Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review
Abstract
1. Introduction
1.1. Inflammation
1.2. Stretching
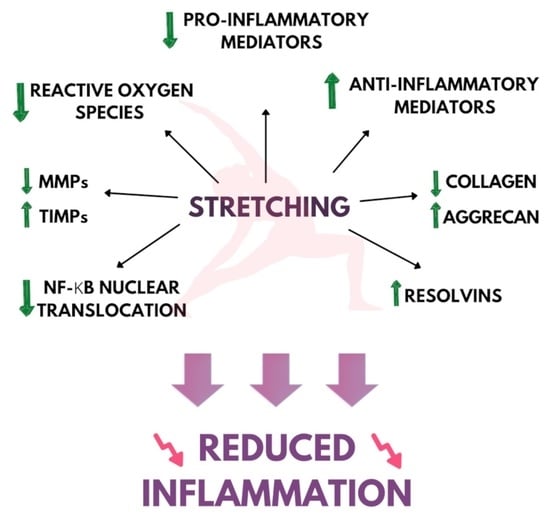
2. Stretching and Inflammation
2.1. Inflammatory Lesion and Tissue Morphology
2.2. Proinflammatory Genes and Cytokine Expression
2.2.1. Patient Studies
2.2.2. Animal Studies
2.2.3. Cell Culture Studies
Mechanically Induced Inflammation
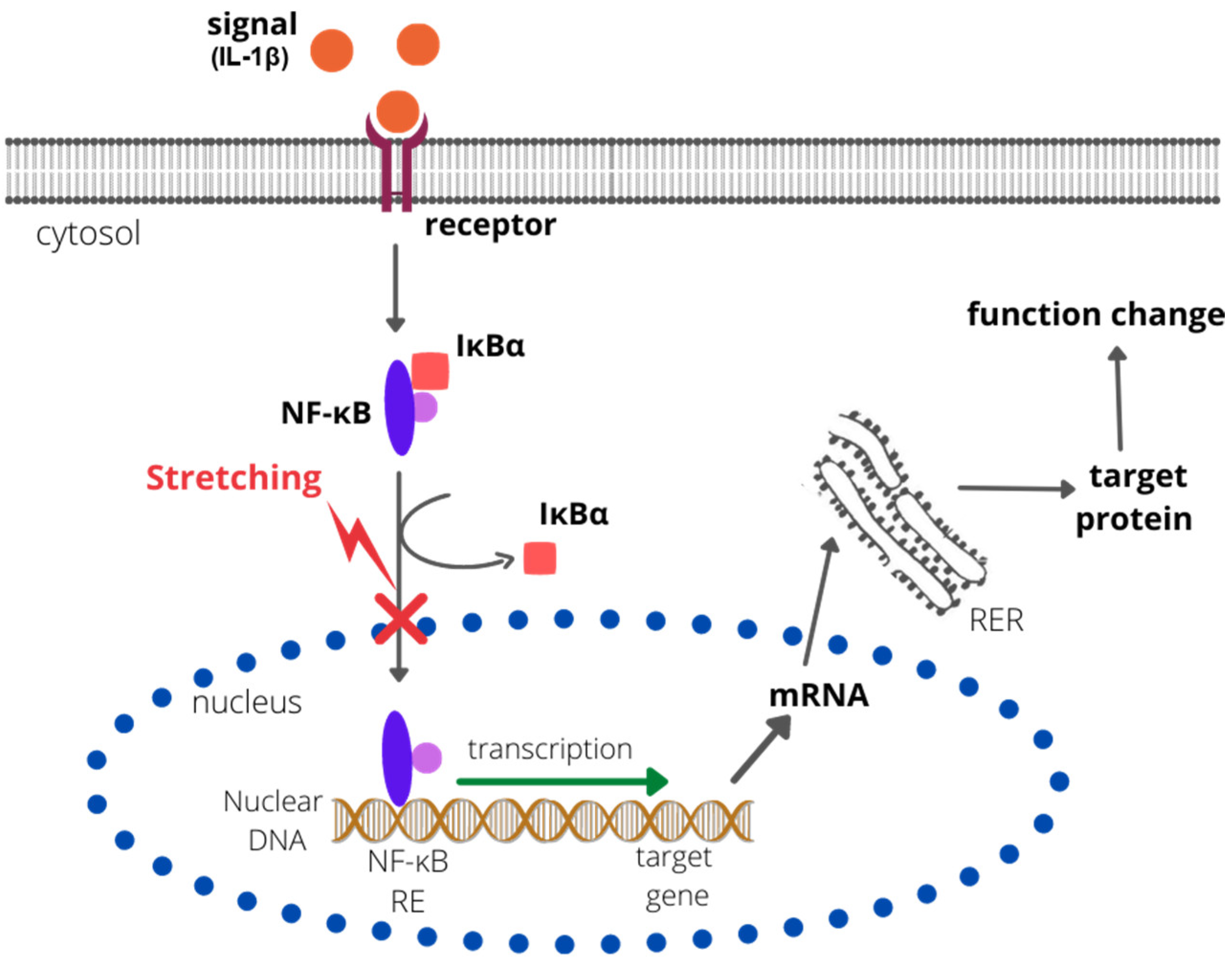
IL-1-Induced Inflammation
3. Stretching and Collagen Metabolism
3.1. Collagen Synthesis and Degradation
3.2. Systemic Sclerosis
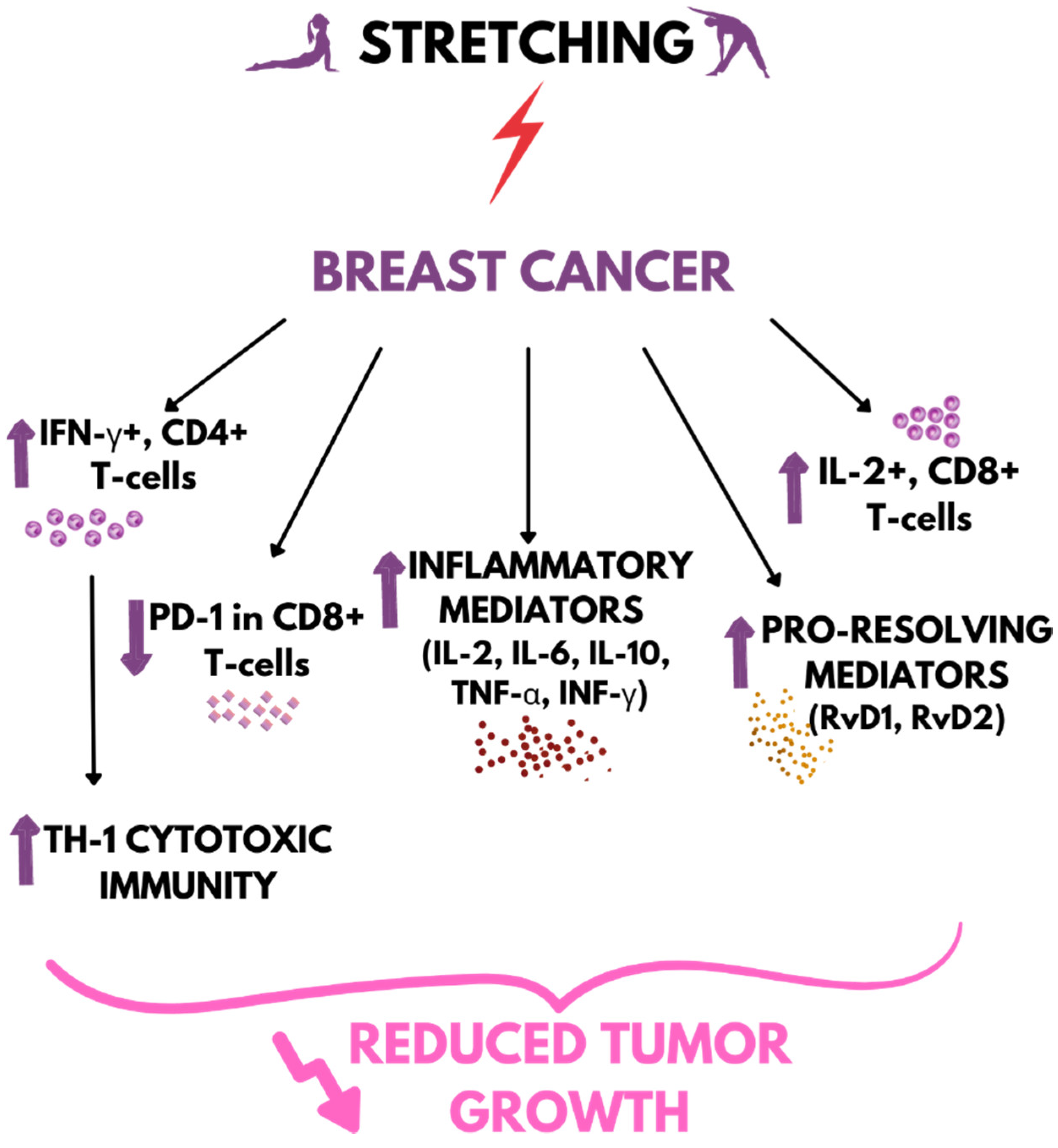
4. Stretching vs. Cancer
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, J.A.; Sharma-Wali, N. Lipoxins: Nature’s Way to Resolve Inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Pahl, A. Cyclooxygenase-1. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–5. ISBN 978-0-08055-232-3. [Google Scholar]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic Study of Constitutive Cyclooxygenase-2 Expression: Role of NF-ΚB and NFAT Transcriptional Pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Kolasa-Wołosiuk, A.; Tarnowski, M.; Baranowska-Bosiacka, I.; Gutowska, I. Long-Term Exposure to Fluoride as a Factor Promoting Changes in the Expression and Activity of Cyclooxygenases (COX1 and COX2) in Various Rat Brain Structures. Neurotoxicology 2019, 74, 81–90. [Google Scholar] [CrossRef]
- Ruan, C.H.; So, S.P.; Ruan, K.H. Inducible COX-2 Dominates over COX-1 in Prostacyclin Biosynthesis: Mechanisms of COX-2 Inhibitor Risk to Heart Disease. Life Sci. 2011, 88, 24–30. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Krueger, J.M. Inflammation and Sleep. In Therapy in Sleep Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 607–616. ISBN 978-1-43771-703-7. [Google Scholar]
- Norman, A.W.; Litwack, G. Prostaglandins. In Hormones; Elsevier: Amsterdam, The Netherlands, 1997; pp. 445–469. ISBN 978-0-12521-441-4. [Google Scholar]
- Anderson, J.M.; Miller, K.M. Biomaterial Biocompatibility and the Macrophage. In The Biomaterials: Silver Jubilee Compendium; Williams, D.F., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 1984; pp. 21–26. ISBN 978-0-08045-154-1. [Google Scholar]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK Signalling Pathways as Molecular Targets for Anti-Inflammatory Therapy--from Molecular Mechanisms to Therapeutic Benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar]
- Mantovani, A. Molecular Pathways Linking Inflammation and Cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer: Inflammation by Remote Control. Nature 2005, 435, 752–753. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 16, 27–41. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Inflammation and Oncogenesis: A Vicious Connection. Curr. Opin. Genet. Dev. 2010, 20, 65–71. [Google Scholar] [CrossRef]
- Tan, T.T.; Coussens, L.M. Humoral Immunity, Inflammation and Cancer. Curr. Opin. Immunol. 2007, 19, 209–221. [Google Scholar] [CrossRef]
- Freire, M.O.; Van Dyke, T.E. Natural Resolution of Inflammation. Periodontology 2013, 63, 149–164. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for Improving Health-Related Quality of Life, Mental Health and Cancer-Related Symptoms in Women Diagnosed with Breast Cancer. Cochrane Database Syst. Rev. 2017, 3, CD010802. [Google Scholar] [CrossRef]
