Regulation of Expression of Cannabinoid CB2 and Serotonin 5HT1A Receptor Complexes by Cannabinoids in Animal Models of Hypoxia and in Oxygen/Glucose-Deprived Neurons
Abstract
:1. Introduction
2. Results
2.1. In Vivo HI-Induced Brain Damage
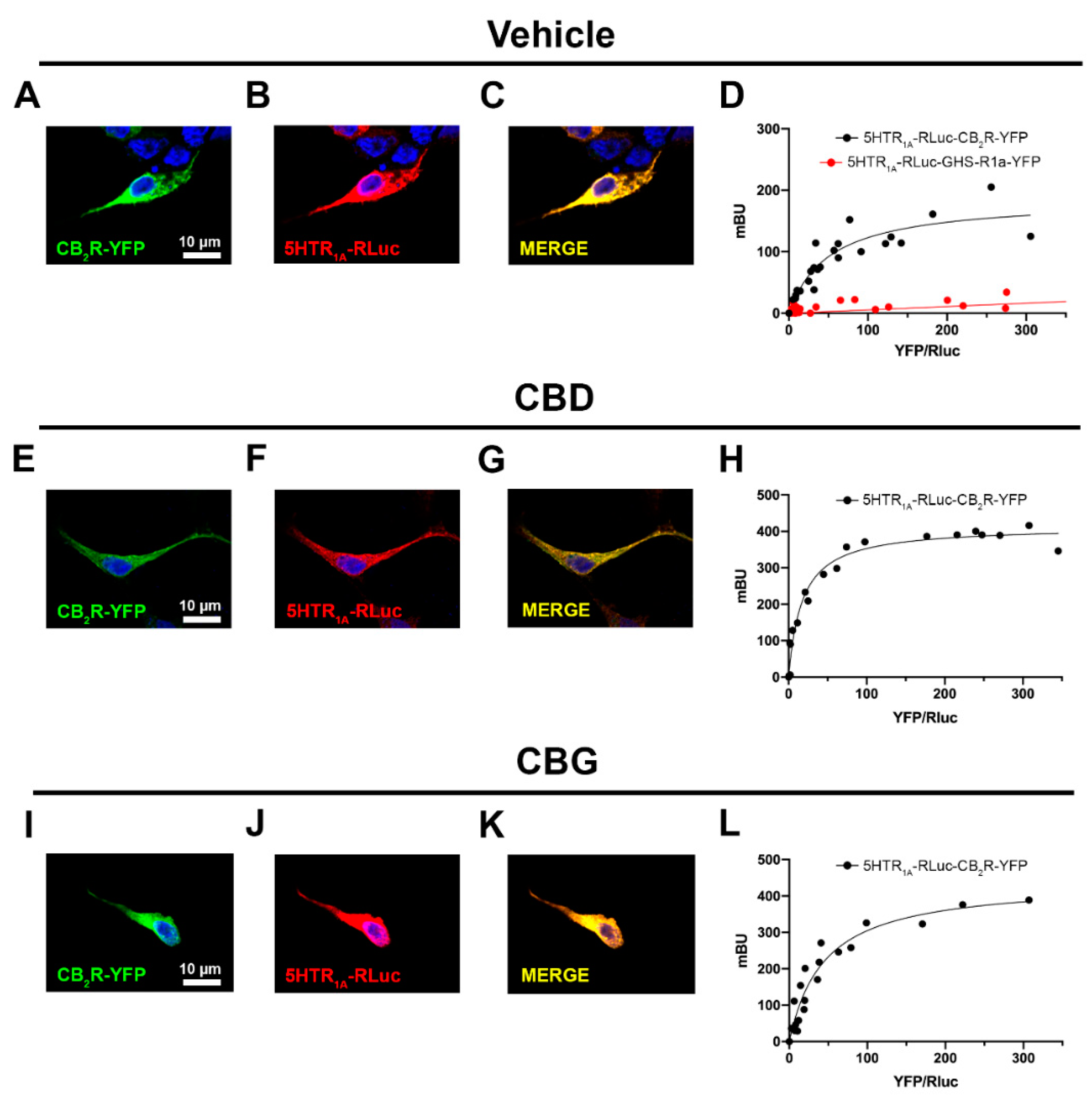
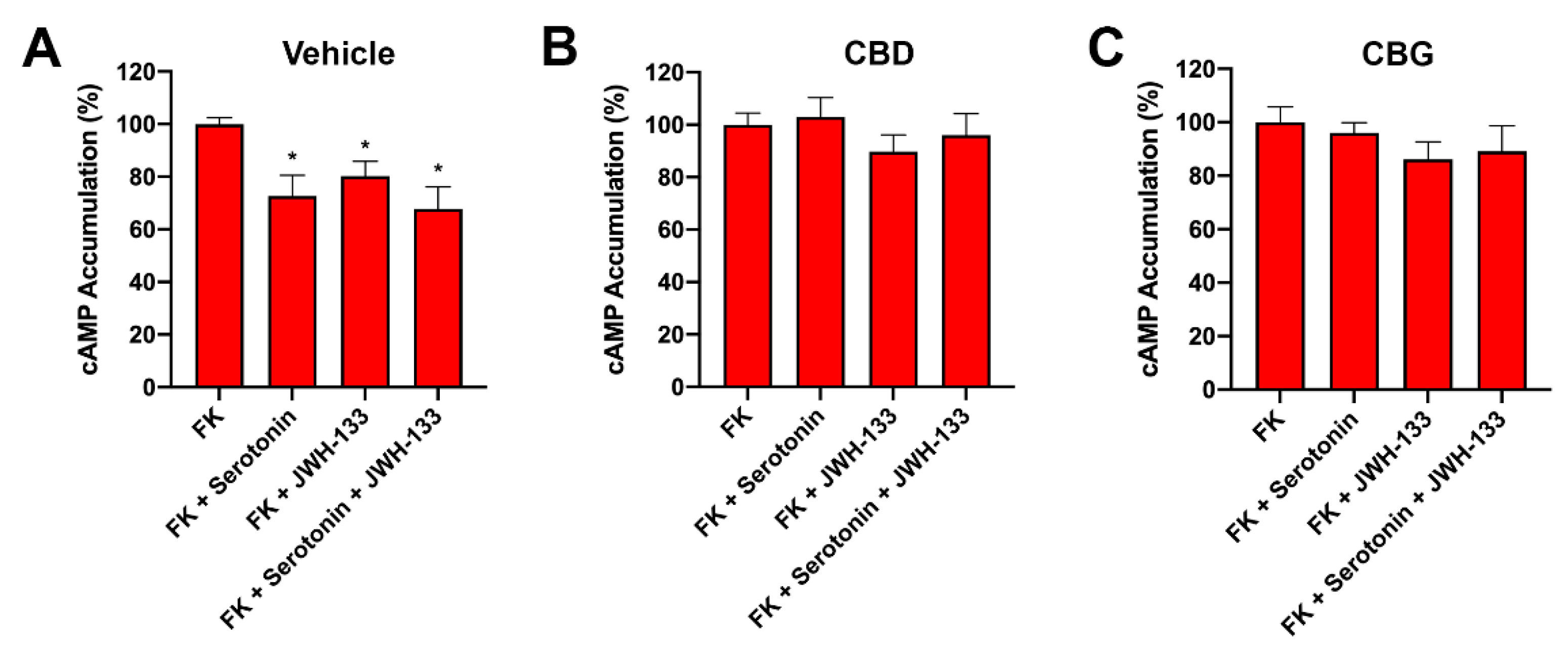
2.2. CBD and CBG Favour CB2–5HT1A Receptor Complex Formation
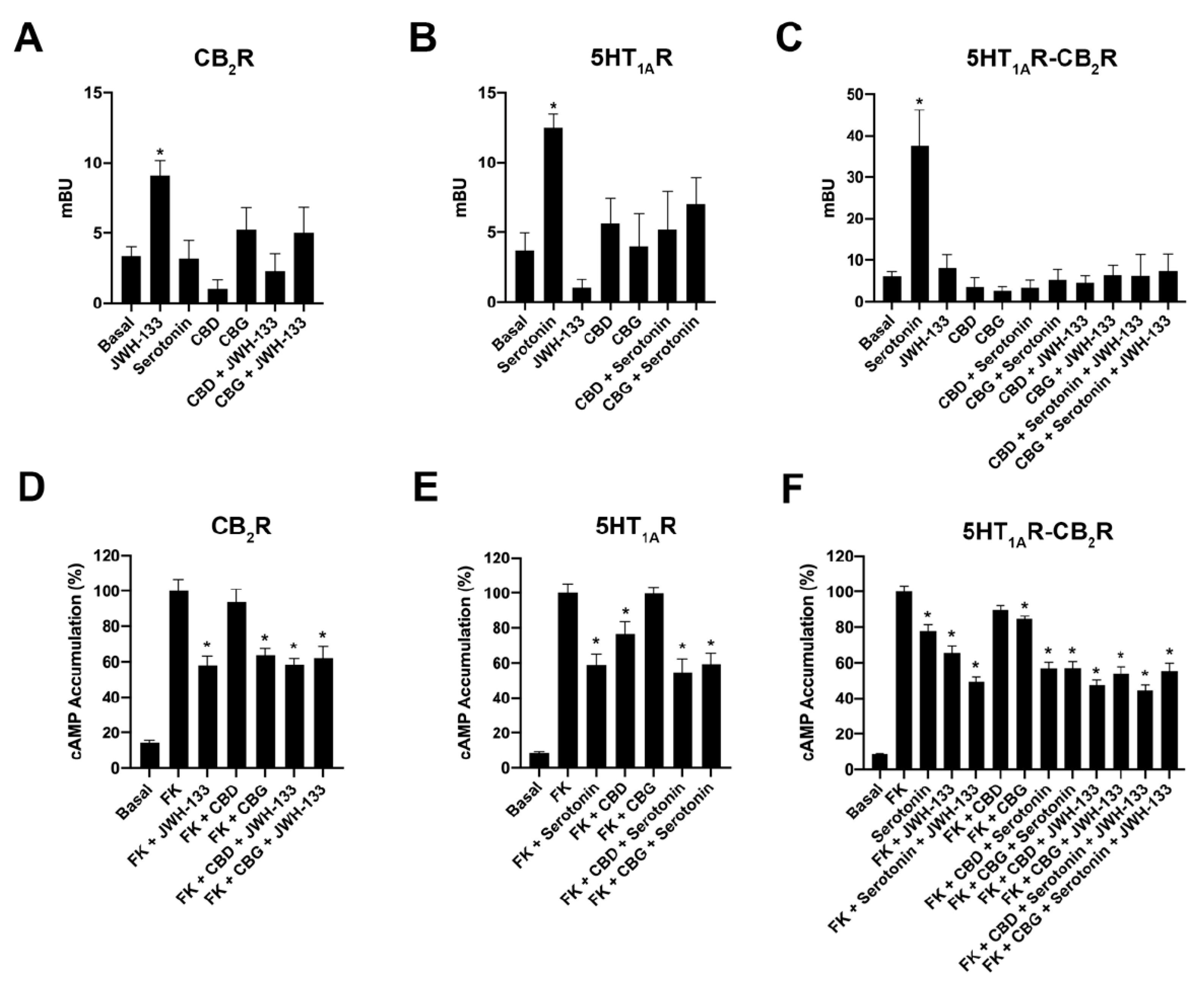
2.3. CBD and CBG Blocked β-Arrestin 2 Recruitment Induced by Serotonin in Cells Expressing CB2–5HT1A-Hets
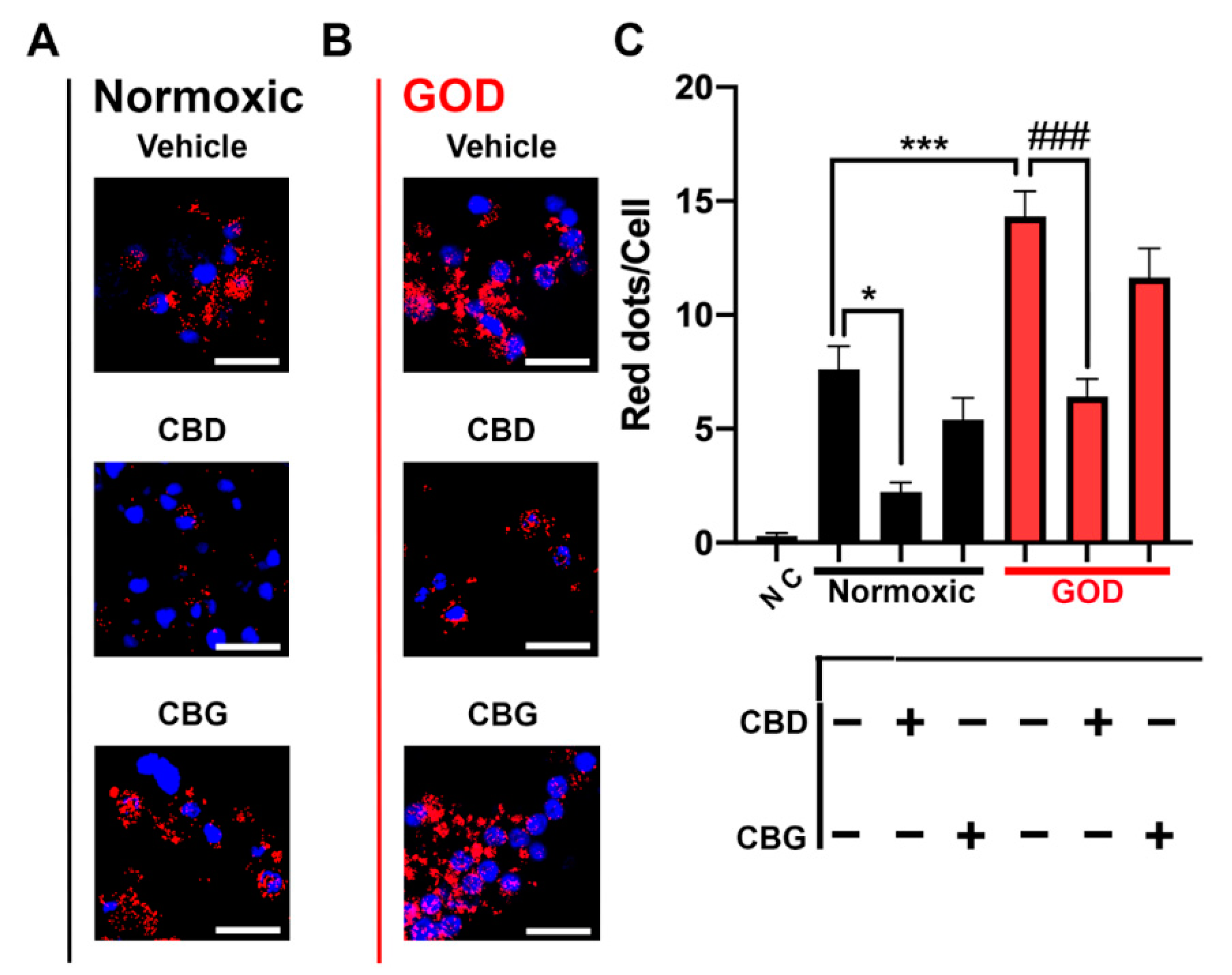
2.4. CB2–5HT1A-Het Expression Was Upregulated in Glucose-Oxygen-Deprived (GOD) Primary Striatal Neurons
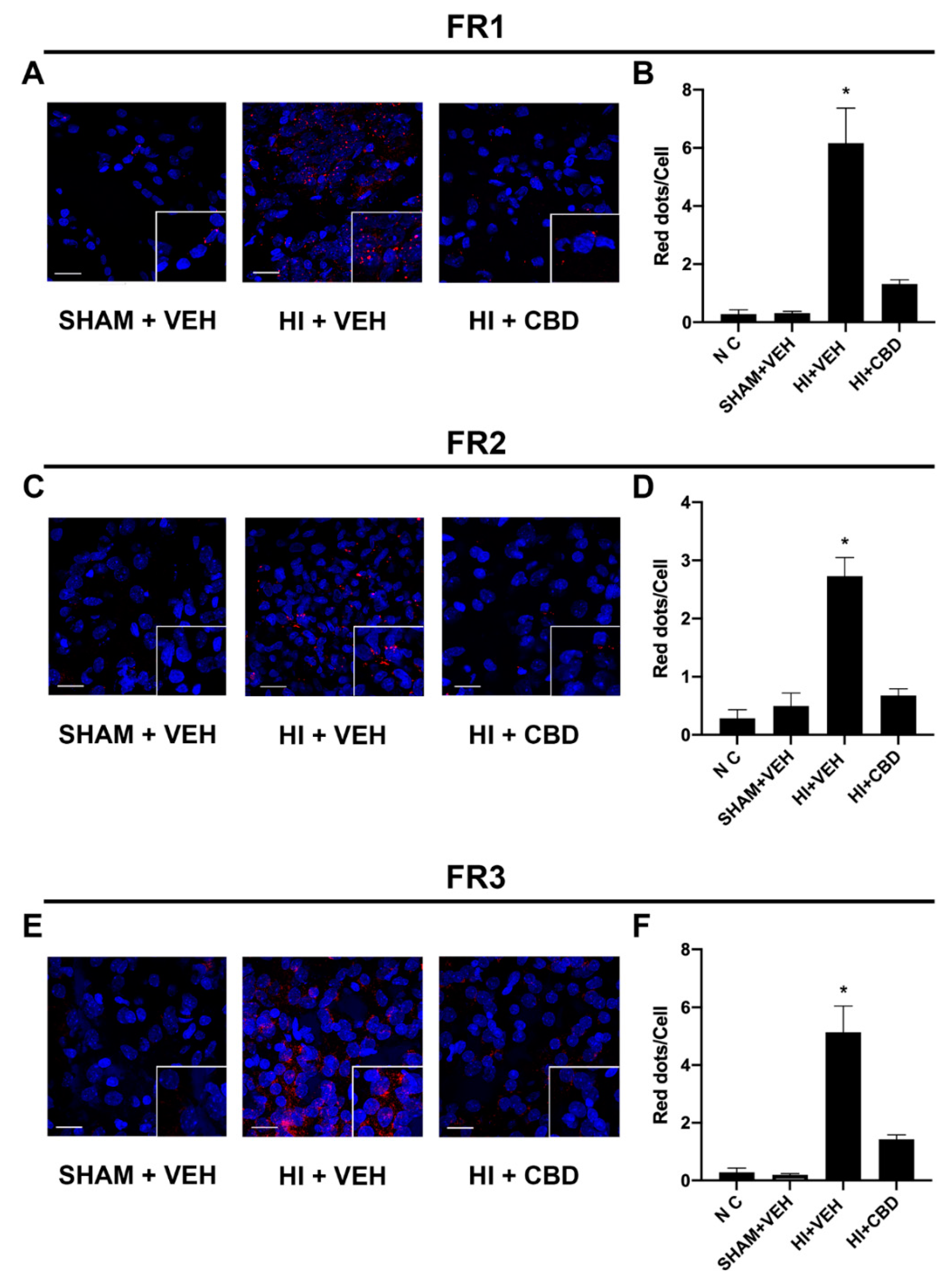
2.5. The CB2–5HT1A-Het Was Overexpressed in Brain Slices from Lesioned Animals
2.6. CBD Abolished CB2–5HT1A-Het Functionality in GOD Striatal and Cortical Neurons
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. HI Brain Damage Induction
4.3. Brain Sampling
4.4. Cell Culture and Transfection
4.5. Neuronal Primary Cultures
4.6. Expression Vectors
4.7. Glucose-Oxygen Deprivation (GOD)
4.8. Immunofluorescence
4.9. Bioluminescence Resonance Energy Transfer (BRET) Assay
4.10. β-Arrestin 2 Recruitment
4.11. cAMP Determination
4.12. Proximity Ligation Assay (PLA)
4.13. Data Handling and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Franco, R.; Rivas-Santisteban, R.; Reyes-Resina, I.; Casanovas, M.; Pérez-Olives, C.; Ferreiro-Vera, C.; Navarro, G.; Sánchez de Medina, V.; Nadal, X. Pharmacological potential of varinic-, minor-, and acidic phytocannabinoids. Pharmacol. Res. 2020, 158, 104801. