Identification of Potential Biomarkers and Metabolic Pathways of Different Levels of Heat Stress in Beef Calves
Abstract
:1. Introduction
2. Results
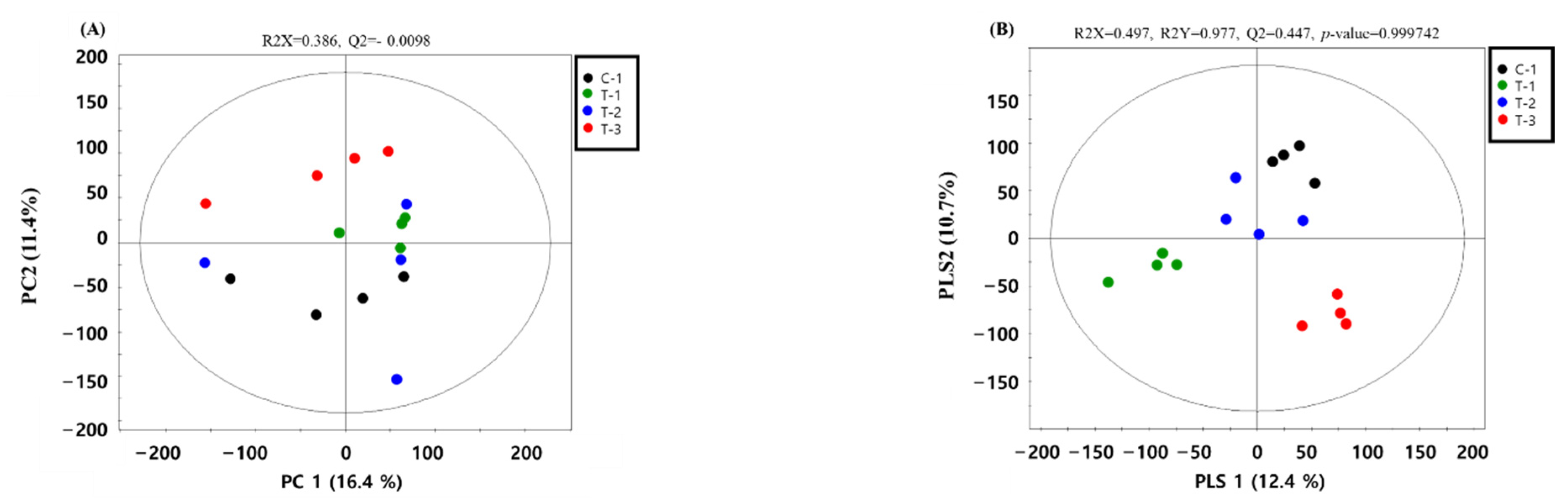
2.1. Metabolic Profiles
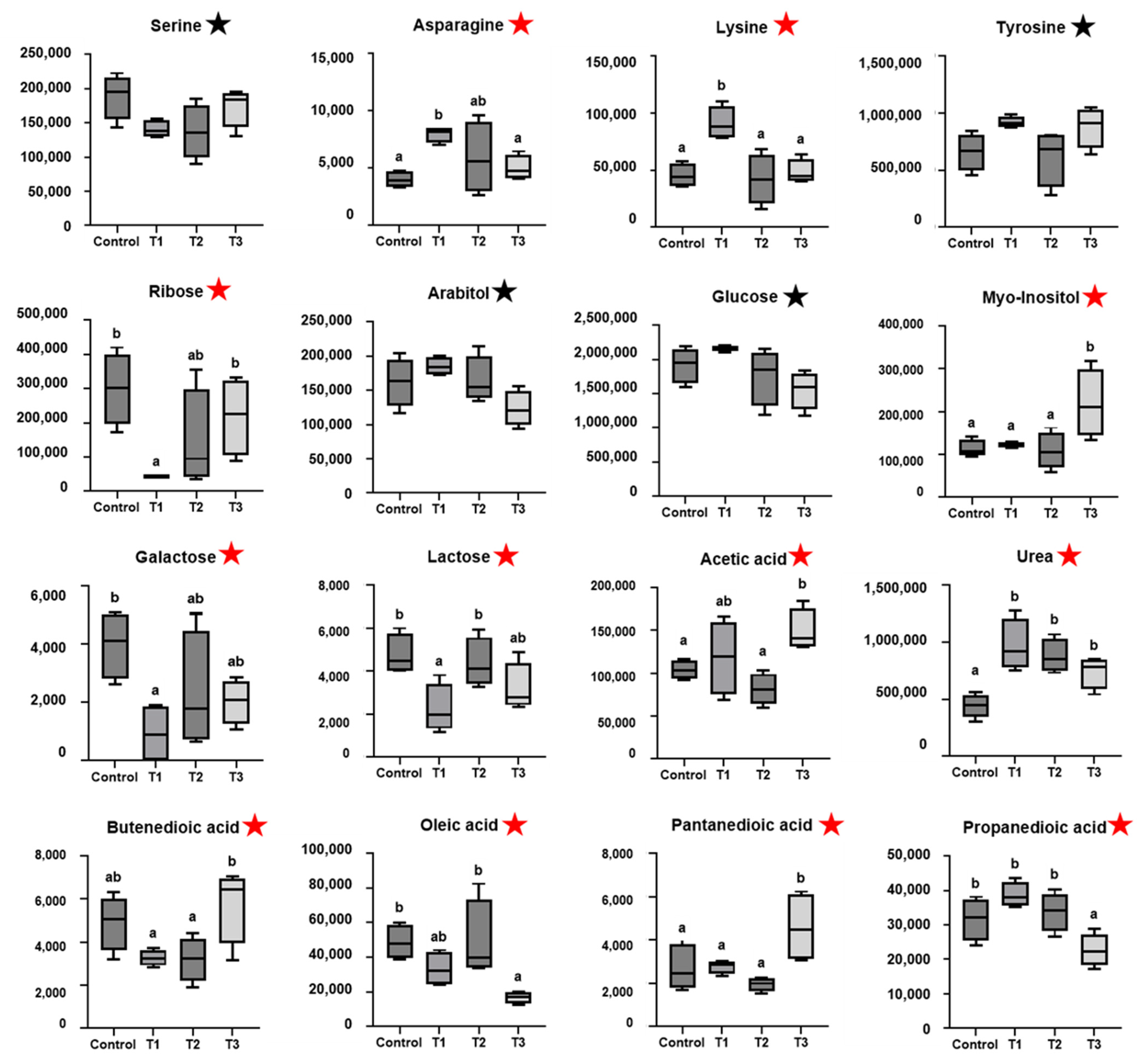
2.2. Detection and Identification of Metabolic Markers
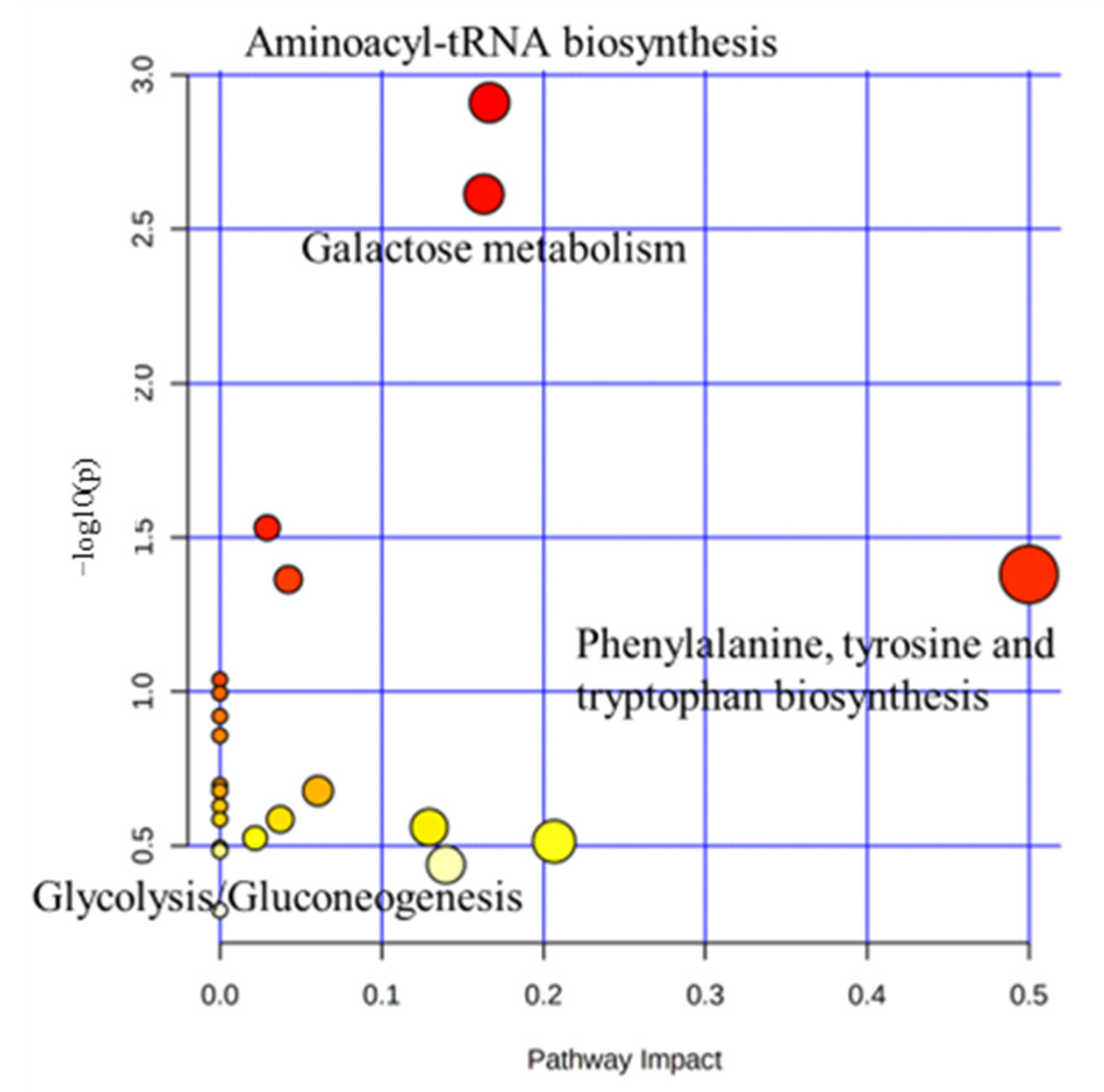
2.3. Characterization and Functional Analysis of Metabolic Pathways in Serum
3. Discussion
4. Materials and Methods
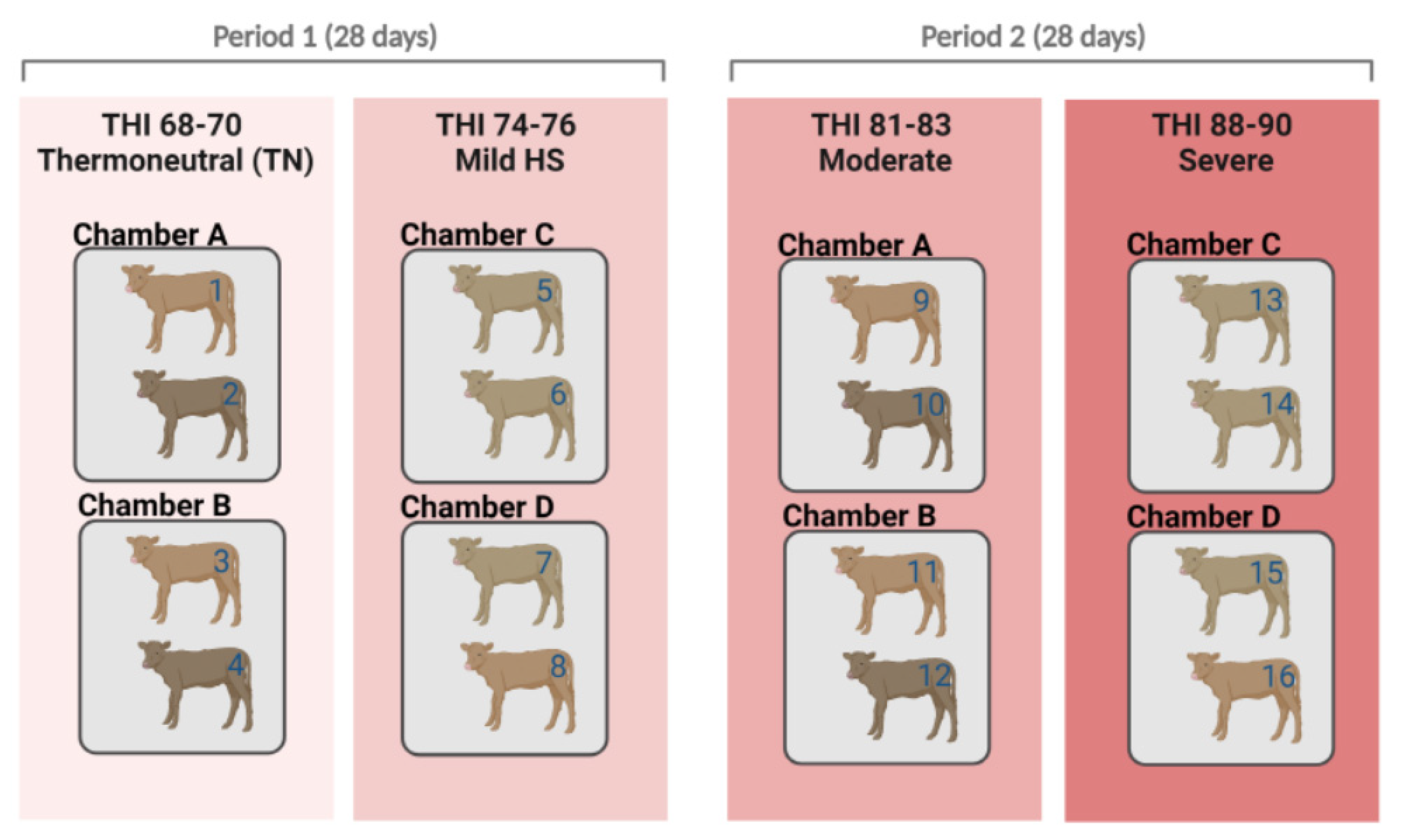
4.1. Animals and Climatic Chamber Details
4.2. Management Conditions and Treatment
4.3. Blood Sample Preparation
4.4. GC-TOF-MS Analysis
4.5. Data Preprocessing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhoads, M.; Rhoads, R.; VanBaale, M.; Collier, R.; Sanders, S.; Weber, W.; Crooker, B.; Baumgard, L. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, J.-S.; Jeon, S.W.; Peng, D.Q.; Kim, Y.S.; Bae, M.H.; Jo, Y.H.; Lee, H.G. Correlation between blood, physiological and behavioral parameters in beef calves under heat stress. Asian Australas. J. Anim. Sci. 2018, 31, 919. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Peng, D.-Q.; Jo, Y.-H.; Nejad, J.G.; Lee, H.-G. Responses of beef calves to long-term heat stress exposure by evaluating growth performance, physiological, blood and behavioral parameters. J. Therm. Biol. 2021, 103033. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, J.; Rhoads, R.; VanBaale, M.; Sanders, S.; Baumgard, L. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Farooq, U.; Samad, H.; Shehzad, F.; Qayyum, A. Physiological responses of cattle to heat stress. World Appl. Sci. J. 2010, 8, 38–43. [Google Scholar]
- O’brien, M.; Rhoads, R.; Sanders, S.; Duff, G.; Baumgard, L. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 2010, 38, 86–94. [Google Scholar] [CrossRef]