- Ghasemi, E.; Khademi-Kalantari, K.; Khalkhali-Zavieh, M.; Rezasoltani, A.; Ghasemi, M.; Baghban, A.A.; Ghasemi, M. The effect of functional stretching exercises on functional outcomes in spastic stroke patients: A randomized controlled clinical trial. J. Bodyw. Mov. Ther. 2018, 22, 1004–1012. [Google Scholar] [CrossRef]
- Hardee, J.P.; Porter, R.R.; Sui, X.; Archer, E.; Lee, I.M.; Lavie, C.J.; Blair, S.N. The Effect of Resistance Exercise on All-Cause Mortality in Cancer Survivors. Mayo Clin. Proc. 2014, 89, 1108–1115. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Jing, F.; Cai, S.; Bao, C.; Wang, J.; Jin, M.; Chen, K. Association between Physical Activity and All Cancer Mortality: Dose-Response Meta-Analysis of Cohort Studies. Int. J. Cancer 2016, 138, 818–832. [Google Scholar] [CrossRef]
- Park, S.H. Effects of Passive Static Stretching on Blood Glucose Levels in Patients with Type 2 Diabetes Mellitus. J. Phys. Ther. Sci. 2015, 27, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Eklund, J.A.; Corlett, E.N.; Johnson, F. A Method for Measuring the Load Imposed on the Back of a Sitting Person. Ergonomics 1983, 26, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 1000, pp. 131–151. [Google Scholar] [CrossRef]
- Hotta, K.; Behnke, B.J.; Arjmandi, B.; Ghosh, P.; Chen, B.; Brooks, R.; Maraj, J.J.; Elam, M.L.; Maher, P.; Kurien, D.; et al. Daily Muscle Stretching Enhances Blood Flow, Endothelial Function, Capillarity, Vascular Volume and Connectivity in Aged Skeletal Muscle. J. Physiol. 2018, 596, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.G.; Kokkonen, J.; Arnall, D.A. Twenty Minutes of Passive Stretching Lowers Glucose Levels in an At-Risk Population: An Experimental Study. J. Physiother. 2011, 57, 173–178. [Google Scholar] [CrossRef]
- James-Palmer, A.; Anderson, E.Z.; Zucker, L.; Kofman, Y.; Daneault, J.-F. Yoga as an Intervention for the Reduction of Symptoms of Anxiety and Depression in Children and Adolescents: A Systematic Review. Front. Pediatr. 2020, 8, 78. [Google Scholar] [CrossRef]
- Hartfiel, N.; Havenhand, J.; Khalsa, S.B.; Clarke, G.; Krayer, A. The Effectiveness of Yoga for the Improvement of Well-Being and Resilience to Stress in the Workplace. Scand. J. Work. Environ. Health 2011, 37, 70–76. [Google Scholar] [CrossRef]
- Kamei, T.; Toriumi, Y.; Kimura, H.; Kumano, H.; Ohno, S.; Kimura, K. Decrease in Serum Cortisol during Yoga Exercise Is Correlated with Alpha Wave Activation. Percept. Mot. Ski. 2000, 90, 1027–1032. [Google Scholar] [CrossRef]
- Sumi, K.; Ashida, K.; Nakazato, K. Repeated stretch-shortening contraction of the triceps surae attenuates muscle atrophy and liver dysfunction in a rat model of inflammation. Exp. Physiol. 2020, 105, 1111–1123. [Google Scholar] [CrossRef]
- Eda, N.; Ito, H.; Shimizu, K.; Suzuki, S.; Lee, E.; Akama, T. Yoga Stretching for Improving Salivary Immune Function and Mental Stress in Middle-Aged and Older Adults. J. Women Aging 2018, 30, 227–241. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R. Mitigating Cellular Inflammation in Older Adults: A Randomized Controlled Trial of Tai Chi Chih. Am. J. Geriatr. Psychiatry 2012, 20, 764–772. [Google Scholar] [CrossRef]
- Telles, S.; Gaur, V.; Balkrishna, A. Effect of a Yoga Practice Session and a Yoga Theory Session on State Anxiety. Percept. Mot. Ski. 2009, 109, 924–930. [Google Scholar] [CrossRef]
