Saliva as Blood Alternative in Therapeutic Monitoring of Teriflunomide—Development and Validation of the Novel Analytical Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Application of the Salivette Device
2.2. Method Validation
2.2.1. Linearity and Selectivity
2.2.2. Accuracy and Precision
2.2.3. Matrix Effect and Recovery
2.2.4. Stability and Dilution Integrity
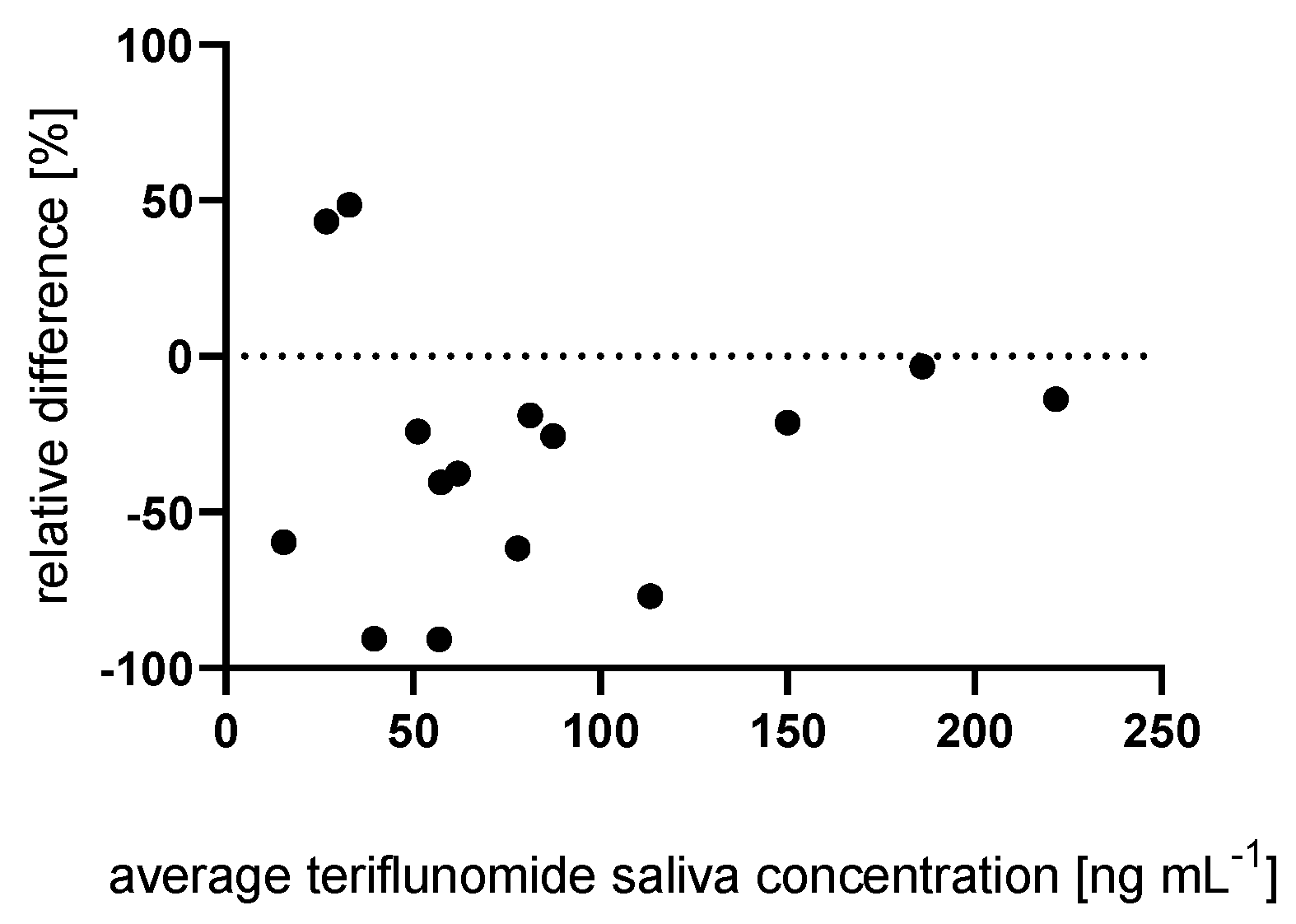
2.3. Influence of Salivary Flow
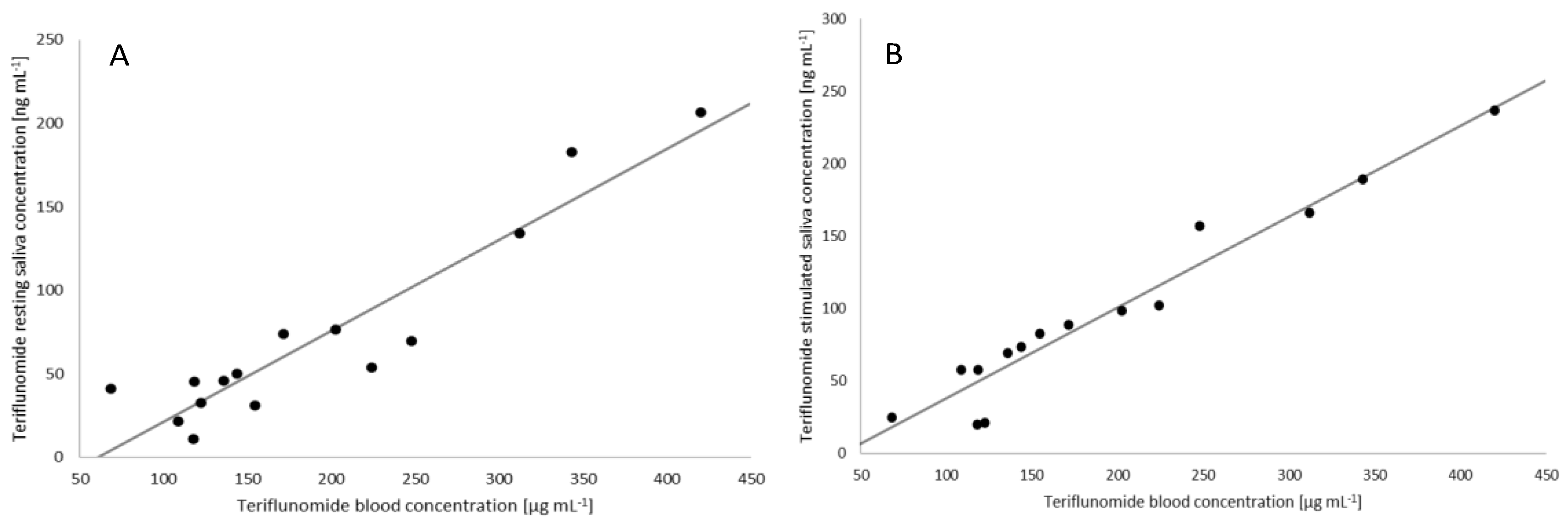
2.4. Correlation between the Plasma and Saliva Concentration of Teriflunomide
3. Materials and Methods
3.1. Chemicals
3.2. Standard Solutions, Calibration Standards and Quality Control Samples
3.3. Clinical Samples
3.4. Sample Extraction
3.5. Chromatographic and Mass Spectrometric Conditions
3.6. Application of the Salivette Device
3.7. Method Validation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.; Loke, Y.; Golder, S.; Kirkham, J.; Jorgensen, A.; Sinha, I.; Hawcutt, D. Overview of systematic reviews of therapeutic ranges: Methodologies and recommendations for practice. BMC Med. Res. Methodol. 2017, 17, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, M.J. Leflunomide Aventis Pharma. Curr. Opin. Investig. Drugs 2001, 2, 222–230. [Google Scholar] [PubMed]
- European Medicines Agency. Arava. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/arava (accessed on 13 July 2022).