- West, J. Interactions of energy and bovine somatotropin with heat stress. J. Dairy Sci. 1994, 77, 2091–2102. [Google Scholar] [CrossRef]
- Zimbelman, R.; Baumgard, L.; Collier, R. Effects of encapsulated niacin on evaporative heat loss and body temperature in moderately heat-stressed lactating Holstein cows. J. Dairy Sci. 2010, 93, 2387–2394. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Mass spectrometry-based metabolomics: Applications to biomarker and metabolic pathway research. Biomed. Chromatogr. 2016, 30, 7–12. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Xue, M.; Wang, D.; Liu, J. Serum metabolome profiling revealed potential biomarkers for milk protein yield in dairy cows. J. Proteom. 2018, 184, 54–61. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics profiling of serum and urine in three beef cattle breeds revealed different levels of tolerance to heat stress. J. Agric. Food. Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads Jr, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, B.; Wang, J.; Liu, H.; Liu, J. Biomarker and pathway analyses of urine metabolomics in dairy cows when corn stover replaces alfalfa hay. J. Anim. Sci. Biotechnol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, Y.; Ren, L.; Hu, L.; Xu, R.; Shen, Y.; Cao, Y.; Gao, Y.; Li, J. Effects of dietary N-carbamylglutamate supplementation on milk production performance, nutrient digestibility and blood metabolomics of lactating Holstein cows under heat stress. Anim. Feed Sci. Technol. 2021, 273, 114797. [Google Scholar] [CrossRef]