- Uebelacker, L.A.; Tremont, G.; Epstein-Lubow, G.; Gaudiano, B.A.; Gillette, T.; Kalibatseva, Z.; Miller, I.W. Open Trial of Vinyasa Yoga for Persistently Depressed Individuals: Evidence of Feasibility and Acceptability. Behav. Modif. 2010, 34, 247–264. [Google Scholar] [CrossRef]
- Wang, C.; Collet, J.P.; Lau, J. The Effect of Tai Chi on Health Outcomes in Patients with Chronic Conditions: A Systematic Review. Arch. Intern. Med. 2004, 164, 493–501. [Google Scholar] [CrossRef]
- Yeole, D.U.L. Effectiveness of Tai-Chi on Balance in Elderly. J. Med. Sci. Clin. Res. 2016, 4, 14848–14854. [Google Scholar] [CrossRef]
- Loughran, M.; Glasgow, P.; Bleakley, C.; McVeigh, J. The Effects of a Combined Static-Dynamic Stretching Protocol on Athletic Performance in Elite Gaelic Footballers: A Randomised Controlled Crossover Trial. Phys. Ther. Sport 2017, 25, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Agata, N.; Sasai, N.; Masumi, I.M.; Kawakami, K.; Hayakawa, K.; Kobayashi, K.; Sokabe, M. Repetitive Stretch Suppresses Denervation-Induced Atrophy of Soleus Muscle in Rats. Muscle Nerve 2009, 39, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.S.; Miyabara, E.H.; Soares, A.G.; Saito, E.T.; Moriscot, A.S. MTOR Pathway Inhibition Attenuates Skeletal Muscle Growth Induced by Stretching. Cell Tissue Res. 2006, 324, 149–156. [Google Scholar] [CrossRef]
- Bamman, M.M.; Roberts, B.M.; Adams, G.R. Molecular Regulation of Exercise-Induced Muscle Fiber Hypertrophy. Cold Spring Harb. Perspect. Med. 2018, 8, a029751. [Google Scholar] [CrossRef]
- Lin, S.S.; Liu, Y.W. Mechanical Stretch Induces MTOR Recruitment and Activation at the Phosphatidic Acid-Enriched Macropinosome in Muscle Cell. Front. Cell Dev. Biol. 2019, 7, 78. [Google Scholar] [CrossRef]
- Moyano, F.R.; Valenza, M.C.; Martin, L.M.; Caballero, Y.C.; Gonzalez-Jimenez, E.; Demet, G.V. Effectiveness of Different Exercises and Stretching Physiotherapy on Pain and Movement in Patellofemoral Pain Syndrome: A Randomized Controlled Trial. Clin. Rehabil. 2013, 27, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, J.; Kautiainen, H.; Wirén, K.; Häkkinen, A. Stretching Exercises vs Manual Therapy in Treatment of Chronic Neck Pain: A Randomized, Controlled Cross-over Trial. J. Rehabil. Med. 2007, 39, 126–132. [Google Scholar] [CrossRef]
- Corey, S.M.; Vizzard, M.A.; Bouffard, N.A.; Badger, G.J.; Langevin, H.M. Stretching of the Back Improves Gait, Mechanical Sensitivity and Connective Tissue Inflammation in a Rodent Model. PLoS ONE 2012, 7, e29831. [Google Scholar] [CrossRef]
- Vergara, D.M.; Berrueta, L.; Carmody, C.; An, X.; Wayne, P.M.; Zavacki, A.M.; Langevin, H.M. Establishment of a Novel Porcine Model to Study the Impact of Active Stretching on a Local Carrageenan-Induced Inflammation. Am. J. Phys. Med. Rehabil. 2020, 99, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Berrueta, L.; Muskaj, I.; Olenich, S.; Butler, T.; Badger, G.J.; Colas, R.A.; Spite, M.; Serhan, C.N.; Langevin, H.M. Stretching Impacts Inflammation Resolution in Connective Tissue. J. Cell. Physiol. 