Intelligent Drug Delivery by Peptide-Based Dual-Function Micelles
Abstract
:1. Introduction
2. Results and Discussion
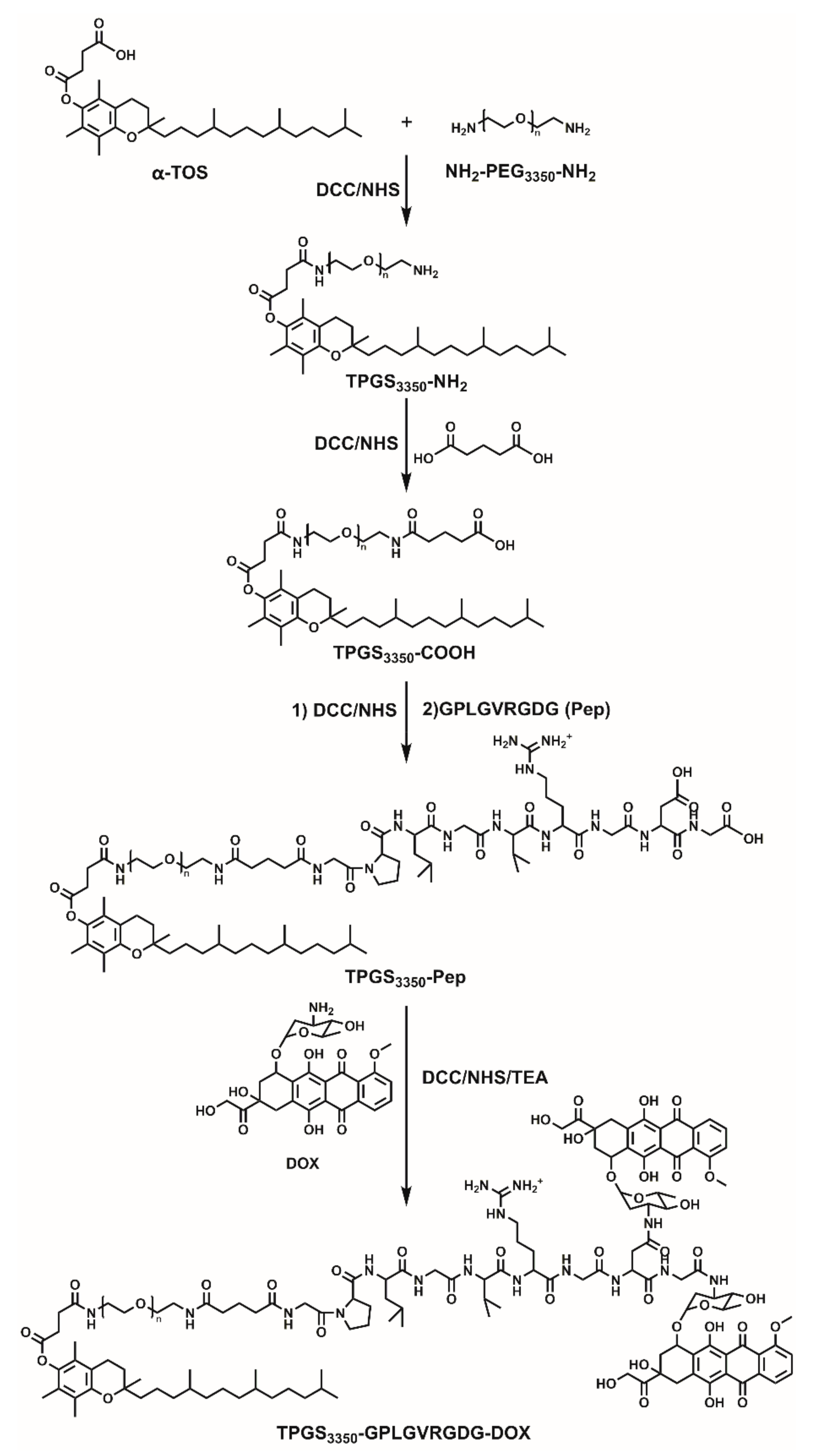
2.1. Synthesis and Characterzation of Polymeric Prodrugs
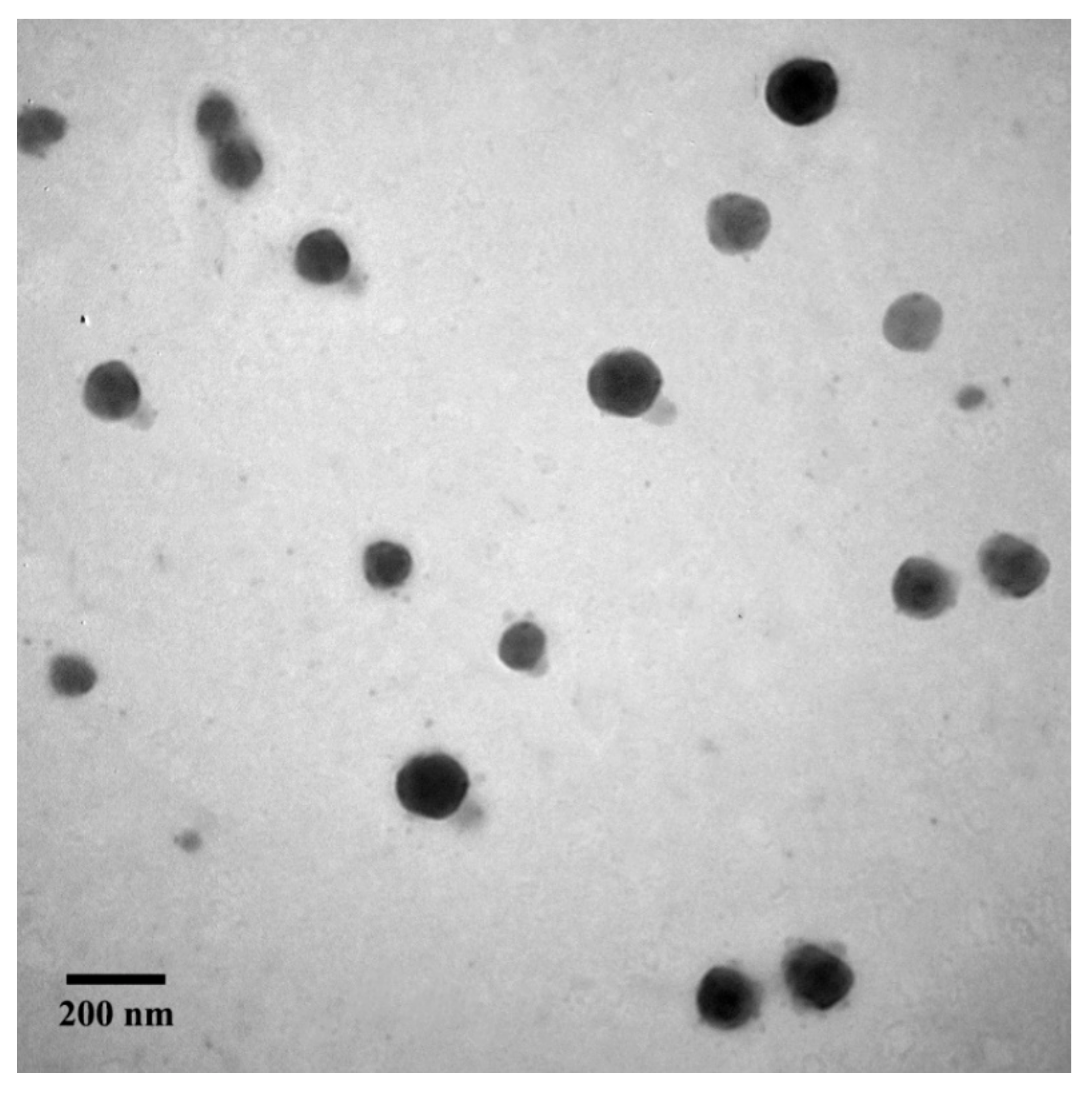
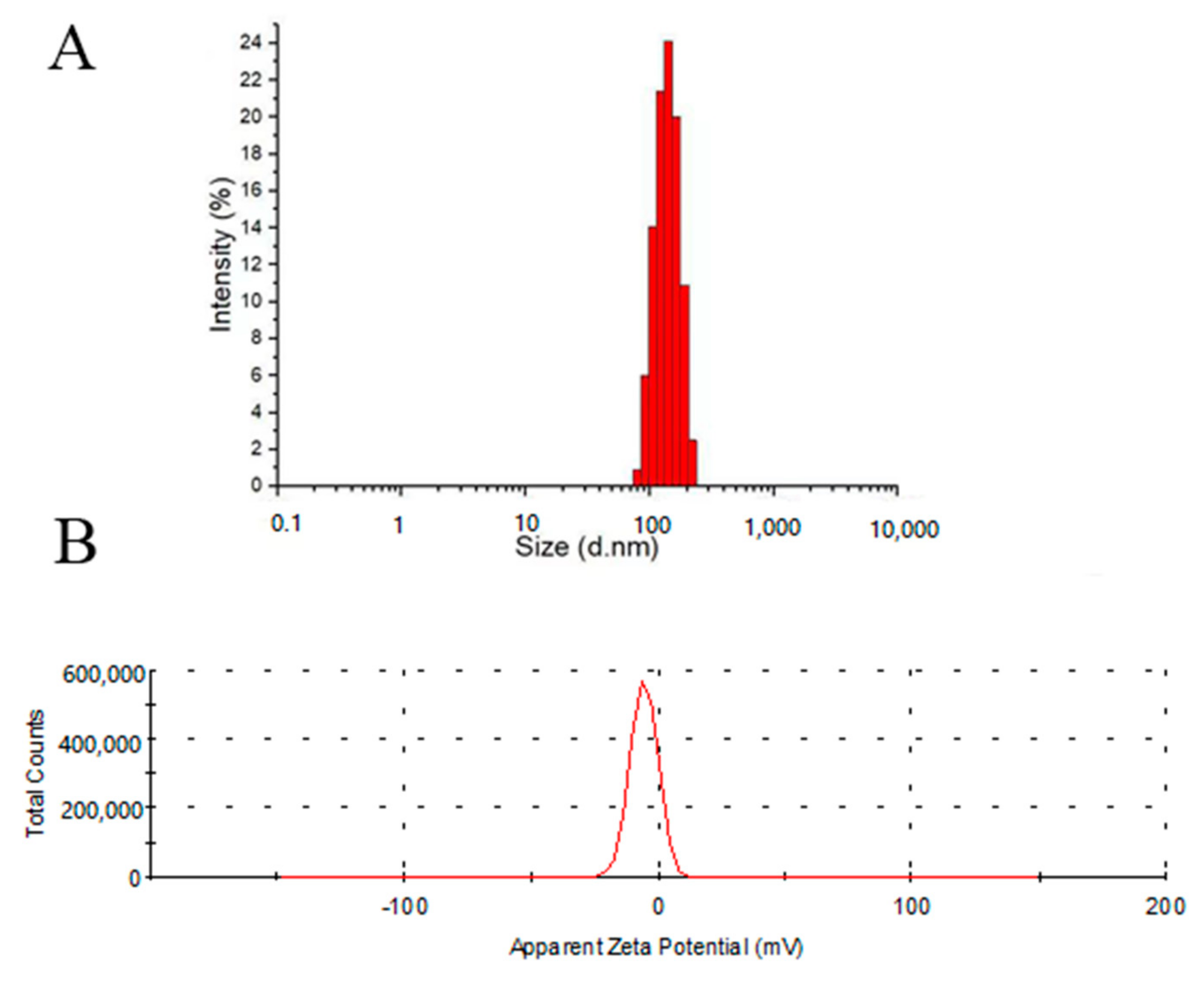
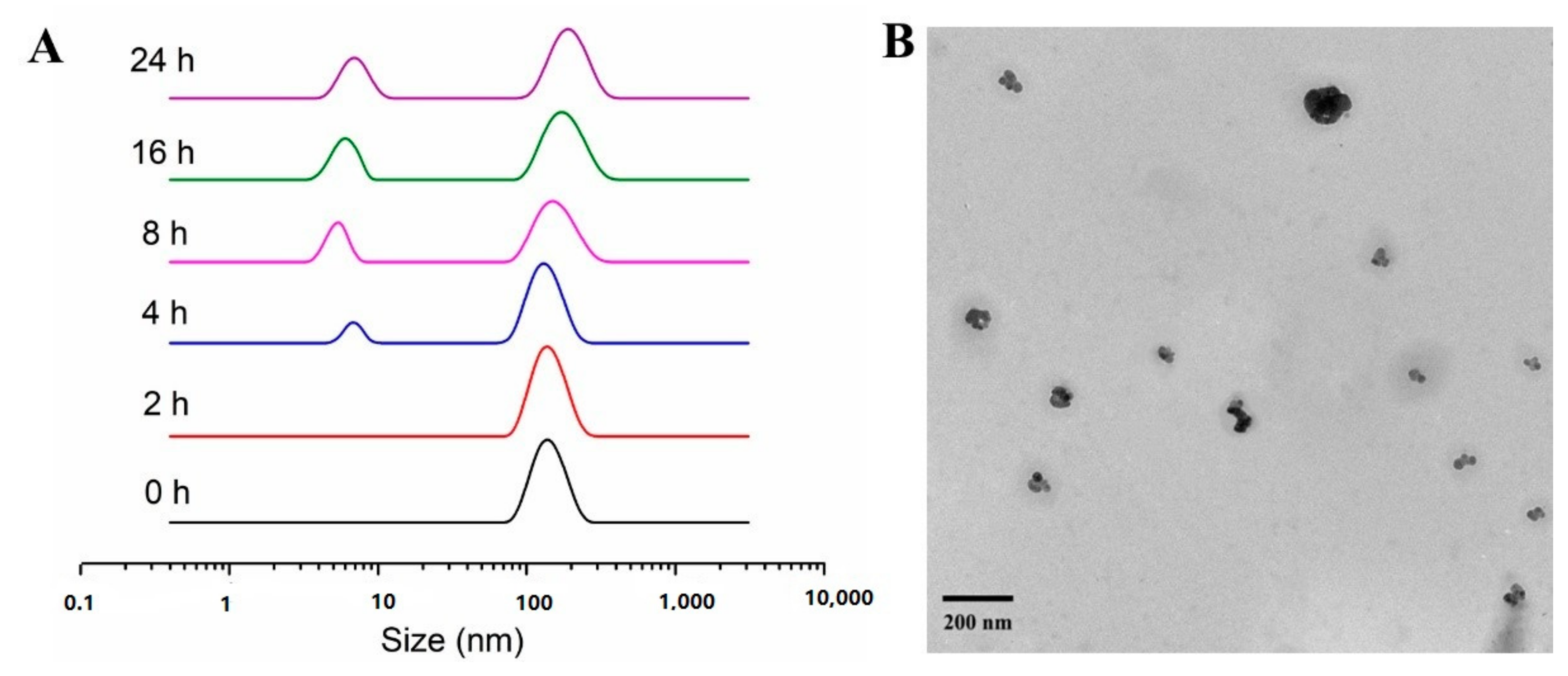
2.2. Characterization of Micelles
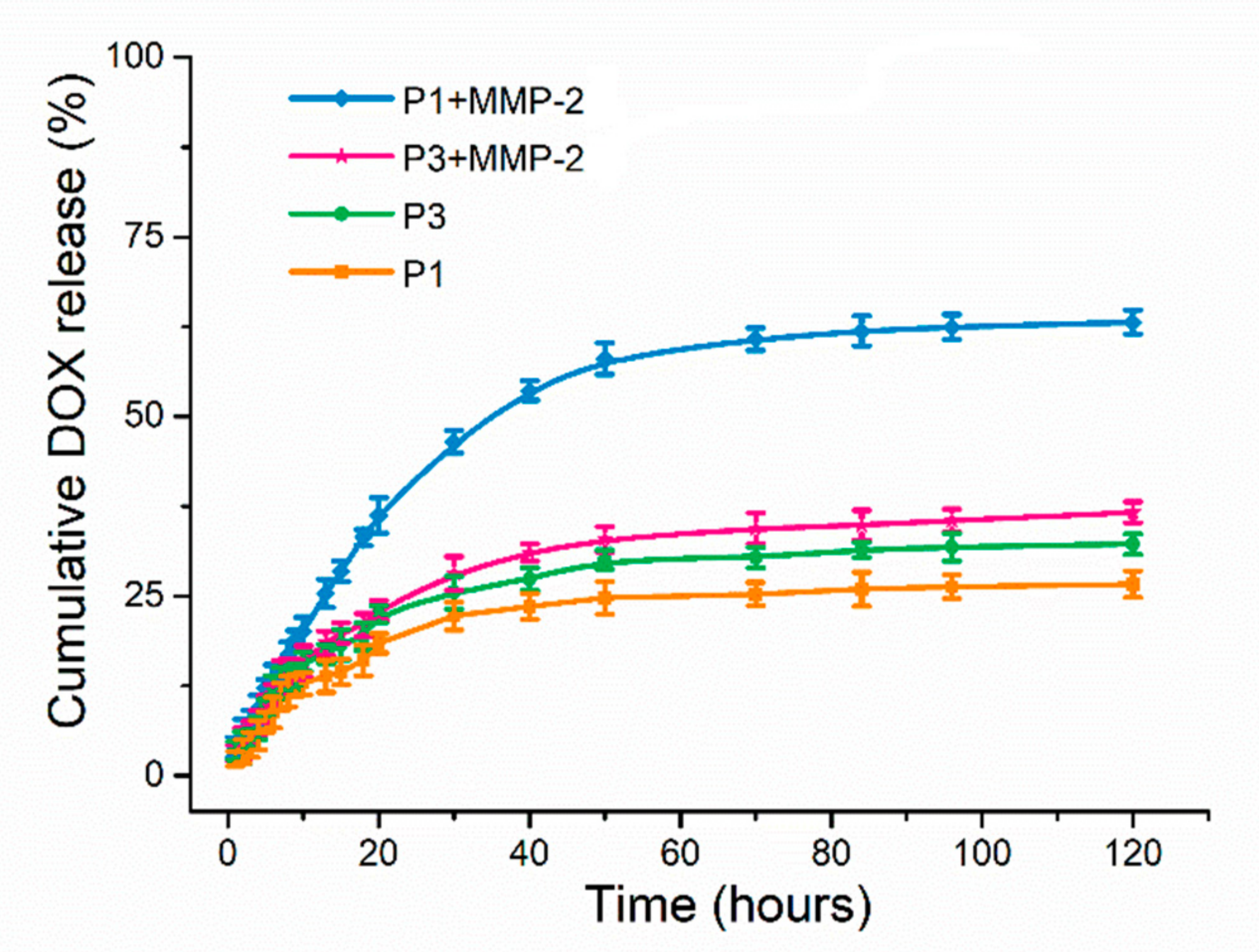
2.3. In Vitro Drug Release
2.4. In Vitro MMP-2 Enzyme Responsiveness
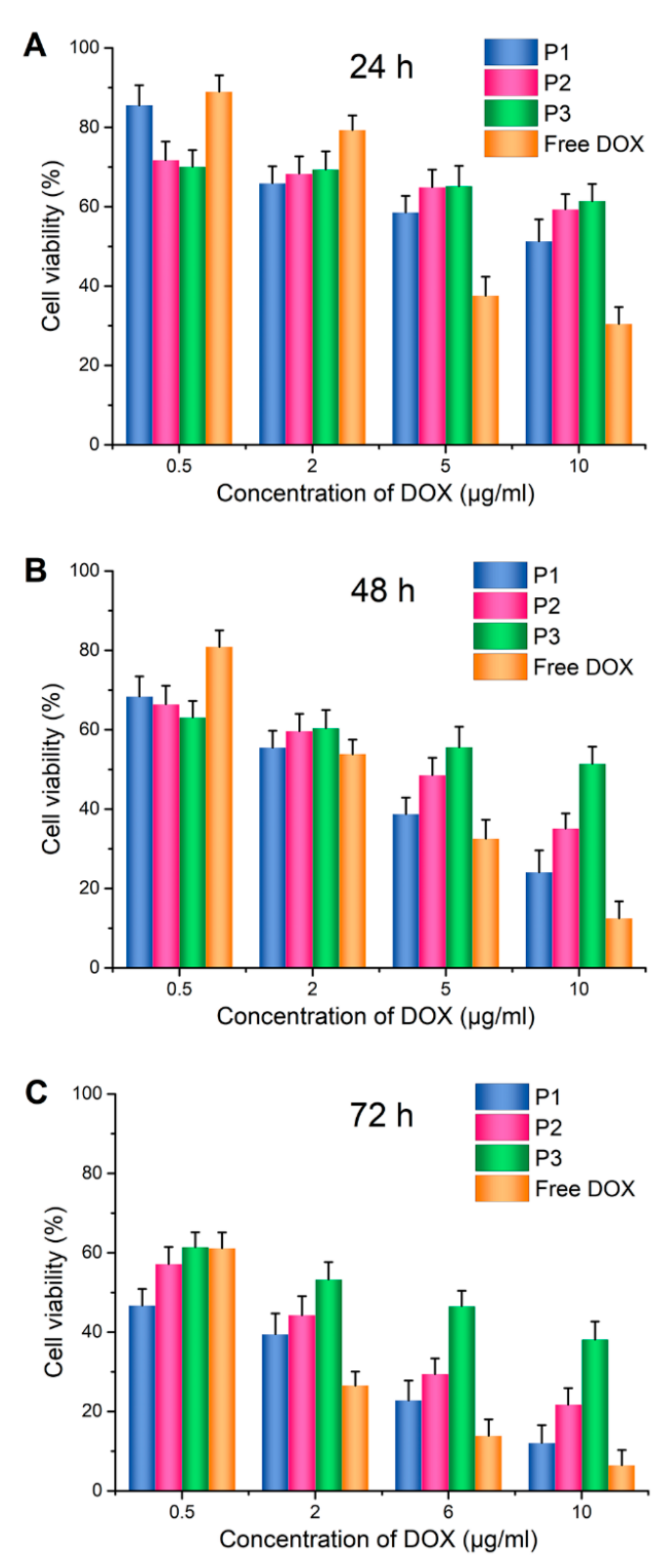
2.5. In Vitro Cytotoxicity
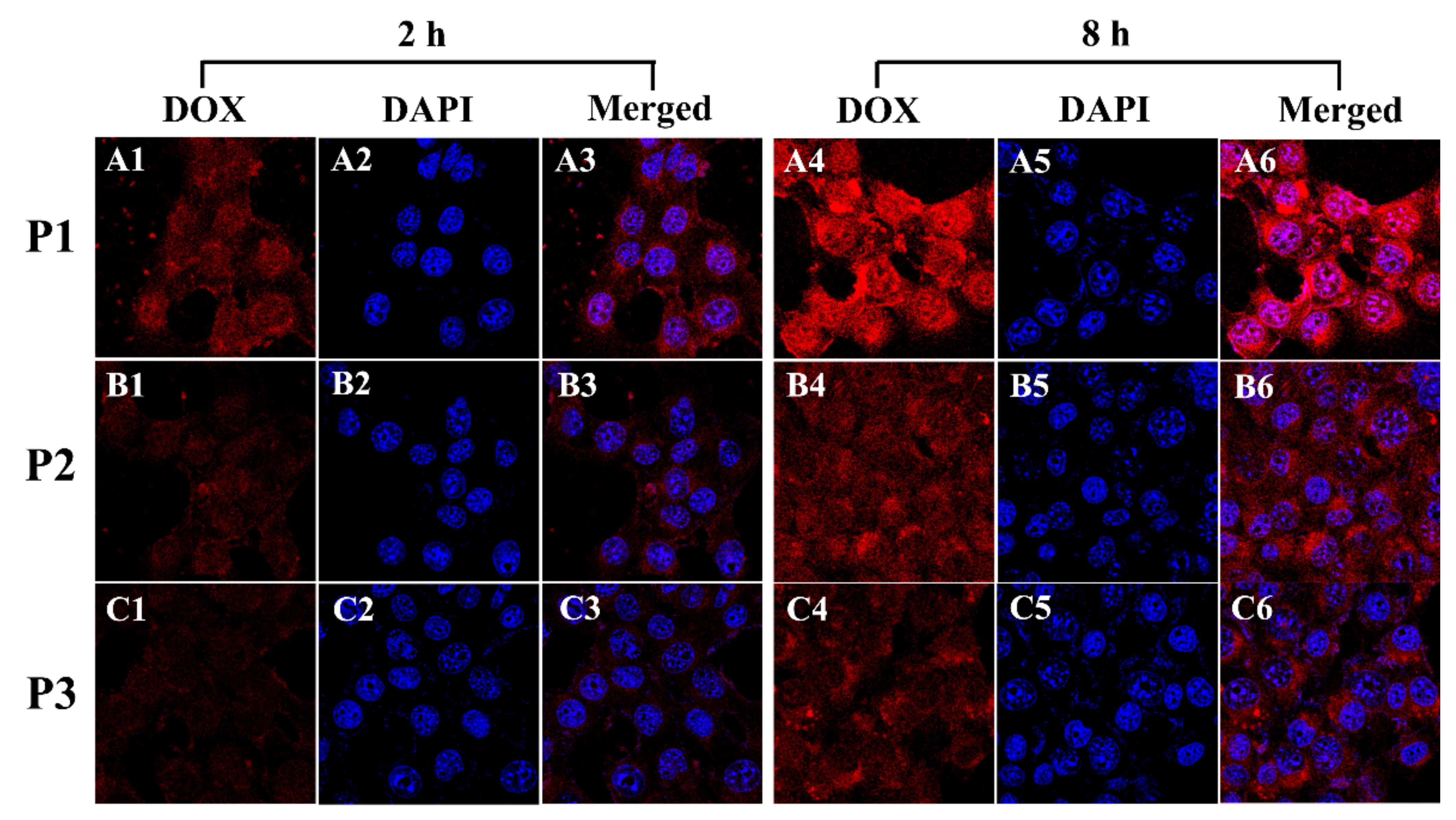
2.6. In Vitro Cellular Uptake
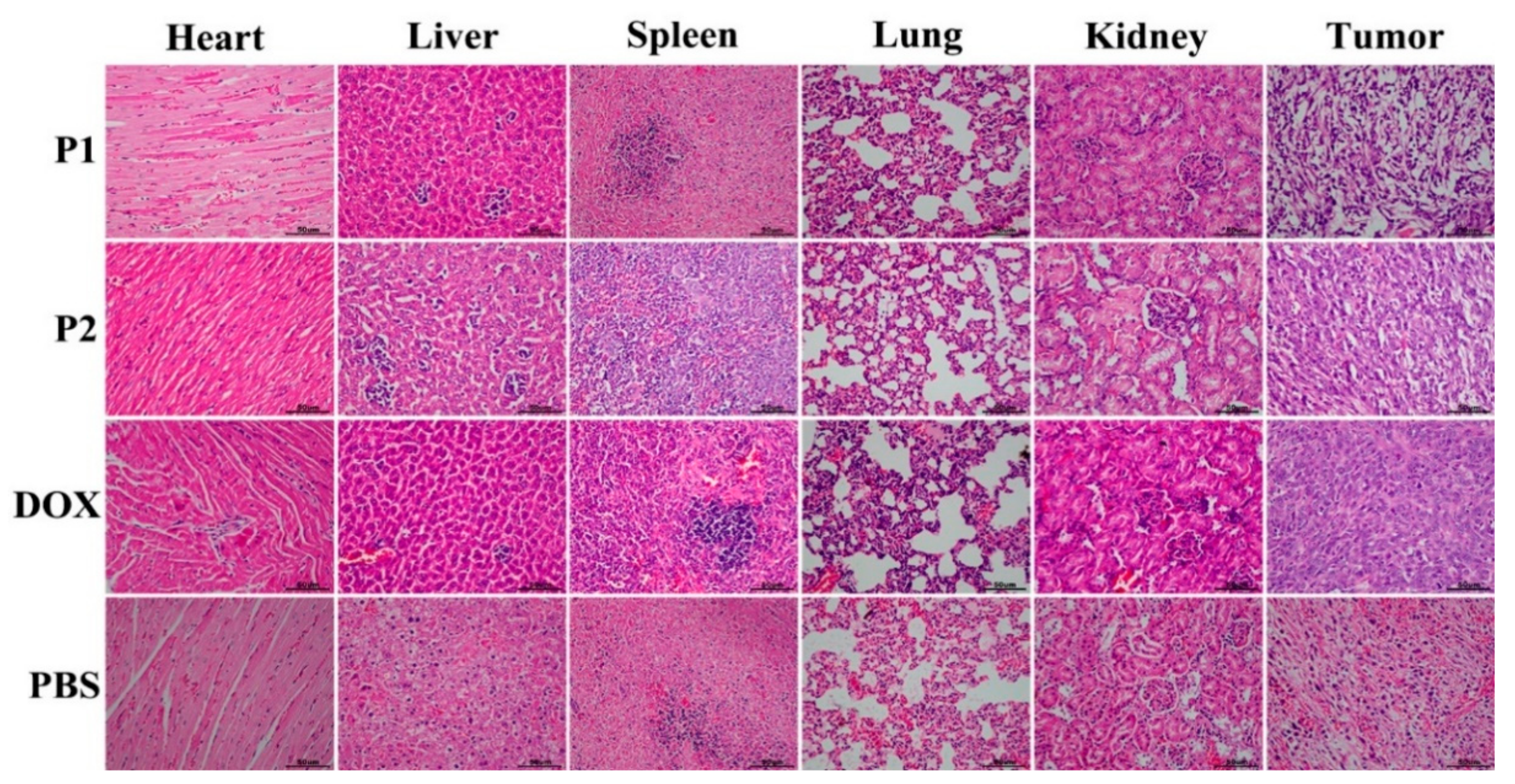
2.7. In Vivo Antitumor Efficacy
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Polymer
3.3. Characterizaton of TPGS3350-GPLGVRGDG-DOX
3.4. Fabricaton and Characterizaton of Micelles
3.5. Characterization of P1
3.6. Drug Release
3.7. In Vitro MMP-2 Enzyme Responsiveness
3.8. In Vitro Cytotoxcity
3.9. In Vitro Cellular Uptake
3.10. In Vivo Antititumor Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMPs | matrix metalloproteinase |
| DOX | doxorubicin |
| TPGS3350 | D-α-tocopherol polyethylene glycol 3350 succinate |
| GPLGVRGDG | Gly-Pro-Leu-Gly-Val-Arg-Gly-Asp-Gly |
| GPLGVRG | Gly-Pro-Leu-Gly-Val-Arg-Gly |
| VRGDG | Val-Arg-Gly-Asp-Gly |
| PEG | polyethylene glycol |
