M.; Waidmann, O.; Zeuzem, S.; Sarrazin, C.; Pfeilschifter, J.; Vermehren, J.; Finkelmeier, F.; Grammatikos, G. Serum sphingolipids predict de novo hepatocellular carcinoma in hepatitis C cirrhotic patients with sustained virologic response. Liver Int. 2019, 39, 2174–2183. [Google Scholar] [CrossRef]
- Freeman, R.B., Jr.; Wiesner, R.H.; Roberts, J.P.; McDiarmid, S.; Dykstra, D.M.; Merion, R.M. Improving liver allocation: MELD and PELD. Am. J. Transplant. 2004, 4 (Suppl. S9), 114–131. [Google Scholar] [CrossRef]
- Peschel, G.; Grimm, J.; Buechler, C.; Gunckel, M.; Pollinger, K.; Aschenbrenner, E.; Kammerer, S.; Jung, E.M.; Haimerl, M.; Werner, J.; et al. Liver stiffness assessed by shear-wave elastography declines in parallel with immunoregulatory proteins in patients with chronic HCV infection during DAA therapy. Clin. Hemorheol. Microcirc. 2021, 79, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Alem, S.A.; Abdellatif, Z.; Mabrouk, M.; Zayed, N.; Elsharkawy, A.; Khairy, M.; Musa, S.; Anwar, I.; Yosry, A. Diagnostic accuracy of acoustic radiation force impulse elastography (ARFI) in comparison to other non-invasive modalities in staging of liver fibrosis in chronic HCV patients: Single-center experience. Abdom. Radiol. 2019, 44, 2751–2758. [Google Scholar] [CrossRef]
- Paranagua-Vezozzo, D.C.; Andrade, A.; Mazo, D.F.; Nunes, V.; Guedes, A.L.; Ragazzo, T.G.; Moutinho, R.; Nacif, L.S.; Ono, S.K.; Alves, V.A.; et al. Concordance of non-invasive mechanical and serum tests for liver fibrosis evaluation in chronic hepatitis C. World J. Hepatol. 2017, 9, 436–442. [Google Scholar] [CrossRef]
- Butt, A.A.; Yan, P.; Simon, T.G.; Chung, R.T.; Abou-Samra, A.B.; ERCHIVES Study Team. Changes in circulating lipids level over time after acquiring HCV infection: Results from ERCHIVES. BMC Infect. Dis. 2015, 15, 510. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Yatsuhashi, H.; Abiru, S.; Yamasaki, K.; Komori, A.; Nagaoka, S.; Saeki, A.; Uchida, S.; Bekki, S.; Kugiyama, Y.; et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS ONE 2016, 11, e0163644. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Wiest, R.; Liebisch, G.; Buechler, C. Associations of systemic sphingolipids with measures of hepatic function in liver cirrhosis are related to cholesterol. Prostaglandins Other Lipid Mediat. 2017, 131, 25–32. [Google Scholar] [CrossRef]
- Probst, A.; Dang, T.; Bochud, M.; Egger, M.; Negro, F.; Bochud, P.Y. Role of hepatitis C virus genotype 3 in liver fibrosis progression—A systematic review and meta-analysis. J. Viral Hepat. 2011, 18, 745–759. [Google Scholar] [CrossRef]
- Shiffman, M.L. Retreatment of patients who do not respond to initial therapy for chronic hepatitis C. Cleve Clin. J. Med. 2004, 71 (Suppl. S3), S13–S16. [Google Scholar] [CrossRef]
- Snoeck, E.; Wade, J.R.; Duff, F.; Lamb, M.; Jorga, K. Predicting sustained virological response and anaemia in chronic hepatitis C patients treated with peginterferon alfa-2a (40KD) plus ribavirin. Br. J. Clin. Pharmacol. 2006, 62, 699–709. [Google Scholar] [CrossRef]