2016, 231, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, K.R.; Cao, T.V.; Schad, J.F.; King, H.; Stoll, S.T.; Standley, P.R. In Vitro Modeling of Repetitive Motion Injury and Myofascial Release. J. Bodyw. Mov. Ther. 2010, 14, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Buckley, M.J.; Evans, C.H.; Agarwal, S. Cyclic Tensile Strain Acts as an Antagonist of IL-1β Actions in Chondrocytes. J. Immunol. 2000, 165, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.R.; Cao, T.V.; Campbell, D.H.; Standley, P.R. Mechanical Strain Applied to Human Fibroblasts Differentially Regulates Skeletal Myoblast Differentiation. J. Appl. Physiol. 2012, 113, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.N.; Best, T.M. Is Self Myofascial Release an Effective Preexercise and Recovery Strategy? A Literature Review. Curr. Sports Med. Rep. 2015, 14, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Franke, J.D.; Fryer, G. Osteopathic Manipulative Treatment for Nonspecific Low Back Pain: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2014, 15, 286. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.J.; Seffinger, M.A.; Hensel, K.L.; Wiseman, R. American Osteopathic Association Guidelines for Osteopathic Manipulative Treatment (OMT) for Patients with Low Back Pain. J. Am. Osteopath. Assoc. 2016, 116, 536–549. [Google Scholar] [CrossRef]
- Giusti, R. (Ed.) Glossary of Osteopathic Terminology, 3rd ed.; AACOM: Bethesda, MD, USA, 2017. [Google Scholar]
- Ajimsha, M.S.; Al-Mudahka, N.R.; Al-Madzhar, J.A. Effectiveness of Myofascial Release: Systematic Review of Randomized Controlled Trials. J. Bodyw. Mov. Ther. 2015, 19, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, S.W.; Kolber, M.J.; Cain, M.; Lee, M. The Effects of Self-Myofascial Release on Joint Range of Motion, Muscle Recovery, and Performance: A Systematic Review. Int. J. Sports Phys. Ther. 2015, 10, 827–838. [Google Scholar] [PubMed]
- Wang, L.; Cui, J.B.; Xie, H.M.; Zuo, X.Q.; He, J.L.; Jia, Z.S.; Zhang, L.N. Effects of Different Static Progressive Stretching Durations on Range of Motion, Myofibroblasts, and Collagen in a Posttraumatic Knee Contracture Rat Model. Phys. Ther. 2022, 102, pzab300. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Wang, H.H.; Chen, C.H.; Hu, H.M. Effectiveness of a Stretching Exercise Program on Low Back Pain and Exercise Self-Efficacy among Nurses in Taiwan: A Randomized Clinical Trial. Pain Manag. Nurs. 2014, 15, 283–291. [Google Scholar] [CrossRef]
- Graff-Radford, S.B.; Bassiur, J.P. Temporomandibular Disorders and Headaches. Neurol. Clin. 2014, 32, 525–537. [Google Scholar] [CrossRef]
- Page, P. Current Concepts in Muscle Stretching for Exercise and Rehabilitation. Int. J. Sports Phys. Ther. 2012, 7, 109–119. [Google Scholar]
- Sherman, K.J.; Cherkin, D.C.; Wellman, R.D.; Cook, A.J.; Hawkes, R.J.; Delaney, K.; Deyo, R.A. A Randomized Trial Comparing Yoga, Stretching, and a Self-Care Book for Chronic Low Back Pain. Arch. Intern. Med. 2011, 171, 2019–2026. [Google Scholar] [CrossRef]
- Tilbrook, H.E.; Cox, H.; Hewitt, C.E.; Kang’ombe, A.R.; Chuang, L.H.; Jayakody, S.; Aplin, J.D.; Semlyen, A.; Trewhela, A.; Watt, I.; et al. Yoga for Chronic Low Back Pain: A Randomized Trial. Ann. Intern. Med. 2011, 155, 569–578. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carrillo, C.; Sadeghi, N.; Breen, E.C.; Witarama, T.; Yokomizo, M.; Lavretsky, H.; Carroll, J.E.; Motivala, S.J.; et al. Cognitive Behavioral Therapy vs. Tai Chi for Late Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial. Sleep 2014, 37, 1543–1552. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Alonso-Blanco, C.; Fernández-Carnero, J.; Carlos Miangolarra-Page, J. The Immediate Effect of Ischemic Compression Technique and Transverse Friction Massage on Tenderness of Active and Latent Myofascial Trigger Points: A Pilot Study. J. Bodyw. Mov. Ther. 2006, 10, 3–9. [Google Scholar] [CrossRef]