| EPR | enhanced permeability and retention |
| FDA | US Food and Drug Administration |
| MMP | matrix metalloproteinase |
| HLB | hydrophilic/lipophilic balance |
| CMC | critical micelle concentration |
| RGD | Arg-Gly-Asp |
| PBS | Phosphate-Buffered Saline |
| P1 | TPGS3350-GPLGVRGDG-DOX & DOX |
| P2 | TPGS3350-GPLGVRG-DOX & DOX |
| P3 | TPGS3350-DOX & DOX |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 359. [Google Scholar]
- Chen, F.; Li, Y.; Lin, X.; Qiu, H.; Yin, S. Polymeric systems containing supramolecular coordination complexes for drug delivery. Polymers 2021, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.M.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Siegel, D.A.; Feuer, E.J.; Firth, A.U.; Kohler, B.A.; Scott, S.; Ma, J.; et al. Annual report to the nation on the status of cancer, Featuring cancer in men and women age 20–49 years. J. Natl. Cancer I 2019, 111, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabou, A.A.; Ahmed, H.H.; Shalby, A.B. Selenium overcomes doxorubicin resistance in their nano-platforms against breast and colon cancers. Biol. Trace Elem. Res. 2020, 193, 377–389. [Google Scholar] [CrossRef]
- Choi, J.-S.; Doh, K.-O.; Kim, B.-K.; Seu, Y.-B. Synthesis of cholesteryl doxorubicin and its anti-cancer activity. Bioorg. Med. Chem. Lett. 2017, 27, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef]
