Abstract
Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) are major elements of the innate immune system that recognize pathogen-associated molecular patterns. Single-nucleotide polymorphisms (SNPs) in the TLR, NLR, and RLR genes may lead to an imbalance in the production of pro- and anti-inflammatory cytokines, changes in susceptibility to infections, the development of diseases, and carcinogenesis. Acute myeloid leukemia (AML) is a bone marrow malignancy characterized by uncontrolled proliferation of transformed myeloid precursors. We retrospectively analyzed 90 AML patients. We investigated the effect of fifteen SNPs located in the genes coding for RLR1 (rs9695310, rs10738889, rs10813831), NOD1 (rs2075820, rs6958571), NOD2 (rs2066845, rs2066847, rs2066844), TLR3 (rs5743305, rs3775296, 3775291), TLR4 (rs4986791, rs4986790), and TLR9 (rs187084, rs5743836). We observed that TLR4 rs4986791, TLR9 rs5743836, and NOD2 rs2066847 were associated with CRP levels, while RLR-1 rs10738889 was associated with LDH level. Furthermore, we found TLR3 rs5743305 AA to be more common in patients with infections. We also found TLR9 rs187084 C to be associated with more favorable risk, and RLR-1 rs9695310 GG with higher age at diagnosis. In conclusion, the current study showed that SNPs in the genes encoding TLRs, NLRs, and RLRs may be potential biomarkers in patients with AML.
1. Introduction
Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) play essential roles in mechanisms of innate immunity. They detect pathogen-associated molecular patterns (PAMPs) from bacteria, viruses, fungi, protozoa, and other microorganisms that enter the human body. Detection of pathogen invasion activates intracellular transmission pathways, ultimately leading to increased production of proinflammatory cytokines and infected-cell death, as well as activation of adaptive immunity mechanisms [,,]. In addition, TLRs, NLRs, and RLRs detect host cell damage-associated molecular patterns (DAMPs), which include free nucleic acids, uric acid crystals, heat shock proteins, and many others. Detection of DAMPs leads to the repair and regeneration of damaged tissues []. In addition to their involvement in immune mechanisms, these receptors are also involved in cell differentiation, maturation, apoptosis, and angiogenesis. The proper regulation of TLR, NLR, and RLR receptor activity and suppression of inflammatory response after infection is extremely important because chronic overproduction of proinflammatory cytokines is responsible for the development of chronic inflammatory, metabolic, neurodegenerative, and autoimmune diseases and cancers [,,].
Single-nucleotide polymorphisms (SNPs) in genes encoding TLRs, NLRs, and RLRs can lead to an imbalance in the production of pro- and anti-inflammatory cytokines, changes in susceptibility to certain infections, and the development of allergic and inflammatory diseases, as well as carcinogenesis [,]. In addition, some SNPs are associated with increased resistance of cancer cells to treatment and apoptosis [,]. The search for associations between the occurrence of specific SNPs and the predisposition to develop various cancers and their impact on disease course and prognosis has been ongoing for several years.
Acute myeloid leukemia (AML) belongs to the group of malignant blood cancers and is the most common acute leukemia among adult patients. It leads to the suppression of normal hematopoiesis in the bone marrow and, therefore, to an increased risk of severe infections, anemia, and thrombocytopenia. AML is heterogeneous and differs in its course, degree of resistance to treatment, ability of blasts to cross the blood–marrow barrier, and formation of extramedullary metastases. Prognosis depends on the presence of specific molecular and cytogenetic mutations in tumor cells, and 5-year patient survival rates range from 20–30% []. Infections associated with neutropenia after chemotherapy are a major problem during AML therapy. It is not uncommon for patients to develop sepsis and septic shock that leads to death. Therefore, it is important to identify patients who are at a high risk of severe infections and to apply infection prevention and treatment measures. In order to achieve better AML treatment outcomes, an individualized approach to the patient is necessary, taking into account factors that may affect the course of therapy.
The aim of this study was to present 15 SNPs in TLRs, NLRs, and RLRs encoding genes in patients with de novo diagnosed AML and their association with clinical features such as infection rate, blood CRP level, cytogenetic risk according to the European Leukemia Net (ELN), and tumor burden (number of blasts in the bone marrow and presence of extramedullary metastases, blood LDH levels) at diagnosis, as well as their relationship with patients’ age and sex. Due to heterogeneity of the course, response to treatment, prognosis, and the survival of young and elderly patients diagnosed with AML, at a later stage of the study, we analyzed the obtained results of two age groups: >55 years of age and ≤55 years of age.