- Hou, C.-R.; Tsai, L.-C.; Cheng, K.-F.; Chung, K.-C.; Hong, C.-Z. Immediate Effects of Various Physical Therapeutic Modalities on Cervical Myofascial Pain and Trigger-Point Sensitivity. Arch. Phys. Med. Rehabil. 2002, 83, 1406–1414. [Google Scholar] [CrossRef]
- Andersson, G.B.J.; Lucente, T.; Davis, A.M.; Kappler, R.E.; Lipton, J.A.; Leurgans, S. A Comparison of Osteopathic Spinal Manipulation with Standard Care for Patients with Low Back Pain. N. Engl. J. Med. 1999, 341, 1426–1431. [Google Scholar] [CrossRef]
- Schad, J.F.; Meltzer, K.R.; Hicks, M.R.; Beutler, D.S.; Cao, T.V.; Standley, P.R. Cyclic Strain Upregulates VEGF and Attenuates Proliferation of Vascular Smooth Muscle Cells. Vasc. Cell 2011, 3, 21. [Google Scholar] [CrossRef]
- Sucher, B.M.; Hinrichs, R.N. Manipulative Treatment of Carpal Tunnel Syndrome: Biomechanical and Osteopathic Intervention to Increase the Length of the Transverse Carpal Ligament. J. Am. Osteopath. Assoc. 1998, 98, 679–686. [Google Scholar] [CrossRef]
- Xiong, Y.; Berrueta, L.; Urso, K.; Olenich, S.; Muskaj, I.; Badger, G.J.; Aliprantis, A.; Lafyatis, R.; Langevin, H.M. Stretching Reduces Skin Thickness and Improves Subcutaneous Tissue Mobility in a Murine Model of Systemic Sclerosis. Front. Immunol. 2017, 8, 124. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Muenchow, M.; Wallig, M.A.; Horn, P.L.; Woods, J.A. Exercise Delays Allogeneic Tumor Growth and Reduces Intratumoral Inflammation and Vascularization. J. Appl. Physiol. 2004, 96, 2249–2256. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; MaCcarrone, M.; Serhan, C.N. Proresolving Lipid Mediators Resolvin D1, Resolvin D2, and Maresin 1 Are Critical in Modulating T Cell Responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Xuan, W.; Fan, G.H. Roles of Resolvins in the Resolution of Acute Inflammation. Cell Biol. Int. 2015, 39, 3–22. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Langevin, H.M.; Bishop, J.; Maple, R.; Badger, G.J.; Fox, J.R. Effect of Stretching on Thoracolumbar Fascia Injury and Movement Restriction in a Porcine Model. Am. J. Phys. Med. Rehabil. 2018, 97, 187–191. [Google Scholar] [CrossRef]
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK Cascades: Signaling Components, Nuclear Roles and Mechanisms of Nuclear Translocation. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.K.; Pavicevic, Z.; Du, Z.; Kim, J.G.; Fan, M.; Jiao, Y.; Rosebush, M.; Samant, S.; Gu, W.; Pfeffer, L.M.; et al. Pro-Inflammatory Genes as Biomarkers and Therapeutic Targets in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2010, 285, 32512–32521. [Google Scholar] [CrossRef] [PubMed]