- Jo, J.-H.; Ghassemi Nejad, J.; Peng, D.-Q.; Kim, H.-R.; Kim, S.-H.; Lee, H.-G. Characterization of Short-Term Heat Stress in Holstein Dairy Cows Using Altered Indicators of Metabolomics, Blood Parameters, Milk MicroRNA-216 and Characteristics. Animals 2021, 11, 722. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Uppal, K.; Tran, V.T.; Jones, D.P.; Filipov, N.M. Metabolomics of fescue toxicosis in grazing beef steers. Food Chem. Toxicol. 2017, 105, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xi, Y.; Li, Y.; Wang, Z.; Zhang, L.; Han, Z. Analysis of astragalus polysaccharide intervention in heat-stressed dairy cows’ serum metabolomics. Animals 2020, 10, 574. [Google Scholar] [CrossRef]
- Kim, W.; Nejad, J.G.; Peng, D.; Jung, U.; Kim, M.; Jo, Y.; Jo, J.; Lee, J.; Lee, H. Identification of heat shock protein gene expression in hair follicles as a novel indicator of heat stress in beef calves. Animal 2020, 14, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Sammad, A.; Hu, L.; Fang, H.; Xu, Q.; Wang, Y. Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle. Metabolites 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Urich, K. Comparative Animal Biochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-inositol: Its metabolism and potential implications for poultry nutrition—A review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Marins, T.N.; Monteiro, A.P.; Weng, X.; Guo, J.; Rivas, R.M.O.; Gao, J.; Bernard, J.K.; Tomlinson, D.J.; DeFrain, J.M.; Tao, S. Effect of supplemental zinc source with and without evaporative cooling on systemic and mammary metabolism of lactating dairy cows during summer. J. Dairy Sci. 2020, 103, 10258–10263. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar]
- Gao, S.; Guo, J.; Quan, S.; Nan, X.; Fernandez, M.S.; Baumgard, L.; Bu, D. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017, 100, 5040–5049. [Google Scholar] [CrossRef]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef]