- Norouzi, M.; Amerian, M.; Amerian, M.; Atyabi, F. Clincal applications of nanomedicine in cancer therapy. Drug Discov. Today 2020, 25, 107–125. [Google Scholar] [CrossRef]
- Luo, L.; Xu, F.; Peng, H.; Luo, Y.; Tian, X.; Battaglia, G.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. J. Control. Release 2020, 318, 124–135. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Wilson, B.K.; Sinko, P.J.; Prud’homme, R.K. Encapsulation and controlled release of a camptothecin prodrug from nanocarriers and microgels: Tuning release rate with nanocarrier excipient composition. Mol. Pharmaceut. 2021, 18, 1093–1101. [Google Scholar] [CrossRef]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C.W. A framework for designing delivery systems. Nat. Nanotechnol. 2020, 15, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Zhang, H.J.; Feng, C.; Liu, C.; Xiao, Y.F.; Zhang, X.C.; Mei, L.; Kim, J.S.; Tao, W.; Ji, X.Y. Arsenene-mediated multiple independently targeted reactive oxygen species burst for cancer therapy. Nat. Commun. 2021, 12, 4777. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.T.; Lin, C.C.; Wang, Y.; Lu, Y.T.; Wang, W.Y.; Li, Z.M.; Zeng, W.W.; Zeng, X.W.; Ji, X.Y.; Mei, L. Heterojunction engineered bioactive chlorella for cascade promoted cancer therapy. J. Control. Release 2022, 345, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Xi, Y.; Li, S.; Pan, J. Progress on nanocarriers in responcsive to tumor microenvironment. Chem. Ind. Eng. 2021, 38, 80–87. [Google Scholar]