- Syed, E.; Rahbin, N.; Weiland, O.; Carlsson, T.; Oksanen, A.; Birk, M.; Davidsdottir, L.; Hagen, K.; Hultcrantz, R.; Aleman, S. Pegylated interferon and ribavirin combination therapy for chronic hepatitis C virus infection in patients with Child-Pugh Class A liver cirrhosis. Scand. J. Gastroenterol. 2008, 43, 1378–1386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, C.; Liu, Q.; Wang, A.; Lei, M. Inhibiting Ceramide Synthesis Attenuates Hepatic Steatosis and Fibrosis in Rats with Non-alcoholic Fatty Liver Disease. Front. Endocrinol. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J.; Bruderly, M. Cirrhosis and chronic liver failure: Part I. Diagnosis and evaluation. Am. Fam. Physician 2006, 74, 756–762. [Google Scholar]
- Liaskou, E.; Hirschfield, G.M. Cirrhosis-associated immune dysfunction: Novel insights in impaired adaptive immunity. EBioMedicine 2019, 50, 3–4. [Google Scholar] [CrossRef]
- McNally, B.D.; Ashley, D.F.; Hanschke, L.; Daou, H.N.; Watt, N.T.; Murfitt, S.A.; MacCannell, A.D.V.; Whitehead, A.; Bowen, T.S.; Sanders, F.W.B.; et al. Long-chain ceramides are cell non-autonomous signals linking lipotoxicity to endoplasmic reticulum stress in skeletal muscle. Nat. Commun. 2022, 13, 1748. [Google Scholar] [CrossRef]
- Correnti, J.; Lin, C.; Brettschneider, J.; Kuriakose, A.; Jeon, S.; Scorletti, E.; Oranu, A.; McIver-Jenkins, D.; Kaneza, I.; Buyco, D.; et al. Liver-specific ceramide reduction alleviates steatosis and insulin resistance in alcohol-fed mice. J. Lipid Res. 2020, 61, 983–994. [Google Scholar] [CrossRef]
- Morales, A.; Mari, M.; Garcia-Ruiz, C.; Colell, A.; Fernandez-Checa, J.C. Hepatocarcinogenesis and ceramide/cholesterol metabolism. Anticancer Agents Med. Chem. 2012, 12, 364–375. [Google Scholar] [CrossRef][Green Version]
- Grammatikos, G.; Schoell, N.; Ferreiros, N.; Bon, D.; Herrmann, E.; Farnik, H.; Koberle, V.; Piiper, A.; Zeuzem, S.; Kronenberger, B.; et al. Serum sphingolipidomic analyses reveal an upregulation of C16-ceramide and sphingosine-1-phosphate in hepatocellular carcinoma. Oncotarget 2016, 7, 18095–18105. [Google Scholar] [CrossRef]
- Park, J.W.; Park, W.J.; Kuperman, Y.; Boura-Halfon, S.; Pewzner-Jung, Y.; Futerman, A.H. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology 2013, 57, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Taniguchi, E.; Itou, M.; Sakata, M.; Sumie, S.; Sata, M. Insulin resistance and chronic liver disease. World J. Hepatol. 2011, 3, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nkontchou, G.; Bastard, J.P.; Ziol, M.; Aout, M.; Cosson, E.; Ganne-Carrie, N.; Grando-Lemaire, V.; Roulot, D.; Capeau, J.; Trinchet, J.C.; et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J. Hepatol. 2010, 53, 827–833. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair; EASL Governing Board Representative; Panel Members. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Yen, Y.H.; Kuo, F.Y.; Chen, C.H.; Hu, T.H.; Lu, S.N.; Wang, J.H.; Hung, C.H. Ultrasound is highly specific in diagnosing compensated cirrhosis in chronic hepatitis C patients in real world clinical practice. Medicine 2019, 98, e16270. [Google Scholar] [CrossRef]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Liebisch, G.; Drobnik, W.; Reil, M.; Trumbach, B.; Arnecke, R.; Olgemoller, B.; Roscher, A.; Schmitz, G. Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). J. Lipid Res. 1999, 40, 1539–1546. [Google Scholar] [CrossRef]