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Hidalgo, B.; González-Mariscal, I.; García-Martín, A.; Prados, M.E.; Ruiz-Pino, F.; Appendino, G.; Tena-Sempere, M.; Muñoz, E. Δ9-Tetrahydrocannabinolic Acid markedly alleviates liver fibrosis and inflammation in mice. Phytomedicine 2021, 81, 153426. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Moreno-Martet, M.; Rodríguez-Cueto, C.; Palomo-Garo, C.; Gómez-Cañas, M.; Valdeolivas, S.; Guaza, C.; Romero, J.; Guzmán, M.; Mechoulam, R.; et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 2011, 163, 1365–1378. [Google Scholar] [CrossRef]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 2009, 132, 3152–3164. [Google Scholar] [CrossRef]
- Morales, P.; Goya, P.; Jagerovic, N. Emerging strategies targeting CB2 cannabinoid receptor: Biased agonism and allosterism. Biochem. Pharmacol. 2018, 157, 8–17. [Google Scholar] [CrossRef]
- Navarro, G.; Morales, P.; Rodríguez-Cueto, C.; Fernández-Ruiz, J.; Jagerovic, N.; Franco, R. Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front. Neurosci. 2016, 10, 406. [Google Scholar] [CrossRef]
- Bow, E.W.; Rimoldi, J.M. The structure-function relationships of classical cannabinoids: CB1/CB2 modulation. Perspect. Medicin. Chem. 2016, 8, 17–39. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. In Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2017; Volume 103, pp. 61–101. [Google Scholar]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2009, 150, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Kendall, D.A. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010, 215, 611–616. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; del Bel, E.A.; Guimarães, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, F.J.; Lafuente, H.; Rey-Santano, M.C.; Mielgo, V.E.; Gastiasoro, E.; Rueda, M.; Pertwee, R.G.; Castillo, A.I.; Romero, J.; Martínez-Orgado, J. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr. Res. 2008, 64, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, H.; Alvarez, F.J.; Pazos, M.R.; Alvarez, A.; Rey-Santano, M.C.; Mielgo, V.; Murgia-Esteve, X.; Hilario, E.; Martinez-Orgado, J. Cannabidiol Reduces Brain Damage and Improves Functional Recovery After Acute Hypoxia-Ischemia in Newborn Pigs. Pediatr. Res. 2011, 70, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia–ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.R.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: Role of 5HT1A and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Ceprián, M.; Jiménez-Sánchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martínez-Orgado, J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology 2017, 116, 151–159. [Google Scholar] [CrossRef]
- Nelson, K.B. Perinatal ischemic stroke. Stroke 2007, 38, 742–745. [Google Scholar] [CrossRef]
- Hayakawa, K.; Irie, K.; Sano, K.; Watanabe, T.; Higuchi, S.; Enoki, M.; Nakano, T.; Harada, K.; Ishikane, S.; Ikeda, T.; et al. Therapeutic time window of cannabidiol treatment on delayed ischemic damage via high-mobility group box1-inhibiting mechanism. Biol. Pharm. Bull. 2009, 32, 1538–1544. [Google Scholar] [CrossRef]
- Mohammed, N.; Ceprian, M.; Jimenez, L.; Pazos, M.R.; Martinez-Orgado, J. Neuroprotective Effects of Cannabidiol In Hypoxic Ischemic Insult: The Therapeutic Window In Newborn Mice. CNS Neurol. Disord. Drug Targets 2017, 16, 102–108. [Google Scholar] [CrossRef]
- Ferré, S.; Baler, R.; Bouvier, M.; Caron, M.G.; Devi, L.A.; Durroux, T.; Fuxe, K.; George, S.R.; Javitch, J.A.; Lohse, M.J.; et al. Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 2009, 5, 131–134. [Google Scholar] [CrossRef]
- Fuxe, K.; Canals, M.; Torvinen, M.; Marcellino, D.; Terasmaa, A.; Genedani, S.; Leo, G.; Guidolin, D.; Diaz-Cabiale, Z.; Rivera, A.; et al. Intramembrane receptor-receptor interactions: A novel principle in molecular medicine. J. Neural Transm. 2007, 114, 49–75. [Google Scholar] [CrossRef]
- Franco, R.; Casadó, V.; Cortés, A.; Mallol, J.; Ciruela, F.; Ferré, S.; Lluis, C.; Canela, E.I.I. G-protein-coupled receptor heteromers: Function and ligand pharmacology. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S90–S98. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Rico, A.J.; Rivas-Santisteban, R.; Lillo, J.; Roda, E.; Navarro, G.; Lanciego, J.L.; Franco, R. Expression of GPR55 and either cannabinoid CB 1 or CB 2 heteroreceptor complexes in the caudate, putamen, and accumbens nuclei of control, parkinsonian, and dyskinetic non-human primates. Brain Struct. Funct. 