- Jiang, Y.; Zhou, Y.; Zhang, C.Y.; Fang, T. Co-delivery of paclitaxel and doxorubicin by pH-responsive prodrug micelles for cancer therapy. Int. J. Nanomed. 2020, 15, 3319–3331. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Sheng, Y.; Yuan, Y.; Liu, C.; Tao, X.; Shan, X.; Xu, F. In vitro macrophage uptake and in vivo biodistribution of PLA-PEG nanoparticles loaded with hemoglobin as blood substtutes: Effect of PEG content. J. Mater. Sci. Mater. Med. 2009, 20, 1881–1891. [Google Scholar] [CrossRef]
- Chen, Y.; Su, M.; Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Zhou, J.; Liu, J.; Jiang, Z. Enzymatic PEG-poly(amine-co-disulfide ester) nanoparticles as pH- and redox-responsive drug nanocarriers for efficient antitumor treatment. ACS Appl. Mater. Inter. 2017, 9, 30519–30535. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Belkind-Gerson, J.; Chogle, A.; Bhuiyan, M.A.N.; Hicks, T.; Misra, S. Probable neuropsychiatric toxicity of polyethylene glycol: Roles of media, internet and the cargivers. Gastrohep 2019, 1, 118–123. [Google Scholar] [CrossRef]
- Konp, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar]