- Liebisch, G.; Lieser, B.; Rathenberg, J.; Drobnik, W.; Schmitz, G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim. Biophys. Acta 2004, 1686, 108–117. [Google Scholar] [CrossRef]

| Laboratory Parameter | Baseline (178 Patients) | 12 Weeks Therapy (176 Patients) | p-Value |
|---|---|---|---|
| Age, years | 54 (24–82) | 54 (24–82) | ns |
| BMI, kg/m2 | 25.6 (17.6–41.6) | 25.6 (17.6–41.6) | ns |
| MELD | 7 (6–21) | 7 (6–21) | ns |
| Platelets, n/nL | 195 (38–402) | 206 (37–407) | ns |
| Ferritin, ng/mL | 128.6 (5.6–2309) | 94.2 (2.9–1161) | 0.003 |
| ALT, U/L | 61 (2–305) | 26 (6–388) | <0.001 |
| AST, U/L | 47 (7–1230) | 22 (6–836) | <0.001 |
| Bilirubin, mg/dL | 1.0 (1.0–4.3) | 1.0 (1.0–7.5) | ns |
| Albumin, g/L | 38 (2–50) | 39 (16–93) | ns |
| INR | 1.05 (1.00–2.44) | 1.04 (1.00–2.22) | ns |
| Creatinine, mg/dL | 0.78 (0.14–14.00) | 0.76 (0.14–14.7) | ns |
| Leukocytes, n/L | 6.5 (2.2–72.4) | 6.8 (2.4–62.9) | ns |
| CRP, mg/L | 2.9 (1.0–55.0) | 2.9 (2.9–20.3) | ns |
| HDL, mg/dL | 52 (19–111) | 50 (13–96) | ns |
| LDL, mg/dL | 95 (23–296) | 119 (33–251) | <0.001 |
| Ceramide Species d18:1;O2/nmol/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 16:0 | 18:0 | 20:0 | 22:0 | 23:0 | 24:0 | 24:1 | 26:0 | |
| BMI, kg/m2 | r | 0.079 | −0.052 | 0.032 | 0.118 | 0.121 | 0.023 | 0.013 | −0.101 |
| p | - | - | - | - | - | - | - | - | |
| Age, years | r | 0.200 | 0.062 | 0.017 | −0.136 | −0.012 | −0.239 | −0.012 | −0.022 |
| p | - | - | - | - | - | 0.011 | - | - | |
| MELD score | r | 0.124 | −0.317 | −0.346 | −0.380 | −0.298 | −0.461 | −0.295 | −0.205 |
| p | - | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.049 | |
| ALT, U/L | r | 0.117 | −0.191 | −0.226 | −0.286 | −0.247 | −0.352 | −0.176 | −0.165 |
| p | - | - | 0.019 | <0.001 | 0.007 | <0.001 | - | - | |
| AST, U/L | r | 0.005 | −0.156 | −0.118 | −0.164 | −0.143 | −0.151 | −0.117 | −0.115 |
| p | - | - | - | - | - | - | - | - | |
| Bilirubin, mg/dL | r | 0.085 | −0.213 | −0.255 | −0.257 | −0.240 | −0.322 | −0.208 | −0.111 |
| p | - | 0.034 | 0.005 | 0.004 | 0.010 | <0.001 | 0.043 | - | |
| Albumin, g/L | r | −0.133 | 0.248 | 0.279 | 0.309 | 0.228 | 0.368 | 0.200 | 0.212 |
| p | - | 0.008 | 0.002 | <0.001 | 0.021 | <0.001 | - | 0.041 | |
| INR | r | 0.174 | −0.289 | −0.320 | −0.395 | −0.336 | −0.496 | −0.308 | −0.237 |
| p | - | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.011 | |
| Creatinine, mg/dL | r | −0.069 | −0.039 | −0.093 | −0.078 | 0.002 | −0.046 | −0.009 | 0.053 |
| p | - | - | - | - | - | - | - | - | |
| Leukocytes, n/L | r | −0.066 | 0.201 | 0.237 | 0.121 | 0.127 | 0.201 | 0.255 | 0.090 |
| p | - | - | 0.011 | - | - | - | 0.004 | - | |
| Platelets, n/nL | r | −0.215 | 0.249 | 0.274 | 0.249 | 0.222 | 0.397 | 0.272 | 0.253 |
| p | 0.031 | 0.006 | 0.002 | 0.007 | 0.023 | <0.001 | 0.002 | 0.005 | |