- Davenport, G.M.; Boling, J.A.; Gay, N. Performance and plasma amino acids of growing calves fed corn silage supplemented with ground soybeans, fishmeal and rumen-protected lysine. J. Anim. Sci. 1990, 68, 3773–3779. [Google Scholar] [CrossRef]
- Richardson, C.; Hatfield, E. The limiting amino acids in growing cattle. J. Anim. Sci. 1978, 46, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Beede, D.; Wilcox, C. Nycterohemeral patterns of acid-base status, mineral concentrations and digestive function of lactating cows in natural or chamber heat stress environments. J. Anim. Sci. 1988, 66, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Wang, W.; Zheng, N.; Li, S.; Zhang, Y.; Zhang, X.; Yuan, Y. Natural period change of heat stress reveals unique “heat-stressed milk protein decrease syndrome” in mid-lactation dairy cows. China Anim. Husb. Vet. Med. 2014, 41, 73–84. [Google Scholar]
- Tian, H.; Wang, W.; Zheng, N.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef]
- Getahun, D.; Getabalew, M.; Zewdie, D.; Alemneh, T.; Akeberegn, D. Urea metabolism and recycling in ruminants. BJSTR 2019, 20, 14790–14796. [Google Scholar]
- Beede, D.; Collier, R. Potential nutritional strategies for intensively managed cattle during thermal stress. J. Anim. Sci. 1986, 62, 543–554. [Google Scholar] [CrossRef]
- Galster, A.D.; Clutter, W.E.; Cryer, P.E.; Collins, J.A.; Bier, D. Epinephrine plasma thresholds for lipolytic effects in man: Measurements of fatty acid transport with [l-13C] palmitic acid. J. Clin. Investig. 1981, 67, 1729–1738. [Google Scholar] [CrossRef]
- Mora-Rodriguez, R.; Coyle, E.F. Effects of plasma epinephrine on fat metabolism during exercise: Interactions with exercise intensity. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E669–E676. [Google Scholar] [CrossRef]