- Huynh, E.; Zheng, G. Cancer nanomedicine: Addressing the dark side of the enhanced permeability and retention effect. Nanomedicine 2015, 10, 1993–1995. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, Y.; Yang, W.; Niu, P.; Li, X.; Chen, Y.; Li, Z.; Liu, Y.; An, Y.; Liu, Y.; et al. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy. Nano Today 2020, 35, 100970. [Google Scholar] [CrossRef]
- Prasad, R.; Jain, N.K.; Conde, J.; Srivastava, R. Localized nanotheranostics: Recent developments in cancer nanomedicine. Mater. Today Adv. 2020, 8, 100087. [Google Scholar] [CrossRef]
- Huda, S.; Alam, M.A.; Sharma, P.K. Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. J. Drug Deliv. Sci. Tec. 2020, 60, 102018. [Google Scholar] [CrossRef]
- Lebaudy, E.; Fournel, S.; Lavalle, P.; Vrana, N.E.; Gribova, V. Recent advances in antiinflammatory material design. Adv. Healthc. Mater. 2020, 10, 2001373. [Google Scholar] [CrossRef]
- Shahriari, M.; Torchilin, V.P.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. “Smart” self-assembled structures: Toward intelligent dual responsive drug delivery systems. Biomater. Sci. 2020, 8, 5787–5803. [Google Scholar] [CrossRef]
- Chen, W.H.; Sun, Z.; Lu, L.H. Targeted Engineering of medicinal chemistry for cancer therapy: Recent advances and perspectives. Angew. Chem. Int. Edit. 2020, 60, 5626. [Google Scholar] [CrossRef]