2020, 225, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Aguinaga, D.; Navarro, G.; Rico, A.J.; Oyarzábal, J.; Sánchez-Arias, J.A.; Lanciego, J.L.; Franco, R. Targeting CB 1 and GPR55 Endocannabinoid Receptors as a Potential Neuroprotective Approach for Parkinson’s Disease. Mol. Neurobiol. 2019, 56, 5900–5910. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Villa, M.; Morales, P.; Reyes-Resina, I.; Gutiérrez-Rodríguez, A.; Jiménez, J.; Jagerovic, N.; Martínez-Orgado, J.; Navarro, G. Increased expression of cannabinoid CB2 and serotonin 5-HT1A heteroreceptor complexes in a model of newborn hypoxic-ischemic brain damage. Neuropharmacology 2019, 152, 58–66. [Google Scholar] [CrossRef]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casadó, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef]
- Navarro, G.; Reyes-Resina, I.; Rivas-Santisteban, R.; Sánchez de Medina, V.; Morales, P.; Casano, S.; Ferreiro-Vera, C.; Lillo, A.; Aguinaga, D.; Jagerovic, N.; et al. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol. 2018, 157, 148–158. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; da Silva, F.F.; Milani, H.; Guimarães, F.S.; Oliveira, R.M.W. Differential contribution of CB1, CB2, 5-HT1A, and PPAR-γ receptors to cannabidiol effects on ischemia-induced emotional and cognitive impairments. Eur. J. Neurosci. 2021, 53, 1738–1751. [Google Scholar] [CrossRef]