2. Results
2.1. SNPs in Toll-like Receptors Genes
We found that AML patients with favorable or intermediate risk at diagnosis (according to ELN) were more likely to have allele rs187084 C in the TLR9 gene than patients with unfavorable risk (p = 0.012), Figure 1. Patients with rs4986791 T in the TLR4 gene had lower CRP levels than CC homozygotes (p = 0.015), with rs4986791 T in the TLR4 gene being more common in those with CRP levels < 5 mg/L (p = 0.028), Figure 2. Another polymorphism associated with lower CRP levels was rs5743836 C in the TLR9 gene. Carriers of this SNP had lower serum CRP than rs5743836 TT homozygotes (p = 0.048), Figure 3. Additionally, patients with rs3775291 G in the TLR3 gene tended to have higher LDH levels at diagnosis than rs3775291 AA homozygotes (p = 0.053). Infectious complications (all types of bacterial, viral, and fungal infections except for SARS-CoV-2 infections) during AML therapy were observed more frequently in TLR3 rs5743305 AA homozygotes (p = 0.015), Figure 4. The overall survival (OS) analysis showed no statistically significant differences in OS among patients with TLR3 rs5743305 AA genotype and the TLR3 rs5743305 AT and TT genotypes—in the group with infections during AML treatment or in the group without infections (p-value, 0.711 and 0.465, respectively), Figure 5 and Figure 6. Extramedullary metastases of AML tended to be more common in patients who carry rs3775296 T in the TLR3 gene and rs4986790 G in the TLR4 gene (p = 0.054 and p = 0.078, respectively). Patients who were diagnosed with disease metastases outside the bone marrow at the time of diagnosis tended to be less likely than patients with disease localized to the bone marrow to carry rs3775291 A in the TLR3 gene and rs187084 C in the TLR9 gene (p = 0.068 and p = 0.053, respectively). A summary of the correlations between different SNPs in genes coding for TLR3, TLR4, and TLR9 and clinical features in patients with AML is presented in Table 1. For statistically significant results, bold font is used.
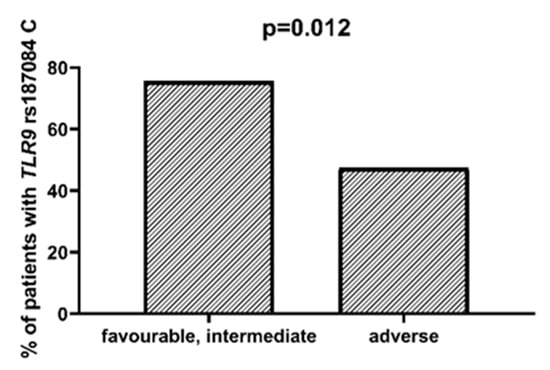
Figure 1.
Association between TLR9 rs187084 C and risk according to ELN.
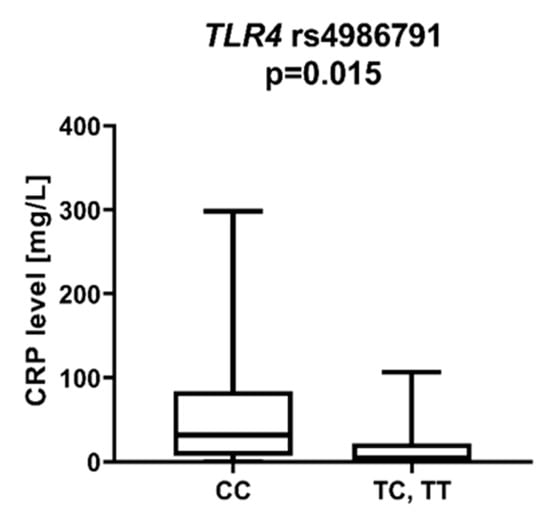
Figure 2.
CRP level in patients with and without TLR4 rs4986791 T.
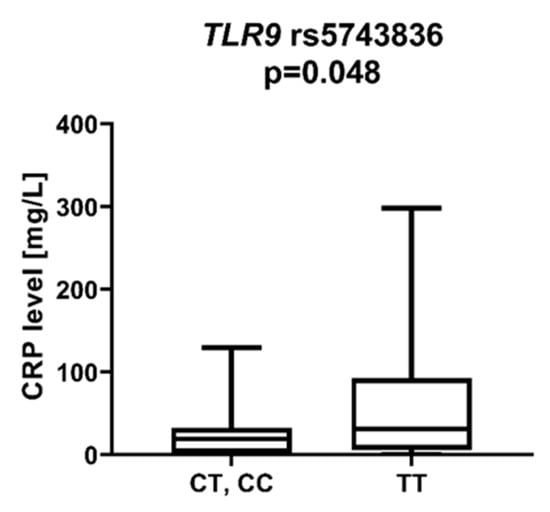
Figure 3.