- Pan, J.; Li, P.J.; Wang, Y.; Chang, L.; Wan, D.; Wang, H. Active targeted drug delivery of MMP-2 sensitive polymeric nanoparticles. Chem. Commun. 2018, 54, 11092–11095. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-responsive ‘smart’ drug delivery and tumor targeting. Trends in Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef]
- Sehgal, I.; Thompson, T.C. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-β in human prostate cancer cell lines. Mol. Biol. Cell 1999, 10, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Shen, S.; Xu, C.-F.; Li, H.-J.; Liu, Y.; Cao, Z.-T.; Yang, X.-Z.; Xia, J.-X.; Wang, J. Tumor acidity-sensitive polyeric vector for active targeted siRNA deliverty. J. Am. Chem. Soc. 2015, 137, 15217–15224. [Google Scholar] [CrossRef] [PubMed]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterilas for advanced drug delivery. Adv. Drug Deliver Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef]
- Wee Gan, C.; Chien, S.; Feng, S.-S. Nanomedicine: Enhancement of chemotherapeutical efficay of docetaxel by using a biodegradable nanoparticle formulation. Curr. Pharm. Design 2010, 16, 2308–2320. [Google Scholar] [CrossRef]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer-drug conjuagte augments tumor penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Zhang, J.; Sun-Waterhouse, D.; Su, G.; Zhao, M. New insight into umami receptor, umami/umami-enhancing peptides and their derivatives: A review. Trends Food Sci. Technol. 2019, 88, 429–438. [Google Scholar] [CrossRef]