| CRP, mg/L | r | 0.233 | 0.100 | 0.147 | 0.076 | 0.064 | 0.053 | 0.177 | 0.088 |
| p | 0.014 | - | - | - | - | - | - | - | |
| Ferritin, ng/mL | r | 0.053 | 0.029 | 0.049 | 0.030 | 0.023 | −0.056 | 0.097 | −0.028 |
| p | - | - | - | - | - | - | - | - | |
| Viral load | r | −0.069 | 0.131 | 0.208 | 0.158 | 0.144 | 0.114 | 0.064 | −0.009 |
| p | - | - | 0.043 | - | - | - | - | - | |
| HDL, mg/dL | r | 0.033 | 0.110 | 0.059 | 0.077 | 0.094 | 0.131 | 0.044 | 0.136 |
| p | - | - | - | - | - | - | - | - | |
| LDL, mg/dL | r | 0.291 | 0.310 | 0.517 | 0.614 | 0.624 | 0.666 | 0.578 | 0.383 |
| p | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Ceramide Species d18:1;O2/nmol/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 16:0 | 18:0 | 20:0 | 22:0 | 23:0 | 24:0 | 24:1 | 26:0 | |
| BMI, kg/m2 | r | 0.055 | 0.037 | 0.101 | 0.196 | 0.167 | 0.067 | 0.027 | −0.092 |
| p | - | - | - | - | - | - | - | - | |
| Age, years | r | 0.252 | 0.183 | 0.187 | 0.025 | 0.152 | −0.103 | 0.147 | 0.063 |
| p | 0.023 | - | - | - | - | - | - | - | |
| MELD score | r | 0.043 | −0.150 | −0.101 | −0.191 | −0.116 | −0.230 | −0.130 | −0.080 |
| p | - | - | - | - | - | - | - | - | |
| ALT, U/L | r | 0.082 | −0.148 | −0.168 | −0.205 | −0.173 | −0.194 | −0.137 | −0.134 |
| p | - | - | - | - | - | - | - | - | |
| AST, U/L | r | −0.003 | −0.105 | −0.119 | −0.168 | −0.127 | −0.234 | −0.077 | −0.119 |
| p | - | - | - | - | - | 0.046 | - | - | |
| Bilirubin, mg/dL | r | −0.026 | −0.058 | −0.076 | −0.046 | −0.022 | −0.024 | −0.004 | 0.015 |
| p | - | - | - | - | - | - | - | - | |
| Albumin, g/L | r | −0.069 | 0.115 | 0.027 | 0.082 | 0.007 | 0.123 | 0.018 | 0.067 |
| p | - | - | - | - | - | - | - | - | |
| INR | r | 0.104 | −0.140 | −0.072 | −0.219 | −0.148 | −0.286 | −0.145 | −0.127 |
| p | - | - | - | - | - | 0.005 | - | - | |
| Creatinine, mg/dL | r | −0.040 | −0.031 | −0.041 | 0.009 | 0.041 | 0.019 | 0.031 | 0.106 |
| p | - | - | - | - | - | - | - | - | |
| Leukocytes, n/L | r | −0.008 | 0.152 | 0.127 | −0.019 | −0.044 | 0.001 | 0.140 | −0.072 |
| p | - | - | - | - | - | - | - | - | |
| Platelets, n/nL | r | −0.196 | 0.124 | 0.052 | 0.041 | 0.012 | 0.156 | 0.101 | 0.119 |
| p | - | - | - | - | - | - | - | - | |
| CRP, mg/L | r | 0.260 | 0.136 | 0.160 | 0.128 | 0.082 | 0.139 | 0.258 | 0.150 |
| p | 0.017 | - | - | - | - | - | 0.018 | ||
| Ferritin, ng/mL | r | 0.034 | −0.017 | −0.010 | −0.023 | 0.001 | −0.093 | 0.037 | 0.001 |
| p | - | - | - | - | - | - | - | - | |
| Viral load | r | −0.031 | 0.064 | 0.134 | 0.100 | 0.115 | 0.009 | 0.028 | −0.010 |
| p | - | - | - | - | - | - | - | - | |
| HDL, mg/dL | r | −0.043 | 0.055 | −0.061 | −0.030 | 0.006 | 0.059 | −0.062 | 0.107 |
| p | - | - | - | - | - | - | - | - | |
| LDL, mg/dL | r | 0.407 | 0.290 | 0.499 | 0.598 | 0.584 | 0.605 | 0.539 | 0.413 |
| p | <0.001 | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Ceramide Species d18:1;O2/nmol/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 16:0 | 18:0 | 20:0 | 22:0 | 23:0 | 24:0 | 24:1 | 26:0 | |
| BMI, kg/m2 | r | 0.073 | −0.159 | −0.031 | 0.167 | 0.181 | 0.202 | 0.113 | −0.022 |
| p | - | - | - | - | - | - | - | - | |