CRP level in patients with and without TLR9 rs5743836 C.
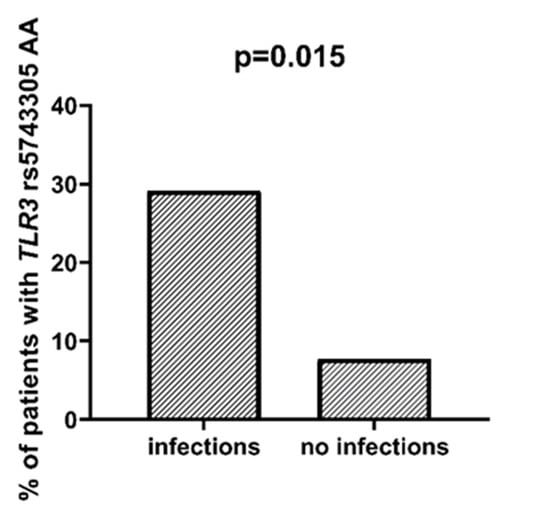
Figure 4.
Association between TLR3 rs5743305 AA and incidence of infection.
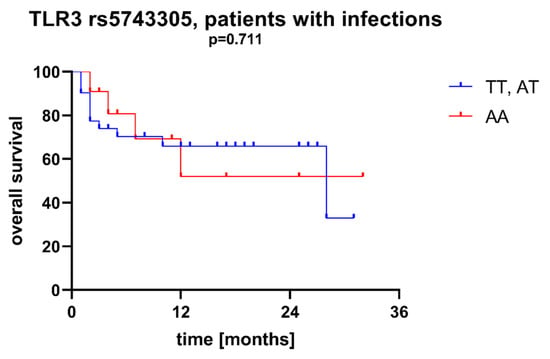
Figure 5.
Overall survival of patients with different SNPs in TLR3 rs5743305—patients with infections during AML therapy.
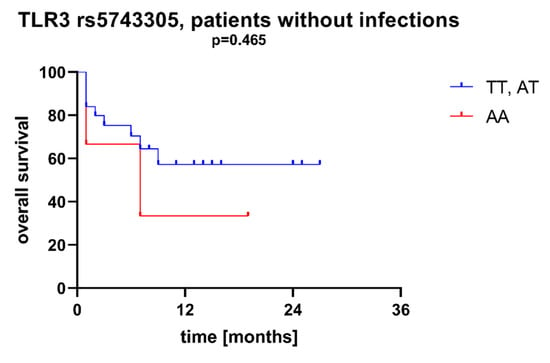
Figure 6.
Overall survival of patients with different SNPs in TLR3 rs5743305—patients without infections during AML therapy.

Table 1.
Summary of correlations between SNPs in genes coding for TLR3, TLR4, and TLR9 and different clinical features in patients with AML. For statistically significant results (p < 0.05), bold font is used.
2.2. SNPs in NOD-like Receptors Genes
We observed a statistically significant difference in patients with the NOD2 rs2066847 ins variant (ins/del, ins/ins), who showed lower CRP protein levels at diagnosis than patients with rs2066847 del/del (p = 0.045), Figure 7. There was also a trend for higher LDH levels in patients with NOD2 rs2066844 T compared to patients without the T allele (p = 0.062).
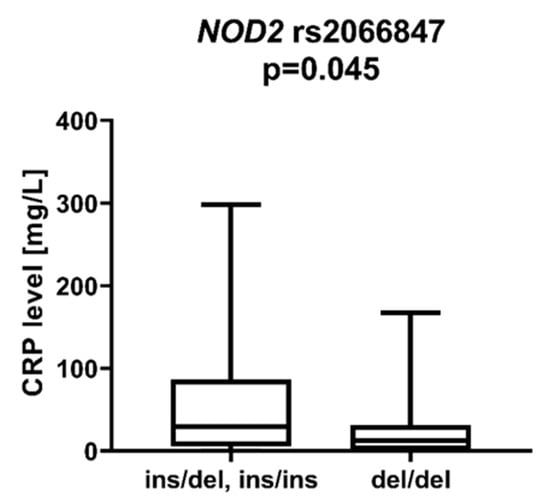
Figure 7.
CRP level in patients with and without rs2066847 insertion in NOD2 gene.