- Jastrebski, S.F.; Lamont, S.J.; Schmidt, C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 2017, 12, e0181900. [Google Scholar] [CrossRef]
- Becker, C.; Collier, R.; Stone, A. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, L.; Dong, R.; Liang, M.; Lu, Y.; Sun, X.; Zhao, X. Comparing responses of dairy cows to short-term and long-term heat stress in climate-controlled chambers. J. Dairy Sci. 2021, 104, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Kim, J.H.; Kim, H.M.; Lee, E.S.; Shin, D.-H.; Kim, S.; Shin, M.; Kim, S.H.; Lee, J.H.; Kim, Y.J. Acetic acid enhances endurance capacity of exercise-trained mice by increasing skeletal muscle oxidative properties. Biosci. Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic Heat Stress Induces Acute Phase Responses and Serum Metabolome Changes in Finishing Pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra-Hijar, G.; Yáñez-Ruiz, D.; Martín-García, A.; Molina-Alcaide, E. Effects of forage: Concentrate ratio and forage type on apparent digestibility, ruminal fermentation, and microbial growth in goats. J. Anim. Sci. 2009, 87, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Ramos, S.; Tejido, M.; Martínez, M.; Ranilla, M.; Carro, M. Microbial protein synthesis, ruminal digestion, microbial populations, and nitrogen balance in sheep fed diets varying in forage-to-concentrate ratio and type of forage. J. Anim. Sci. 2009, 87, 2924–2934. [Google Scholar] [CrossRef]
- Sutton, J.; Dhanoa, M.; Morant, S.; France, J.; Napper, D.; Schuller, E. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets. J. Dairy Sci. 2003, 86, 3620–3633. [Google Scholar] [CrossRef]
- Zimbelman, R.; Rhoads, R.; Rhoads, M.; Duff, G.; Baumgard, L.; Collier, R. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the Southwest Nutrition Conference, Tucson, AZ, USA, 26–27 February 2009; pp. 158–169. [Google Scholar]