- Sarvottam, K.; Magan, D.; Yadav, R.K.; Mehta, N.; Mahapatra, S.C. Adiponectin, Interleukin-6, and Cardiovascular Disease Risk Factors Are Modified by a Short-Term Yoga-Based Lifestyle Intervention in Overweight and Obese Men. J. Altern. Complement. Med. 2013, 19, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.; Irwin, M.R.; Chung, M.; Wang, C. The Effects of Mind-Body Therapies on the Immune System: Meta-Analysis. PLoS ONE 2014, 9, e100903. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines IL-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, Obesity, Stress and Coronary Heart Disease: Is Interleukin-6 the Link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Benabdoun, H.A.; Kulbay, M.; Rondon, E.P.; Vallières, F.; Shi, Q.; Fernandes, J.; Fahmi, H.; Benderdour, M. In Vitro and in Vivo Assessment of the Proresolutive and Antiresorptive Actions of Resolvin D1: Relevance to Arthritis. Arthritis Res. Ther. 2019, 21, 72. [Google Scholar] [CrossRef]
- Cao, D.; Pi, J.; Shan, Y.; Tang, Y.; Zhou, P. Anti-Inflammatory Effect of Resolvin D1 on LPS-Treated MG-63 Cells. Exp. Ther. Med. 2018, 16, 4283–4288. [Google Scholar] [CrossRef]
- Markworth, J.F.; Brown, L.A.; Lim, E.; Floyd, C.; Larouche, J.; Castor-Macias, J.A.; Sugg, K.B.; Sarver, D.C.; Macpherson, P.C.D.; Davis, C.; et al. Resolvin D1 Supports Skeletal Myofiber Regeneration via Actions on Myeloid and Muscle Stem Cells. JCI Insight 2020, 5, e137713. [Google Scholar] [CrossRef]
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 Promotes Insulin Resistance in Obese Mice by Acting on Macrophages, Hepatocytes and Myocytes. Nat. Med. 2015, 21, 239–247. [Google Scholar] [CrossRef]
- Meltzer, K.R.; Standley, P.R. Modeled Repetitive Motion Strain and Indirect Osteopathic Manipulative Techniques in Regulation of Human Fibroblast Proliferation and Interleukin Secretion. J. Am. Osteopath. Assoc. 2007, 107, 527–536. [Google Scholar] [CrossRef]
- Campbell, S.M.; Winkelmann, R.R.; Walkowski, S. Osteopathic Manipulative Treatment: Novel Application to Dermatological Disease. J. Clin. Aesthet. Dermatol. 2012, 5, 24–32. [Google Scholar]
- Steel, A.; Sundberg, T.; Reid, R.; Ward, L.; Bishop, F.L.; Leach, M.; Cramer, H.; Wardle, J.; Adams, J. Osteopathic Manipulative Treatment: A Systematic Review and Critical Appraisal of Comparative Effectiveness and Health Economics Research. Musculoskelet. Sci. Pract. 2017, 27, 165–175. [Google Scholar] [CrossRef]
- Eagan, T.S.; Meltzer, K.R.; Standley, P.R. Importance of Strain Direction in Regulating Human Fibroblast Proliferation and Cytokine Secretion: A Useful in Vitro Model for Soft Tissue Injury and Manual Medicine Treatments. J. Manip. Physiol. Ther. 2007, 30, 584–592. [Google Scholar] [CrossRef]
- Sokolowski, J.D.; Chabanon-Hicks, C.N.; Han, C.Z.; Heffron, D.S.; Mandell, J.W. Fractalkine Is a “Find-Me” Signal Released by Neurons Undergoing Ethanol-Induced Apoptosis. Front. Cell. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef]
- Engel, D.R.; Krause, T.A.; Snelgrove, S.L.; Thiebes, S.; Hickey, M.J.; Boor, P.; Kitching, A.R.; Kurts, C. CX 3 CR1 Reduces Kidney Fibrosis by Inhibiting Local Proliferation of Profibrotic Macrophages. J. Immunol. 2015, 194, 1628–1638. [Google Scholar] [CrossRef]
- White, G.E.; Tan, T.C.C.; John, A.E.; Whatling, C.; McPheat, W.L.; Greaves, D.R. Fractalkine Has Anti-Apoptotic and Proliferative Effects on Human Vascular Smooth Muscle Cells via Epidermal Growth Factor Receptor Signalling. Cardiovasc. Res. 2010, 85, 825–835. [Google Scholar] [CrossRef]