- Mishima, K.; Hayakawa, K.; Abe, K.; Ikeda, T.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Cannabidiol Prevents Cerebral Infarction Via a Serotonergic 5-Hydroxytryptamine1A Receptor-Dependent Mechanism. Stroke 2005, 36, 1071–1076. [Google Scholar] [CrossRef]
- Morales, P.; Gómez-Cañas, M.; Navarro, G.; Hurst, D.P.; Carrillo-Salinas, F.J.; Lagartera, L.; Pazos, R.; Goya, P.; Reggio, P.H.; Guaza, C.; et al. Chromenopyrazole, a Versatile Cannabinoid Scaffold with in Vivo Activity in a Model of Multiple Sclerosis. J. Med. Chem. 2016, 59, 6753–6771. [Google Scholar] [CrossRef]
- Morales, P.; Navarro, G.; Gómez-Autet, M.; Redondo, L.; Fernández-Ruiz, J.; Pérez-Benito, L.; Cordomí, A.; Pardo, L.; Franco, R.; Jagerovic, N. Discovery of Homobivalent Bitopic Ligands of the Cannabinoid CB2 Receptor. Chem. Eur. J. 2020, 26, 15839–15842. [Google Scholar] [CrossRef]
- Navarro, G.; Gonzalez, A.; Sánchez-Morales, A.; Casajuana-Martin, N.; Gómez-Ventura, M.; Cordomí, A.; Busqué, F.; Alibés, R.; Pardo, L.; Franco, R. Design of Negative and Positive Allosteric Modulators of the Cannabinoid CB2 Receptor Derived from the Natural Product Cannabidiol. J. Med. Chem. 2021, 64, 9354–9364. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; de Medina, V.S.; Rivas-Santisteban, R.; Callado, C.S.C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.G.; Limbird, L.E.; Lefkowitz, R.J. Biochemical characterization of the beta-adrenergic receptor of the frog erythrocyte. Mol. Cell. Biochem. 1979, 28, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Engel, J.A.; Jackson, D.M.; Johansson, C.; Svensson, L. (-)Alprenolol potentiates the disrupting effects of dizocilpine on sensorimotor function in the rat. Psychopharmacology 1997, 132, 281–288. [Google Scholar] [CrossRef]