2.3. SNPs in the RIG-I-like Receptors Genes
DDX58 is a gene encoding the DeXD/H-Box Helicase 58 or RIG-I receptor. Statistically significant associations were observed between different SNPs and clinically relevant features of AML. We observed that the rs10738889 G allele was associated with lower LDH levels than rs10738889 AA (p = 0.027, Figure 8). There was also a trend showing a difference in LDH levels in patients with rs10813831 G compared to patients with the AA genotype (p = 0.056). Regarding CRP levels, we observed that DDX58 rs10813831 A tended to be less common in patients with CRP < 5 than in those with CRP > 5 (p = 0.098). The rs10738889 A allele was more common in male than in female AML patients (p = 0.033; Figure 9), and the rs9695310 C variant was associated with lower age at diagnosis than GG, Figure 10. Furthermore, while not statistically significant, a difference in the number of blast cells in the bone marrow at the diagnosis of AML was observed. A higher percentage of blast cells was present in patients with the rs10813831 G allele than rs10813831 AA (p = 0.065), while a lower percentage of blast cells was present in patients with rs10738889 G than rs10738889 AA (p = 0.082).
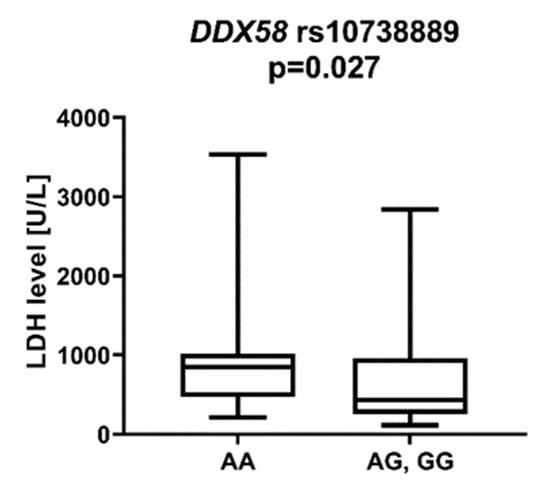
Figure 8.
LDH level in patients with DDX58 rs10738889 G allele.
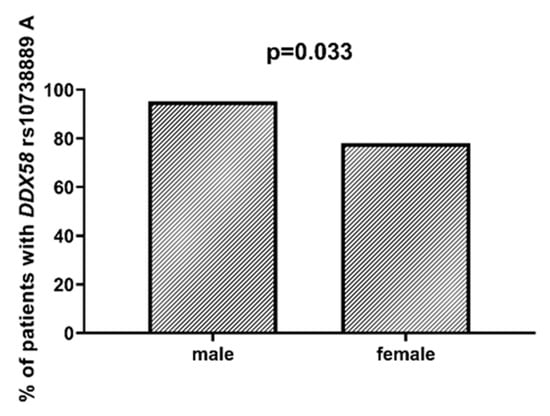
Figure 9.
Association between DDX58 rs10738889 A and gender.
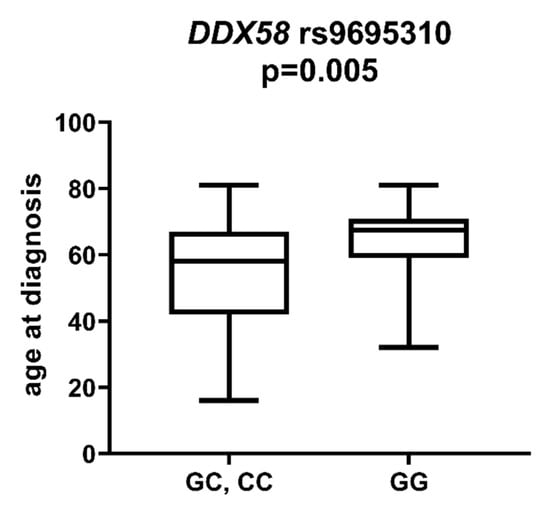
Figure 10.
Age at diagnosis in patients with and without DDX58 rs9695310 C allele.
2.4. SNPs in TLRs, NLRs, and RLRs in Groups of Patients >55 yo and ≤55 yo
After dividing the study group into a group of younger (≤55 years old) and older (>55 years old) patients, the statistical analysis showed the following associations: DDX58 rs10813831 A was more common among patients with infections than without infections in the younger (p = 0.046) but not in the elderly (p = 0.566) group. TLR4 rs4986791 T was associated with a higher LDH level than CC (p = 0.044) among young but not in elderly patients (p = 0.171). The presence of extramedullary metastases among young patients was associated with a higher prevalence of genotypes TLR3 rs3775296 T (p = 0.036) and TLR4 rs4986790 G (p = 0.020). These correlations did not occur in the elderly group (p = 0.593 and p = 0.593, respectively). Additionally, TLR9 rs187084 C was more common in patients without extramedullary lesions in younger patients (p = 0.035), but not in older patients (p = 0.623). In the group of elderly patients, we showed statistically significant associations: TLR9 rs187084 C was more common among patients with favorable and intermediate risk according to ELN (p = 0.005); TLR4 rs4986791 T and TLR9 rs5743836 C were associated with lower CRP level at diagnosis of AML (p = 0.009 and p = 0.003, respectively). In the group of younger patients, the above associations were not statistically significant (p = 0.481, p = 0.204, and p = 0.523, respectively).