- Wang, Z.R.; Chen, J.W.; Little, N.; Lu, J.Q. Self-assembling prodrug nanotherapeutcs for synergistc tumor targeted drug delvery. Acta Biomater. 2020, 111, 20–28. [Google Scholar] [CrossRef]
- Ghalehshahi, H.G.; Balalaie, S.; Aliahmadi, A. Peptide N-connected to hydroxycoumarin and cinnamic acid derivatives: Synthesis and fluorescene spectroscopic, antioxidant and antimicrobial properties. New J. Chem. 2018, 42, 8831–8842. [Google Scholar] [CrossRef]
- Cheng, W.; Liang, C.; Xu, L.; Liu, G.; Gao, N.; Tao, W.; Luo, L.; Zuo, Y.; Wang, X.; Zhang, X.; et al. TPGS-functonalized polydopamne-modified mesoporous silica as drug nanocarriers for enhacend lung cancer chemotherapy against multidrug resistance. Small 2017, 13, 1700623. [Google Scholar] [CrossRef]
- Han, M.; Huang-Fu, M.-Y.; Guo, W.-W.; Guo, N.-N.; Chen, J.; Liu, H.-N.; Xie, Z.-Q.; Lin, M.-T.; Wei, Q.-C.; Gao, J.-Q. MMP-2-sensitive HA end-conjugated poly(amidoamine) dendrimers via click reaction to enhace drug penetration into solid tumor. ACS Appl. Mater. Inter. 2017, 9, 42459–42470. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Xu, X.; Ma, Y.; Zhang, S.; Zhang, S. RGD peptide-based non-viral gene delivery vectors targeting integrin αvβ3 for cancer therapy. J. Drug Target. 