- Jiang, L.; Zhang, W.; Li, W.; Ling, C.; Jiang, M. Anti-inflammatory drug, leflunomide and its metabolite teriflunomide inhibit NSCLC proliferation in vivo and in vitro. Toxicol. Lett. 2018, 282, 154–165. [Google Scholar] [CrossRef]
- Mattar, T.; Kochhar, K.; Bartlett, R.; Bremer, E.G.; Finnegan, A. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett. 1993, 334, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Scholar, E.M. Role of Tyrosine Kinase Inhibitors in Cancer Therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Chong, A.S.; Zeng, H.; Knight, D.A.; Shen, J.; Meister, G.T.; Williams, J.W.; Waldman, W.J. Concurrent antiviral and immunosuppressive activities of leflunomide in vivo. Am. J. Transpl. 2006, 6, 69–75. [Google Scholar] [CrossRef]
- Hu, K.; Wang, M.; Zhao, Y.; Zhang, Y.; Wang, T.; Zheng, Z.; Li, X.; Zeng, S.; Zhao, D.; Li, H.; et al. A Small-Scale Medication of Leflunomide as a Treatment of COVID-19 in an Open-Label Blank-Controlled Clinical Trial. Virol. Sin. 2020, 35, 725. [Google Scholar] [CrossRef]
- Chon, W.J.; Josephson, M.A. Leflunomide in renal transplantation. Expert Rev. Clin. Immunol. 2011, 7, 273–281. [Google Scholar] [CrossRef]
- Leger, D.Y.; Liagre, B.; Beneytout, J.L. Low dose leflunomide activates PI3K/Akt signalling in erythroleukemia cells and reduces apoptosis induced by anticancer agents. Apoptosis 2006, 11, 1747–1760. [Google Scholar] [CrossRef]
- Alamri, R.D.; Elmeligy, M.A.; Albalawi, G.A.; Alquayr, S.M.; Alsubhi, S.S.; El-Ghaiesh, S.H. Leflunomide an immunomodulator with antineoplastic and antiviral potentials but drug-induced liver injury: A comprehensive review. Int. Immunopharmacol. 2011, 93, 107398. [Google Scholar] [CrossRef] [PubMed]
- Multiple Sclerosis Association of America. Available online: https://mymsaa.org/news/aubagio/ (accessed on 13 July 2022).
- European Medicines Agency. Aubagio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/aubagio (accessed on 13 July 2022).
- Ng, J.C.Y.; Leung, M.; Wright, A.J.; Ensom, M.H.H. Clinical Pharmacokinetic Monitoring of Leflunomide in Renal Transplant Recipients with BK Virus Reactivation: A Review of the Literature. Clin. Pharmacokinet. 2017, 56, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.S.; Gebel, H.; Finnegan, A.; Petraitis, E.E.; Jiang, X.L.; Sankary, H.N.; Foster, P.; Williams, J.W. Leflunomide, a novel immunomodulatory agent: In vitro analyses of the mechanism of immunosuppression. In Transplantation Proceedings; Elsevier Science: New York, NY, USA, 1993; Volume 25, pp. 747–749. [Google Scholar]
- Madak, J.T.; Bankhead, A.; Cuthbertson, C.R.; Showalter, H.D.; Neamati, N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol. Ther. 2019, 195, 111–131. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014, 74, 659–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud, E.L.; Jessurun, N.T.; van Hunsel, F.P.A.M.; van Puijenbroek, E.P.; van Tubergen, A.; Klooster, P.M.T.; Vonkeman, H.E. Frequency of real-world reported adverse drug reactions in rheumatoid arthritis patients. Expert Opin. Drug Saf. 2020, 19, 1617–1624. [Google Scholar] [CrossRef]
- European Medicines Agency. Annex I Summary of Product Characteristics. Available online: https://ec.europa.eu/health/documents/community-register/2004/200406108014/anx_8014_en.pdf (accessed on 13 July 2022).
- EMEA. EMEA Public Statement On Leflunomide (ARAVA)-Severe And Serious Hepatic Reactions. Available online: https://www.ema.europa.eu/en/documents/public-statement/public-statement-leflunomide-arava-severe-serious-hepatic-reactions_en.pdf (accessed on 13 July 2022).