3. Discussion
The results of our study presented here are among the first to describe so extensively the associations between SNPs in genes encoding pattern recognition receptors belonging to the innate immune system and clinical features of patients with AML. Currently, little is known about the impact of SNPs in genes encoding TLRs, NLRs, and RLRs on the course and prognosis of hematologic malignancies. M. Quirino et al. [] studied the effect of polymorphisms in TLRs genes on the development of myeloproliferative neoplasms (MPNs). In that study, they found that TLR9-1486 CT may be associated with a lower susceptibility to developing polycythemia vera (PV), and in the haplotype frequency analysis, TLR9-1237T/-1486C was less common in men compared to controls, as well as in men negative for JAK V617F. Y. Zhou et al. [] showed that inflammasome-related genes (NLRP3, NF-κB1, CARD8, IL-1β, and IL-18) were highly expressed in patients with MPNs and that the NF-κB1 polymorphism (rs28362491) was more common in MPN patients than in the study group. The effects of SNPs on the NLRP3 inflammasome system have also been studied as risk factors for the development and severity of AML. In a study by H. Wang et al. [], IL-1B (rs16944) GA was more prevalent in patients with cytogenetic favorable risk, while the number of bone marrow blasts in patients with IL-18 (rs1946518) GG or GT genotypes was higher than in TT patients. Furthermore, the IL-18 rs1946518 GT genotype was statistically significantly associated with worse AML overall survival.
Patients diagnosed with AML and undergoing chemotherapy are a group of patients at particularly high risk of developing bacterial and fungal infections. Approximately 80% of patients develop neutropenic fever (NF) during AML therapy, with the etiologic agent being detected in less than 50% of cases []. The most commonly observed infectious complications are pneumonia, gastrointestinal infections, urinary tract infections, and central vascular catheter-related infections []. Invasive fungal infections are a major problem when treating patients with AML. Their occurrence is favored by prolonged neutropenia, older patient age, and low albumin levels []. The treatment of fungal infections in the era of new anticancer drugs poses many difficulties due to the interaction of most new molecules with antifungal drugs []. Mechanisms of innate immunity are the body’s first line of defense against microbial invasion; their efficient functioning provides protection against the development of severe infections. In the present study, a significant statistical association between the TLR3 rs5743305 AA genotype and an increased frequency of infectious complications (non-SARS-CoV-2 infections) was detected. The TLR3 receptor is responsible for recognizing viral dsRNA in cell endosomes. TLR3 activation leads to the production of increased amounts of interferon type I and the death of infected cells []. TLR3 rs5743305 homozygosity may cause malfunction and make cells much more susceptible to viral infections. Antiviral prophylaxis should be implemented in this group of patients, and they should be closely monitored for the development of viral infections that can lead to the patient’s death.
Age at diagnosis is a clinically relevant parameter that indirectly influences the treatment plan of AML patients. In younger patients, intensive chemotherapy protocols are generally possible, whereas in older patients (>65–70 years of age), less intensive regimens are applicable []. In a study [] of 13,283 patients diagnosed with AML, Acharya et al. showed that older patient age and male gender were associated with worse 3-year overall survival in univariate analysis, and these parameters remained independent prognostic factors in multivariate analyses. In our study group, the mean age was 56 years. Patients with the DDX58 rs9695310 CC genotype had the lowest age at diagnosis (51 years), GC heterozygotes 55 years, and GG homozygotes 64 years. Polymorphisms in the NOD1 and NOD2 genes (rs6958571 AA and rs2066847 ins, respectively) also showed an association with lower patient age at AML diagnosis, but this value was not statistically significant. Regarding gender, we observed that the DDX58 rs10738889 A allele was more frequently present among male than female patients with AML.
Moreover, we found that the TLR9 rs187084 C allele was less frequent among patients with unfavorable cytogenetic and molecular prognosis than in other risk groups. Patients with unfavorable risk according to ELN are a group that requires particularly intensive treatment, as a resistance to chemotherapy and rapid relapse are more common [,].