- Bertoni, S.; Arcaro, V.; Vivo, V.; Rapalli, A.; Tognolini, M.; Cantoni, A.M.; Saccani, F.; Flammini, L.; Domenichini, G.; Ballabeni, V.; et al. Suppression of inflammatory events associated to intestinal ischemia-reperfusion by 5-HT1A blockade in mice. Pharmacol. Res. 2014, 81, 17–25. [Google Scholar] [CrossRef]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferré, S.; Casadó, V.; Agnati, L.; Cortés, A.; Mallol, J.; Fuxe, K.; Canela, E.I.E.I.; et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods 2008, 5, 727–733. [Google Scholar] [CrossRef]
- Navarro, G.; Hradsky, J.; Lluís, C.; Casadó, V.; McCormick, P.J.; Kreutz, M.R.; Mikhaylova, M. NCS-1 associates with adenosine A 2A receptors and modulates receptor function. Front. Mol. Neurosci. 2012, 5, 53. [Google Scholar] [CrossRef]
- Hradsky, J.; Raghuram, V.; Reddy, P.P.; Navarro, G.; Hupe, M.; Casado, V.; McCormick, P.J.; Sharma, Y.; Kreutz, M.R.; Mikhaylova, M. Post-translational membrane insertion of tail-anchored transmembrane EF-hand Ca2+sensor calneurons requires the TRC40/Asna1 protein chaperone. J. Biol. Chem. 2011, 286, 36762–36776. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lillo, J.; Raïch, I.; Silva, L.; Zafra, D.A.; Lillo, A.; Ferreiro-Vera, C.; Sánchez de Medina, V.; Martínez-Orgado, J.; Franco, R.; Navarro, G. Regulation of Expression of Cannabinoid CB2 and Serotonin 5HT1A Receptor Complexes by Cannabinoids in Animal Models of Hypoxia and in Oxygen/Glucose-Deprived Neurons. Int. J. Mol. Sci. 2022, 23, 9695. https://doi.org/10.3390/ijms23179695