- Brent, R.L. Teratogen update: Reproductive risks of leflunomide (Arava); a pyrimidine synthesis inhibitor: Counseling women taking leflunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a child. Teratology 2001, 63, 106–112. [Google Scholar] [CrossRef]
- Chan, V.; Charles, B.G.; Tett, S.E. Population pharmacokinetics and association between A77 1726 plasma concentrations and disease activity measures following administration of leflunomide to people with rheumatoid arthritis. Br. J. Clin. Pharmacol. 2005, 60, 257. [Google Scholar] [CrossRef]
- Rule, G.S.; Rockwood, A.L.; Johnson-Davis, K.L. Quantitation of Teriflunomide in Human Serum/Plasma Across a 40,000-Fold Concentration Range by LC/MS/MS. Methods Mol. Biol. 2016, 1383, 195–203. [Google Scholar] [CrossRef]
- Williamson, S.; Munro, C.; Pickler, R.; Grap, M.J.; Elswick, R.K. Comparison of Biomarkers in Blood and Saliva in Healthy Adults. Nurs. Res. Pract. 2012, 2012, 246178. [Google Scholar] [CrossRef]
- Hutchinson, L.; Sinclair, M.; Reid, B.; Burnett, K.; Callan, B. A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br. J. Clin. Pharmacol. 2018, 84, 1089. [Google Scholar] [CrossRef]
- Aps, J.K.; Martens, L.C. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Mendonza, A.; Gohh, R.; Akhlaghi, F. Determination of cyclosporine in saliva using liquid chromatography-tandem mass spectrometry. Ther. Drug Monit. 2004, 26, 569–575. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Moon, J.; Shin, Y.-W.; Lee, S.-T.; Jung, K.-H.; Park, K.-I.; Jung, K.-Y.; Kim, M.; Lee, S.H.; Yu, K.-S.; et al. Usefulness of saliva for perampanel therapeutic drug monitoring. Epilepsia 2020, 61, 1120–1128. [Google Scholar] [CrossRef]
- Brandt, C.; Bien, C.G.; Helmer, R.; May, T.W. Assessment of the correlations of lacosamide concentrations in saliva and serum in patients with epilepsy. Epilepsia 2018, 59, e34–e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grim, S.A.; Ryan, M.; Miles, M.V.; Tang, P.H.; Strawsburg, R.H.; deGrauw, T.J.; Fakhoury, T.A.; Baumann, R.J. Correlation of levetiracetam concentrations between serum and saliva. Ther. Drug Monit. 2003, 25, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.; Gohh, R.Y.; Akhlaghi, F. Tacrolimus Concentration in Saliva of Kidney Transplant Recipients: Factors Influencing the Relationship with Whole Blood Concentrations. Clin. Pharmacokinet. 2018, 57, 1199–1210. [Google Scholar] [CrossRef]
- Shen, B.; Li, S.; Zhang, Y.; Yuan, X.; Fan, Y.; Liu, Z.; Hu, Q.; Yu, C. Determination of total, free and saliva mycophenolic acid with a LC-MS/MS method: Application to pharmacokinetic study in healthy volunteers and renal transplant patients. J. Pharm. Biomed. Anal. 2009, 50, 515–521. [Google Scholar] [CrossRef]