2018, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Zhou, Z.X.; Zhao, Z.H.; Li, Q.Y.; Wu, Y.L.; Yan, S.; Shen, Y.Q.; Huang, P.T. Enzyme-triggered transcytosis of dendrimer-drug conjugate for deep penetration into pancreatic tumors. ACS Nano 2020, 14, 4890–4904. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Liu, C.; Song, H.; Li, X.; Li, N. Solid phase synthesis of marine cyclopeptide phakellistatin 13. Chem. Nat. Comp. 2018, 54, 745–748. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, D.; Liu, Y.; Guo, X.; Zhang, J.; Pan, J. Intelligent Drug Delivery by Peptide-Based Dual-Function Micelles. Int. J. Mol. Sci. 2022, 23, 9698. https://doi.org/10.3390/ijms23179698
Wan D, Liu Y, Guo X, Zhang J, Pan J. Intelligent Drug Delivery by Peptide-Based Dual-Function Micelles. International Journal of Molecular Sciences. 2022; 23(17):9698. https://doi.org/10.3390/ijms23179698
Chicago/Turabian StyleWan, Dong, Yujun Liu, Xinhao Guo, Jianxin Zhang, and Jie Pan. 2022. "Intelligent Drug Delivery by Peptide-Based Dual-Function Micelles" International Journal of Molecular Sciences 23, no. 17: 9698. https://doi.org/10.3390/ijms23179698
APA StyleWan, D., Liu, Y., Guo, X., Zhang, J., & Pan, J. (2022). Intelligent Drug Delivery by Peptide-Based Dual-Function Micelles. International Journal of Molecular Sciences, 23(17), 9698. https://doi.org/10.3390/ijms23179698