Lillo J, Raïch I, Silva L, Zafra DA, Lillo A, Ferreiro-Vera C, Sánchez de Medina V, Martínez-Orgado J, Franco R, Navarro G. Regulation of Expression of Cannabinoid CB2 and Serotonin 5HT1A Receptor Complexes by Cannabinoids in Animal Models of Hypoxia and in Oxygen/Glucose-Deprived Neurons. International Journal of Molecular Sciences. 2022; 23(17):9695. https://doi.org/10.3390/ijms23179695
Chicago/Turabian StyleLillo, Jaume, Iu Raïch, Laura Silva, David A. Zafra, Alejandro Lillo, Carlos Ferreiro-Vera, Verónica Sánchez de Medina, José Martínez-Orgado, Rafael Franco, and Gemma Navarro. 2022. "Regulation of Expression of Cannabinoid CB2 and Serotonin 5HT1A Receptor Complexes by Cannabinoids in Animal Models of Hypoxia and in Oxygen/Glucose-Deprived Neurons" International Journal of Molecular Sciences 23, no. 17: 9695. https://doi.org/10.3390/ijms23179695
APA StyleLillo, J., Raïch, I., Silva, L., Zafra, D. A., Lillo, A., Ferreiro-Vera, C., Sánchez de Medina, V., Martínez-Orgado, J., Franco, R., & Navarro, G. (2022). Regulation of Expression of Cannabinoid CB2 and Serotonin 5HT1A Receptor Complexes by Cannabinoids in Animal Models of Hypoxia and in Oxygen/Glucose-Deprived Neurons. International Journal of Molecular Sciences, 23(17), 9695. https://doi.org/10.3390/ijms23179695