- Wiesen, M.H.; Farowski, F.; Feldkötter, M.; Hoppe, B.; Müller, C. Liquid chromatography-tandem mass spectrometry method for the quantification of mycophenolic acid and its phenolic glucuronide in saliva and plasma using a standardized saliva collection device. J. Chromatogr. A 2012, 1241, 52–59. [Google Scholar] [CrossRef]
- Van den Elsen, S.H.J.; Oostenbrink, L.M.; Heysell, S.K.; Hira, D.; Touw, D.J.; Akkerman, O.W.; Bolhuis, M.S.; Alffenaar, J.-W.C. Systematic Review of Salivary Versus Blood Concentrations of Antituberculosis Drugs and Their Potential for Salivary Therapeutic Drug Monitoring. Ther. Drug Monit. 2018, 40, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Chan, V.; Charles, B.G.; Tett, S.E. Rapid determination of the active leflunomide metabolite A77 1726 in human plasma by high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 803, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Schwind, B.; Gillich, M.; Brune, K.; Hinz, B. Simultaneous determination of leflunomide and its active metabolite, A77 1726, in human plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2003, 17, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Van Roon, E.N.; Yska, J.P.; Raemaekers, J.; Jansen, T.L.T.A.; van Wanrooy, M.; Brouwers, J.R.B.J. A rapid and simple determination of A77 1726 in human serum by high-performance liquid chromatography and its application for optimization of leflunomide therapy. J. Pharm. Biomed. Anal. 2004, 36, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, A.; Raja, R.K. Comparison of LC-UV and LC-MS methods for simultaneous determination of teriflunomide, dimethyl fumarate and fampridine in human plasma: Application to rat pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1371–1377. [Google Scholar] [CrossRef]
- Sobhani, K.; Garrett, D.A.; Liu, D.-P.; Rainey, P.M. A rapid and simple high-performance liquid chromatography assay for the leflunomide metabolite, teriflunomide (A77 1726), in renal transplant recipients. Am. J. Clin. Pathol. 2010, 133, 454–457. [Google Scholar] [CrossRef]
- Parekh, J.M.; Vaghela, R.N.; Sutariya, D.K.; Sanyal, M.; Yadav, M.; Shrivastav, P.S. Chromatographic separation and sensitive determination of teriflunomide, an active metabolite of leflunomide in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 2217–2225. [Google Scholar] [CrossRef]
- Rakhila, H.; Rozek, T.; Hopkins, A.; Proudman, S.; Cleland, L.; James, M.; Wiese, M. Quantitation of total and free teriflunomide (A77 1726) in human plasma by LC-MS/MS. J. Pharm. Biomed. Anal. 2011, 55, 325–331. [Google Scholar] [CrossRef]