The number of blasts in the bone marrow at diagnosis of AML does not show a significant impact on disease course, treatment, and prognosis. Nevertheless, we observed an association between individual polymorphisms in the DDX58 gene and a higher percentage of blasts in the bone marrow at diagnosis. Patients with the DDX58 rs10813831 G allele and DDX58 rs10738889 AA genotypes tended to have a higher percentage of tumor cells in the bone marrow than carriers of DDX58 rs10813831 AA and DDX58 rs10738889 G. The DDX58 gene encodes the intracellular receptor RIG-I, which is responsible for detecting foreign double-stranded RNA (dsRNA). Activation of RIG-I leads to the increased production of proinflammatory cytokines (mainly interferon) as well as inflammasome formation and the induction of pyroptosis-induced death of infected cells [,]. The aforementioned SNPs in the DDX58 gene may be associated with a predisposition to increased and prolonged production of proinflammatory cytokines, which stimulate bone marrow stem cells to mutate into cancer cells and create the right conditions in the bone marrow microenvironment for mutant cells to proliferate [,].
In our present study, AML extramedullary involvement (EMI) metastases were found in 13 patients (14% of all patients). EMI metastases are most commonly found in the skin, central nervous system, lymph nodes, and less commonly, in internal organs and bones. L. Fianchi et al. [] described factors that increase the risk of extramedullary metastases in AML. EMI was more common in patients with monocytic and myelomonocytic AML subtypes, and blasts were characterized by a lack of CD117 on immunophenotyping; molecularly, MLL gene (mixed-lineage leukemia gene) rearrangement was most commonly diagnosed, and cytogenetically, trisomy of chromosome 8 was most commonly detected. The same study confirmed an unfavorable prognosis in patients with extramedullary metastases with a median survival of 11.6 months. The factors that improve the survival of patients with EMI are intensive chemotherapy treatment, achieving complete remission, and performing an alloHSCT procedure. Our analysis found an association between SNPs in genes encoding TLR3, TLR4, and TLR9 receptors and the frequency of extramedullary metastases. The TLR3 rs3775296 T allele and the TLR4 rs4986790 G allele were more frequent in patients with EMI than in those without extramedullary metastases, whereas the TLR3 3775291 A allele and the TLR9 rs187084 C allele tended to be less frequent in patients with EMI than in those without extramedullary manifestations of AML. Statistical significance was not demonstrated for all determinations. The study group size would need to be increased to confirm these preliminary correlations.
CRP is an acute-phase protein whose serum levels correlate closely with the severity of the inflammatory response. Inflammation has been a known carcinogen for many years. It contributes to tumor growth, cancer cell invasion, migration, and metastasis []. Shrotriya et al. [] analyzed 271 articles on the correlation of CRP levels with prognosis in patients with solid tumors. High CRP levels were associated with increased mortality in 90% of cases, especially among patients with gastrointestinal and renal cancers. In addition, high CRP levels were associated with poor response to treatment and increased relapse rates. We found much less data on the association of CRP levels with prognosis for patients with AML. Gradel et al. [] found that high CRP levels at the time of AML diagnosis are associated with poorer patient general status. Patients in WHO 3/4 general status had 68 mg/L higher CRP levels than patients in WHO 0 status. We observed associations between the TLR4 rs4986791 T allele and the TLR9 rs5743836 C allele and lower CRP levels compared to TLR4 rs4986791 CC and TLR9 rs5743836 TT. Among NOD-like receptors, a statistically significant correlation was described for NOD2 rs2066847 ins, which was associated with lower CRP than NOD2 rs2066847 del/del. The DDX58 rs10813831 A allele tended to be more frequent in patients with CRP > 5 mg/dL at AML diagnosis, although this was not statistically significant.
Lactate dehydrogenase (LDH) is an enzyme released from ruptured cells. In aggressive diseases with rapid cell turnover, blood LDH levels increase significantly. High LDH levels at diagnosis have been described as a factor for poor prognosis in patients with AML [,], correlated with shorter 1-year overall survival and increased 60-day mortality compared to patients with slightly elevated blood LDH levels. In the present study, we found that the DDX58 rs10738889 G allele was associated with lower LDH levels than rs1078889 AA. Similarly, DDX58 rs10738889 GG was associated with lower LDH levels than rs10738889 AG/AA. Furthermore, we observed a trend of higher LDH levels in patients with the DDX58 rs10813831 G allele, NOD2 rs2066844 T allele, and TLR3 3775291 G allele. Patients with high LDH levels should be closely monitored and may require more intensive treatment protocols.
4. Materials and Methods
4.1. Subjects
In our study, we retrospectively analyzed 90 patients diagnosed with AML treated at the Department of Hematology, Blood Neoplasms and Bone Marrow Transplantation of Wroclaw Medical University between 2018 and 2020. There were 42 men and 48 women in the study group. The median age was 61 years (range 21–81 years), and the number of patients aged over 55 years was 54. All patients suffered from primary AML. The characteristics of the study group can be found in Table 2. The research was approved by the Bioethics Committee at the Wroclaw Medical University. All participants signed informed consent forms to participate in the study.

Table 2.