- National Research Council. A Guide to Environmental Research on Animals; National Academy of Sciences: Washington, DC, USA, 1971. [Google Scholar]
- Kim, W.-S.; Ghassemi Nejad, J.; Roh, S.-G.; Lee, H.-G. Heat-shock proteins gene expression in peripheral blood mononuclear cells as an indicator of heat stress in beef calves. Animals 2020, 10, 895. [Google Scholar] [CrossRef]
- Lommen, A. MetAlign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef]
| Tentative Metabolite a | RT b | VIP | MS | Mass Fragment Pattern c | TMS d | ID e |
|---|---|---|---|---|---|---|
| Carbohydrates | ||||||
| Glycerol | 7.25 | 0.72 | 205 | 45, 73, 103, 147, 205, 218 | 3 | STD/MS |
| Ribitol | 9.37 | 0.80 | 217 | 73, 103, 147, 217, 319 | 5 | MS |
| Arabinose | 10.54 | 0.94 | 307 | 73, 103, 147, 217, 307 | 4 | MS |
| Ribose | 10.75 | 1.91 | 103 | 73, 103, 147, 189, 217, 307 | 4 | STD/MS |
| Arabitol | 11.07 | 1.86 | 217 | 73, 103, 147, 205, 217, 307 | 5 | MS |
| Sorbose | 12.18 | 1.05 | 307 | 73, 103, 147, 217, 307 | 5 | MS |
| Glucose | 12.53 | 1.85 | 160 | 73, 103, 147, 205, 319 | 5 | STD/MS |
| Myo-Inositol | 13.62 | 1.65 | 305 | 73, 147, 191, 217, 305, 367 | 6 | STD/MS |
| Galactose | 13.94 | 1.52 | 319 | 73, 103, 147, 205, 319 | 5 | STD/MS |
| Lactose | 16.97 | 1.58 | 204 | 73, 103, 147, 204, 217, 361 | 8 | STD/MS |
| Amino acid | ||||||
| Alanine | 5.52 | 0.78 | 116 | 73, 116, 147, 190, 218 | 2 | STD/MS |
| Valine | 6.69 | 1.46 | 218 | 73, 100, 144, 218, 246 | 2 | STD/MS |
| Leucine | 7.23 | 0.92 | 158 | 73, 147, 158, 218 | 2 | STD/MS |
| Isoleucine | 7.44 | 0.80 | 158 | 45, 73, 100, 158, 218, 260 | 2 | STD/MS |
| Proline | 7.49 | 1.08 | 142 | 73, 142, 216 | 2 | STD/MS |
| Glycine | 7.57 | 1.37 | 174 | 73, 86, 100, 147, 174, 248 | 3 | STD/MS |