- Filali-Ansary, A.; Lunven, C.; Turpault, S.; Beyer, Y.-J.; O’Brien, A.; Delfolie, A.; Boyanova, N.; Sanderink, G.-J.; Baldinetti, F. Dried Blood Spot Methodology in Combination with Liquid Chromatography/Tandem Mass Spectrometry Facilitates the Monitoring of Teriflunomide. Ther. Drug Monit. 2016, 38, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Rule, G.S.; Rockwood, A.L.; Johnson-Davis, K.L. LC-MS/MS Method for the Quantification of the Leflunomide Metabolite, Teriflunomide, in Human Serum/Plasma. Methods Mol. Biol. 2019, 1872, 75–83. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Y.; Song, L.; Jiang, J.; Xiao, F.; Liu, D.; Hu, P. Development of a simple HPLC-MS/MS method to simultaneously determine teriflunomide and its metabolite in human plasma and urine: Application to clinical pharmacokinetic study of teriflunomide sodium and leflunomide. Biomed. Chromatogr. 2019, 33, e4420. [Google Scholar] [CrossRef]
- Sobczak, Ł.; Goryński, K. Evaluation of swabs from 15 commercially available oral fluid sample collection devices for the analysis of commonly abused substances: Doping agents and drugs of abuse. Analyst 2020, 145, 7279–7288. [Google Scholar] [CrossRef] [PubMed]
- Mezzullo, M.; Fanelli, F.; Fazzini, A.; Gambineri, A.; Vicennati, V.; Dalmazi, G.D.; Pelusi, C.; Mazza, R.; Pagotto, U.; Pasquali, R. Validation of an LC-MS/MS salivary assay for glucocorticoid status assessment: Evaluation of the diurnal fluctuation of cortisol and cortisone and of their association within and between serum and saliva. J. Steroid Biochem. Mol. Biol. 2016, 163, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cohier, C.; Mégarbane, B.; Roussel, O. Illicit Drugs in Oral Fluid: Evaluation of Two Collection Devices. J. Anal. Toxicol. 2017, 41, 71–76. [Google Scholar] [CrossRef]
- Wille, S.M.R.; Di Fazio, V.; del Mar Ramírez-Fernandez, M.; Kummer, N.; Samyn, N. Driving under the influence of cannabis: Pitfalls, validation, and quality control of a UPLC-MS/MS method for the quantification of tetrahydrocannabinol in oral fluid collected with StatSure, Quantisal, or Certus collector. Ther. Drug Monit. 2013, 35, 101–111. [Google Scholar] [CrossRef]
- Hall, A.-J.; Warner, J.V.; Henman, M.G.; Ferguson, W.E. Recovery of drugs of abuse from Dräger DCD5000 oral fluid collection device in Australia. J. Anal. Toxicol. 2015, 39, 140–143. [Google Scholar] [CrossRef]
- Gröschl, M.; Köhler, H.; Topf, H.-G.; Rupprecht, T.; Rauh, M. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J. Pharm. Biomed. Anal. 2008, 47, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, H.; Abdel-Rehim, M. Saliva as an alternative specimen to plasma for drug bioanalysis: A review. TrAC Trends Anal. Chem. 2016, 83, 70–79. [Google Scholar] [CrossRef]
- Muddugangadhar, B.C.; Sangur, R.; Rudraprasad, I.V.; Nandeeshwar, D.B.; Kumar, B.H.D. A clinical study to compare between resting and stimulated whole salivary flow rate and pH before and after complete denture placement in different age groups. J. Indian Prosthodont. Soc. 2015, 15, 356. [Google Scholar] [CrossRef]
- Wiese, M.D.; Rowland, A.; Polasek, T.M.; Sorich, M.J.; O’Doherty, C. Pharmacokinetic evaluation of teriflunomide for the treatment of multiple sclerosis. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1025–1035. [Google Scholar] [CrossRef]