Study group characteristics. LDH—lactate dehydrogenase, ELN—European Leukemia Net.
4.2. DNA Isolation, Genotyping
Genomic DNA was isolated from peripheral blood taken on EDTA from 90 AML patients using the commercial GeneMATRIX Quick Blood DNA Purification kit from EurX (Gdańsk, Poland) and NucleoSpin Blood kits (MACHEREY-NAGEL GmbH & Co. KG, Dueren, Germany) according to the manufacturers’ protocols. DNA concentration and purity were assessed on the DeNovix DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA). Isolated DNA was stored at −20 °C for further use.
Patients were genotyped for the DDX58 (rs9695310, rs10738889, rs10813831), NOD1 (rs2075820, rs6958571), NOD2 (rs2066845, rs2066847, rs2066844), TLR3 (rs5743305, rs3775296, 3775291), TLR4 (rs4986791, rs4986790), and TLR9 (rs187084, rs5743836) genetic variants using LightSNiP assays (TIB MOLBIOL, Berlin, Germany), and real-time PCR was performed on a LightCycler 480 II device (Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturers’ instructions.
4.3. Statistical Analysis
Statistical analysis of the results obtained was performed using the Real Statistics Resource Pack for Microsoft Excel 2013 (version 15.0.5023.1000, Microsoft, Redmont, WA, USA). Mann–Whitney U test was used to determine the associations between individual SNPs and CRP level, LDH level, and blast cell count in the bone marrow, while Fisher’s exact test was used to determine the associations between different SNPs and age of onset, presence of extramedullary metastases, presence of infection during treatment, and to illustrate the associations of SNPs with risk groups according to ELN. Kaplan–Meier curves and the Gehan-Breslow-Wilcoxon test were used for overall survival analysis. p-values < 0.05 were regarded as statistically significant. Graphs showing the obtained results were created using GraphPad Prism (GraphPad Software, La Jolla, CA, USA, version 8.0.1).
5. Conclusions
In conclusion, we demonstrated correlations between various SNPs in genes encoding Toll-like, NOD-like, and RIG-I-like receptors and specific clinical features of AML patients that may affect prognosis. These polymorphisms may be used as prognostic markers in AML in the future, but their potential implementation into clinical practice requires further studies on larger groups of patients.
Author Contributions
Conceptualization: K.W.-P., K.B.-K. and J.R.; methodology: K.W.-P. and J.R.; software: P.Ł. and M.D.; formal analysis: B.J., P.Ł., M.D. and K.B.-K.; investigation: K.W.-P., B.K., J.R. and T.W.; resources: B.J., P.Ł. and M.D.; data curation: K.W.-P., B.K., J.R. and P.Ł.; writing—original draft preparation: K.W.-P. and J.R.; writing—review and editing: J.R., T.W. and K.B.-K.; visualization: P.Ł.; supervision: J.R., T.W. and K.B-K.; project administration: K.W-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Health subvention according to number STM.C140.18.028 from the IT Simple system of Wroclaw Medical University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Wroclaw Medical University for studies involving humans (No KB-574/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-like receptors in infection, immunity, and diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Origin and List of DAMPS. Immune Netw. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Ponzetta, A.; Inforzato, A.; Jaillon, S. Innate Immunity, Inflammation and Tumor Progression: Double Edged Swords. J. Intern. Med. 2019, 285, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Cen, X.; Liu, S.; Cheng, K. The role of toll-like receptor in inflammation and tumor immunity. Front. Pharmacol. 2018, 9, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Q.; Liu, L.; Liu, Y.; Zhang, K. TLR-2 gene polymorphisms and susceptibility to cancer: Evidence from meta-analysis. Genet. Test. Mol. Biomark. 2013, 17, 864–872. [Google Scholar] [CrossRef]
- Mukherjee, S.; Huda, S.; Sinha Babu, S.P. Toll-like receptor polymorphism in host immune response to infectious diseases: A review. Scand. J. Immunol. 2019, 90, e12771. [Google Scholar] [CrossRef] [Green Version]
- Trejo-De La, O.A.; Hernández-Sancén, P.; Maldonado-Bernal, C. Relevance of single-nucleotide polymorphisms in human TLR genes to infectious and inflammatory diseases and cancer. Genes Immun. 2014, 15, 199–209. [Google Scholar] [CrossRef]