- Parker, D.; Ferreri, K.; Nakajima, T.; LaMorte, V.J.; Evans, R.; Koerber, S.C.; Hoeger, C.; Montminy, M.R. Phosphorylation of CREB at Ser-133 Induces Complex Formation with CREB-Binding Protein via a Direct Mechanism. Mol. Cell. Biol. 1996, 16, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Németh, M.; Koloszár, I.; Markó, L.; Przybyl, L.; Jinno, K.; Szigeti, C.; Heffer, M.; Gebhardt, M.; Szeberényi, J.; et al. Overexpression of CREB Protein Protects from Tunicamycin-Induced Apoptosis in Various Rat Cell Types. Apoptosis 2014, 19, 1080–1098. [Google Scholar] [CrossRef] [PubMed]
- Kurenova, E.; Xu, L.-H.; Yang, X.; Baldwin, A.S.; Craven, R.J.; Hanks, S.K.; Liu, Z.; Cance, W.G. Focal Adhesion Kinase Suppresses Apoptosis by Binding to the Death Domain of Receptor-Interacting Protein. Mol. Cell. Biol. 2004, 24, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Anloague, A.; Mahoney, A.; Ogunbekun, O.; Hiland, T.A.; Thompson, W.R.; Larsen, B.; Loghmani, M.T.; Hum, J.M.; Lowery, J.W. Mechanical Stimulation of Human Dermal Fibroblasts Regulates Pro-Inflammatory Cytokines: Potential Insight into Soft Tissue Manual Therapies. BMC Res. Notes 2020, 13, 400. [Google Scholar] [CrossRef]
- Nazet, U.; Grässel, S.; Jantsch, J.; Proff, P.; Schröder, A.; Kirschneck, C. Early OA Stage Like Response Occurs after Dynamic Stretching of Human Synovial Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3874. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin Inhibits IL-1β-Induced Inflammation in Rat Chondrocytes and Attenuates Osteoarthritis Progression in a Rat Model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Pan, T.; Wu, D.; Cai, N.; Chen, R.; Shi, X.; Li, B.; Pan, J. Alpha-Mangostin Protects Rat Articular Chondrocytes against IL-1β-Induced Inflammation and Slows the Progression of Osteoarthritis in a Rat Model. Int. Immunopharmacol. 2017, 52, 34–43. [Google Scholar] [CrossRef]
- Tu, C.; Ma, Y.; Song, M.; Yan, J.; Xiao, Y.; Wu, H. Liquiritigenin Inhibits IL-1β-Induced Inflammation and Cartilage Matrix Degradation in Rat Chondrocytes. Eur. J. Pharmacol. 2019, 858, 172445. [Google Scholar] [CrossRef]
- Yang, X.M.; Downey, J.M.; Cohen, M.V.; Housley, N.A.; Alvarez, D.F.; Audia, J.P. The Highly Selective Caspase-1 Inhibitor VX-765 Provides Additive Protection Against Myocardial Infarction in Rat Hearts When Combined with a Platelet Inhibitor. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 574–578. [Google Scholar] [CrossRef]
- Griffin, T.M.; Guilak, F. The Role of Mechanical Loading in the Onset and Progression of Osteoarthritis. Exerc. Sport Sci. Rev. 2005, 33, 195–200. [Google Scholar] [CrossRef]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schünke, M. Pathomechanisms of Cartilage Destruction by Mechanical Injury. Ann. Anat. 2005, 187, 473–485. [Google Scholar] [CrossRef]
- Giacomelli, R.; Ruscitti, P.; Alvaro, S.; Ciccia, F.; Liakouli, V.; Di Benedetto, P.; Guggino, G.; Berardicurti, O.; Carubbi, F.; Triolo, G.; et al. IL-1β at the Crossroad between Rheumatoid Arthritis and Type 2 Diabetes: May We Kill Two Birds with One Stone? Expert Rev. Clin. Immunol. 2016, 12, 849–855. [Google Scholar] [CrossRef]
- Davies, C.M.; Guilak, F.; Weinberg, J.B.; Fermor, B. Reactive Nitrogen and Oxygen Species in Interleukin-1-Mediated DNA Damage Associated with Osteoarthritis. Osteoarthr. Cartil. 2008, 16, 624–630. [Google Scholar] [CrossRef]