- Smeets, M.M.; Vandenbossche, P.; Duijst, W.L.; van Mook, W.N.; Leers, M.P.G. Validation of a new method for saliva cortisol testing to assess stress in first responders. Emerg. Med. J. 2021, 38, 297–302. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation, Committee for Medicinal Products for Human Use (CHMP), EMEA/CHMP/EWP/192217/2009. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 13 July 2022).
- Food and Drug Administration. Bioanalytical Method Validation, Guidance for Industry, Center for Drug Evaluation and Research (CDER). Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 13 July 2022).
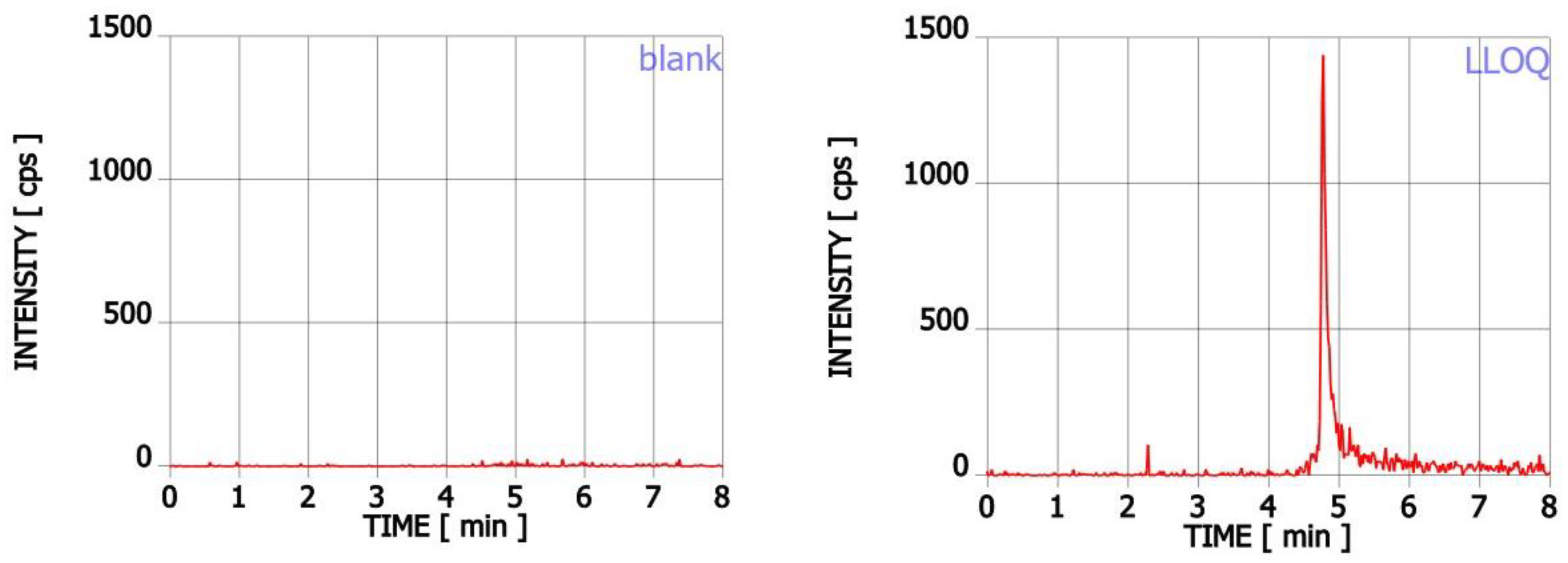
| Nominal Concentration (ng mL−1) | 2 (LLOQ) | 10 (QClow) | 250 (QCmedium) | 450 (QChigh) |
|---|---|---|---|---|
| Within-run precision (%) (n = 5) | 3.0–9.0 a | 2.0–7.0 | 3.0–12 | 4.0–9.0 |
| Within-run accuracy (%) (n = 5) | 101 b | 109 | 104 | 104 |
| Between-run precision (%) (n = 15) | 12 a | 7.0 | 9.0 | 7.0 |
| Between-run accuracy (%) (n = 15) | 112 b | 105 | 96 | 92 |
| Stability Test (%) | 10 ng mL−1 | 450 ng mL−1 | |||
|---|---|---|---|---|---|
| Stability | Accuracy | Stability | Accuracy | ||
| Freeze/thaw stability | 95 | 113 | 94 | 95 | |
| Stability in autosampler | after 24 h | 104 | 103 | 100 | 92 |
| after 48 h | 102 | 100 | 100 | 93 | |
| Short-term stability | 102 | 101 | 97 | 107 | |
| Long-term stability | 91 | 104 | 103 | 101 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankowski, B.; Michorowska, S.; Raćkowska, E.; Sikora, M.; Giebułtowicz, J. Saliva as Blood Alternative in Therapeutic Monitoring of Teriflunomide—Development and Validation of the Novel Analytical Method. Int. J. Mol. Sci. 2022, 23, 9544. https://doi.org/10.3390/ijms23179544
Sankowski B, Michorowska S, Raćkowska E, Sikora M, Giebułtowicz J. Saliva as Blood Alternative in Therapeutic Monitoring of Teriflunomide—Development and Validation of the Novel Analytical Method. International Journal of Molecular Sciences. 2022; 23(17):9544. https://doi.org/10.3390/ijms23179544
Chicago/Turabian StyleSankowski, Bartłomiej, Sylwia Michorowska, Emilia Raćkowska, Mariusz Sikora, and Joanna Giebułtowicz. 2022. "Saliva as Blood Alternative in Therapeutic Monitoring of Teriflunomide—Development and Validation of the Novel Analytical Method" International Journal of Molecular Sciences 23, no. 17: 9544. https://doi.org/10.3390/ijms23179544
APA StyleSankowski, B., Michorowska, S., Raćkowska, E., Sikora, M., & Giebułtowicz, J. (2022). Saliva as Blood Alternative in Therapeutic Monitoring of Teriflunomide—Development and Validation of the Novel Analytical Method. International Journal of Molecular Sciences, 23(17), 9544. https://doi.org/10.3390/ijms23179544