| Serine | 8.06 | 1.40 | 218 | 73, 100, 147, 204, 218, 278 | 3 | STD/MS |
| Asparatic acid | 9.44 | 1.19 | 232 | 73, 100, 147, 218, 232 | 3 | STD/MS |
| Methionine | 9.45 | 1.55 | 176 | 61, 73, 128, 147, 176, 293 | 2 | STD/MS |
| 5-Oxoproline | 9.49 | 0.84 | 156 | 73, 147, 156, 230, 258 | 2 | STD/MS |
| Phenylalanine | 10.33 | 1.19 | 218 | 73, 100, 147, 192, 218, 266 | 2 | STD/MS |
| Asparagine | 10.65 | 1.95 | 116 | 73, 116, 132, 231 | 3 | STD/MS |
| Ornithine | 11.72 | 0.71 | 174 | 73, 142, 174, 200, 420 | 4 | MS |
| Lysine | 12.45 | 2.26 | 317 | 73, 92, 128, 174, 230, 317 | 4 | STD/MS |
| Tyrosine | 12.58 | 1.34 | 218 | 73, 100, 147, 179, 218, 280 | 3 | STD/MS |
| Tryptophan | 14.35 | 0.93 | 73 | 45, 73, 202, 291 | 3 | STD/MS |
| Fatty acids | ||||||
| Oleic acid | 14.18 | 1.64 | 339 | 73, 117, 129, 145, 339 | 1 | STD/MS |
| Oleamide | 15.32 | 0.87 | 75 | 75, 116, 128, 131, 144, 338 | 1 | STD/MS |
| Organic compounds | ||||||
| Acetic acid | 5.24 | 1.37 | 147 | 45, 66, 73, 147, 177, 205 | 2 | MS |
| Urea | 6.77 | 1.66 | 147 | 45, 73, 147, 171, 189, 204 | 2 | MS |
| Butenedioic acid | 7.87 | 1.84 | 245 | 45, 73, 147, 245 | 2 | MS |
| Threonic acid | 9.81 | 0.83 | 292 | 73, 117, 147, 205, 220, 292 | 4 | STD/MS |
| Glyoxylic acid | 14.90 | 1.01 | 203 | 75, 113, 147, 203 | 2 | MS |
| Nucleotides | ||||||
| Pyrimidine | 7.86 | 1.93 | 241 | 45, 73, 99, 147, 241, 256 | 2 | MS |
| etc. | ||||||
| Pentanedioic acid | 9.89 | 1.52 | 198 | 73, 147, 198, 304 | 2 | MS |
| Propanedioic acid | 11.12 | 2.25 | 305 | 73, 147, 221, 305 | 3 | MS |
| Phosphoric acid | 11.35 | 0.79 | 299 | 73, 103, 147, 357, 399, 445 | 4 | MS |
| 9, 12-Octadecadienoic acid | 14.16 | 1.32 | 338 | 75, 81, 95, 117, 129, 337 | 1 | MS |
| Metabolite Pathway | p-Value | Impact | Metabolites |
|---|---|---|---|