| Age, years | r | −0.218 | 0.083 | 0.084 | −0.172 | −0.149 | −0.168 | −0.081 | 0.003 |
| p | - | - | - | - | - | - | - | - | |
| MELD score | r | 0.032 | −0.326 | −0.390 | −0.257 | −0.287 | −0.392 | −0.345 | −0.294 |
| p | - | - | - | - | - | - | - | - | |
| ALT, U/L | r | 0.017 | −0.225 | 0.111 | −0.017 | 0.006 | −0.006 | −0.015 | −0.008 |
| p | - | - | - | - | - | - | - | - | |
| AST, U/L | r | 0.019 | −0.209 | <0.001 | −0.242 | −0.242 | −0.271 | −0.109 | −0.039 |
| p | - | - | - | - | - | - | - | - | |
| Bilirubin, mg/dL | r | 0.066 | −0.194 | −0.172 | −0.251 | −0.308 | −0.372 | −0.240 | −0.146 |
| p | - | - | - | - | - | - | - | - | |
| Albumin, g/L | r | 0.040 | 0.281 | 0.380 | 0.427 | 0.454 | 0.531 | 0.309 | 0.347 |
| p | - | - | - | 0.048 | 0.026 | 0.003 | - | - | |
| INR | r | −0.008 | −0.350 | −0.491 | −0.384 | −0.495 | −0.586 | −0.480 | −0.352 |
| p | - | - | 0.010 | - | 0.009 | 0.001 | 0.014 | - | |
| Creatinine, mg/dL | r | −0.231 | 0.030 | −0.078 | −0.101 | 0.079 | −0.007 | 0.018 | 0.072 |
| p | - | - | - | - | - | - | - | - | |
| Leukocytes, n/L | r | −0.043 | −0.009 | 0.176 | 0.177 | 0.357 | 0.402 | 0.297 | 0.436 |
| p | - | - | - | - | - | - | - | 0.040 | |
| Platelets, n/nL | r | −0.119 | −0.074 | 0.241 | 0.214 | 0.367 | 0.443 | 0.238 | 0.445 |
| p | - | - | - | - | - | 0.034 | - | 0.032 | |
| CRP, mg/L | r | 0.163 | 0.194 | 0.258 | 0.124 | 0.106 | 0.057 | 0.169 | −0.019 |
| p | - | - | - | - | - | - | - | - | |
| Ferritin, ng/mL | r | 0.176 | 0.102 | 0.283 | 0.226 | 0.147 | 0.105 | 0.367 | −0.140 |
| p | - | - | - | - | - | - | - | - | |
| Viral load | r | −0.083 | 0.049 | −0.006 | −0.064 | −0.072 | −0.021 | −0.150 | −0.196 |
| p | - | - | - | - | - | - | - | - | |
| HDL, mg/dL | r | 0.433 | 0.243 | 0.391 | 0.340 | 0.286 | 0.301 | 0.279 | 0.122 |
| p | - | - | - | - | - | - | - | - | |
| LDL, mg/dL | r | 0.414 | 0.384 | 0.430 | 0.615 | 0.654 | 0.674 | 0.588 | 0.191 |
| p | - | - | 0.042 | <0.001 | <0.001 | <0.001 | 0.001 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höring, M.; Peschel, G.; Grimm, J.; Krautbauer, S.; Müller, M.; Weigand, K.; Liebisch, G.; Buechler, C. Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection. Int. J. Mol. Sci. 2022, 23, 9806. https://doi.org/10.3390/ijms23179806
Höring M, Peschel G, Grimm J, Krautbauer S, Müller M, Weigand K, Liebisch G, Buechler C. Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection. International Journal of Molecular Sciences. 2022; 23(17):9806. https://doi.org/10.3390/ijms23179806
Chicago/Turabian StyleHöring, Marcus, Georg Peschel, Jonathan Grimm, Sabrina Krautbauer, Martina Müller, Kilian Weigand, Gerhard Liebisch, and Christa Buechler. 2022. "Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection" International Journal of Molecular Sciences 23, no. 17: 9806. https://doi.org/10.3390/ijms23179806
APA StyleHöring, M., Peschel, G., Grimm, J., Krautbauer, S., Müller, M., Weigand, K., Liebisch, G., & Buechler, C. (2022). Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection. International Journal of Molecular Sciences, 23(17), 9806. https://doi.org/10.3390/ijms23179806