- Medvedev, A.E. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J. Interf. Cytokine Res. 2013, 33, 467–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell, L.F.; Cook, R.J. Advances in acute myeloid leukemia. BMJ 2021, 375, n2026. [Google Scholar] [CrossRef] [PubMed]
- Quirino, M.G.; Macedo, L.C.; Pagnano, K.B.B.; Pagliarini-e-Silva, S.; Sell, A.M.; Visentainer, J.E.L. Toll-like receptor gene polymorphisms in patients with myeloproliferative neoplasms. Mol. Biol. Rep. 2021, 48, 4995–5001. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, S.; Liu, N.; He, N.; Zhang, A.; Meng, S.; Ji, C.; Ma, D.; Ye, J. Genetic polymorphisms and expression of NLRP3 inflammasome-related genes are associated with Philadelphia chromosome-negative myeloproliferative neoplasms. Hum. Immunol. 2020, 81, 606–613. [Google Scholar] [CrossRef]
- Wang, H.; Hua, M.; Wang, S.; Yu, J.; Chen, C.; Zhao, X.; Zhang, C.; Zhong, C.; Wang, R.; He, N.; et al. Genetic polymorphisms of IL-18 rs1946518 and IL-1β rs16944 are associated with prognosis and survival of acute myeloid leukemia. Inflamm. Res. 2017, 66, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.; Koura, D.; Taplitz, R. Updates in infection risk and management in acute leukemia. Hematology (U. S.) 2020, 20, 135–139. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, M.; Singh, H.; Kumar, L.; Sharma, A.; Bakhshi, S.; Raina, V.; Thulkar, S. Infections in acute myeloid leukemia: An analysis of 382 febrile episodes. Med. Oncol. 2010, 27, 1037–1045. [Google Scholar] [CrossRef]
- Nganthavee, V.; Phutthasakda, W.; Atipas, K.; Tanpong, S.; Pungprasert, T.; Dhirachaikulpanich, D.; Krithin, S.; Tanglitanon, S.; Jutidamronphang, W.; Owattanapanich, W.; et al. High incidence of invasive fungal infection during acute myeloid leukemia treatment in a resource-limited country: Clinical risk factors and treatment outcomes. Support. Care Cancer 2019, 27, 3613–3622. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Kadia, T.M.; DiNardo, C.D.; Welch, M.A.; Ravandi, F. Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach. Cancer 2021, 127, 1186–1207. [Google Scholar] [CrossRef]
- Acharya, U.H.; Halpern, A.B.; Wu, Q.; Voutsinas, J.M.; Walter, R.B.; Yun, S.; Kanaan, M.; Estey, E.H. Impact of region of diagnosis, ethnicity, age, and gender on survival in acute myeloid leukemia (AML). J. Drug Assess. 2018, 7, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Rintahaka, J.; Wiik, D.; Kovanen, P.E.; Alenius, H.; Matikainen, S. Cytosolic Antiviral RNA Recognition Pathway Activates Caspases 1 and 3. J. Immunol. 2008, 180, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Karin, M.; Sun, B. Targeting cancer-promoting inflammation—Have anti-inflammatory therapies come of age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Fianchi, L.; Quattrone, M.; Criscuolo, M.; Bellesi, S.; Dragonetti, G.; Maraglino, A.M.E.; Bonanni, M.; Chiusolo, P.; Sica, S.; Pagano, L. Extramedullary Involvement in Acute Myeloid Leukemia. A Single Center Ten Years’ Experience. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021030. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; O’Brien, P.E.; Ghatak, S. Inflammation and Cancer. Wound Heal. Stem Cells Repair Restor. Basic Clin. Asp. 2018, 420, 239–274. [Google Scholar] [CrossRef]
- Shrotriya, S.; Walsh, D.; Bennani-Baiti, N.; Thomas, S.; Lorton, C. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: A systematic review. PLoS ONE 2015, 10, e0143080. [Google Scholar] [CrossRef]
- Gradel, K.O.; Póvoa, P.; Garvik, O.S.; Vinholt, P.J.; Nielsen, S.L.; Jensen, T.G.; Chen, M.; Dessau, R.B.; Møller, J.K.; Coia, J.E.; et al. Longitudinal trajectory patterns of plasma albumin and C-reactive protein levels around diagnosis, relapse, bacteraemia, and death of acute myeloid leukaemia patients. BMC Cancer 2020, 20, 249. [Google Scholar] [CrossRef]
- Shaaban, Y.; Taalab, M.M.; Aref, S.; Mabed, M. AML-029: The Prognostic Significance of Serum Lactate Dehydrogenase Level in Egyptian AML Patients. Clin. Lymphoma Myeloma Leuk. 2020, 20, S174. [Google Scholar] [CrossRef]
- Xiao, Z.; Gong, R.; Chen, X.; Xiao, D.; Luo, S.; Ji, Y. Association between serum lactate dehydrogenase and 60-day mortality in Chinese Hakka patients with acute myeloid leukemia: A cohort study. J. Clin. Lab. Anal. 2021, 35, e24049. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).