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 0.042 | 0.5 | Lysine |
| Aminoacyl-tRNA biosynthesis | 0.001 | 0.17 | Asparagine, Lysine |
| Galactose metabolism | 0.002 | 0.16 | Lactose, Galactose, Myo-Inositiol |
| Glyoxylate and dicarboxylate metabolism | 0.043 | 0.04 | Acetic acid |
| Glycolysis/Gluconeogenesis | 0.029 | 0.03 | Acetic acid |
| Inositol phosphate metabolism | 0.317 | 0.13 | Myo-Inositol |
| Pyruvate metabolism | 0.210 | 0.06 | Acetic acid |
| Phosphatidylinositiol signaling system | 0.260 | 0.04 | Myo-Inositol |
| Cystein and methionine metabolism | 0.299 | 0.02 | Asparagine, Lysine |
| Glycine, serine and threonine metabolism | 0.306 | 0.21 | Serine, Lysine |
| Tyrosine metabolism | 0.364 | 0.14 | Tyrosine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-S.; Kim, J.; Lee, H.-G. Identification of Potential Biomarkers and Metabolic Pathways of Different Levels of Heat Stress in Beef Calves. Int. J. Mol. Sci. 2022, 23, 10155. https://doi.org/10.3390/ijms231710155
Kim W-S, Kim J, Lee H-G. Identification of Potential Biomarkers and Metabolic Pathways of Different Levels of Heat Stress in Beef Calves. International Journal of Molecular Sciences. 2022; 23(17):10155. https://doi.org/10.3390/ijms231710155
Chicago/Turabian StyleKim, Won-Seob, Jongkyoo Kim, and Hong-Gu Lee. 2022. "Identification of Potential Biomarkers and Metabolic Pathways of Different Levels of Heat Stress in Beef Calves" International Journal of Molecular Sciences 23, no. 17: 10155. https://doi.org/10.3390/ijms231710155
APA StyleKim, W.-S., Kim, J., & Lee, H.-G. (2022). Identification of Potential Biomarkers and Metabolic Pathways of Different Levels of Heat Stress in Beef Calves. International Journal of Molecular Sciences, 23(17), 10155. https://doi.org/10.3390/ijms231710155