- Madhavan, S.; Anghelina, M.; Rath-Deschner, B.; Wypasek, E.; John, A.; Deschner, J.; Piesco, N.; Agarwal, S. Biomechanical Signals Exert Sustained Attenuation of Proinflammatory Gene Induction in Articular Chondrocytes. Osteoarthr. Cartil. 2006, 14, 1023–1032. [Google Scholar] [CrossRef][Green Version]
- Dossumbekova, A.; Anghelina, M.; Madhavan, S.; He, L.; Quan, N.; Knobloch, T.; Agarwal, S. Biomechanical Signals Inhibit IKK Activity to Attenuate NF-ΚB Transcription Activity in Inflamed Chondrocytes. Arthritis Rheum. 2007, 56, 3284–3296. [Google Scholar] [CrossRef]
- Branski, R.C.; Perera, P.; Verdolini, K.; Rosen, C.A.; Hebda, P.A.; Agarwal, S. Dynamic Biomechanical Strain Inhibits IL-1β-Induced Inflammation in Vocal Fold Fibroblasts. J. Voice 2007, 21, 651–660. [Google Scholar] [CrossRef]
- Chowdhury, T.T.; Bader, D.L.; Lee, D.A. Dynamic Compression Inhibits the Synthesis of Nitric Oxide and PGE2 by IL-1β-Stimulated Chondrocytes Cultured in Agarose Constructs. Biochem. Biophys. Res. Commun. 2001, 285, 1168–1174. [Google Scholar] [CrossRef]
- Ferretti, M.; Madhavan, S.; Deschner, J.; Rath-Deschner, B.; Wypasek, E.; Agarwal, S. Dynamic Biophysical Strain Modulates Proinflammatory Gene Induction in Meniscal Fibrochondrocytes. Am. J. Physiol. Cell Physiol. 2006, 290, C1610–C1615. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and Function of Aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Castillo-Briceño Patricia, P.; Bihan, D.; Nilges, M.; Hamaia, S.; Meseguer, J.; García-Ayala, A.; Farndale, R.W.; Mulero, V. A Role for Specific Collagen Motifs during Wound Healing and Inflammatory Response of Fibroblasts in the Teleost Fish Gilthead Seabream. Mol. Immunol. 2011, 48, 826–834. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef]
- Caterson, B.; Flannery, C.R.; Hughes, C.E.; Little, C.B. Mechanisms Involved in Cartilage Proteoglycan Catabolism. Matrix Biol. 2000, 19, 333–344. [Google Scholar] [CrossRef]
- Roughley, P.J.; Mort, J.S. The Role of Aggrecan in Normal and Osteoarthritic Cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef]
- Abusharkh, H.A.; Reynolds, O.M.; Mendenhal, l.J.; Gozen, B.A.; Tingstad, E.; Idone, V.; Abu-Lail, N.I.; Van Wie, B.J. Combining stretching and gallic acid to decrease inflammation indices and promote extracellular matrix production in osteoarthritic human articular chondrocytes. Exp. Cell Res. 2021, 408, 112841. [Google Scholar] [CrossRef]
- Bouffard, N.A.; Cutroneo, K.R.; Badger, G.J.; White, S.L.; Buttolph, T.R.; Ehrlich, H.P.; Stevens-Tuttle, D.; Langevin, H.M. Tissue Stretch Decreases Soluble TGF-Β1 and Type-1 Procollagen in Mouse Subcutaneous Connective Tissue: Evidence from Ex Vivo and In Vivo Models. J. Cell. Physiol. 2008, 214, 389–395. [Google Scholar] [CrossRef]
- Roberts, A.B.; Flanders, K.C.; Heine, U.I.; Jakowlew, S.; Kondaiah, P.; Kim, S.J.; Sporn, M.B. Transforming Growth Factor-Beta: Multifunctional Regulator of Differentiation and Development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 327, 145–154. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-β Signaling and the Fibrotic Response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Kang, J.H.; Jung, M.Y.; Choudhury, M.; Leof, E.B. Transforming Growth Factor Beta Induces Fibroblasts to Express and Release the Immunomodulatory Protein PD-L1 into Extracellular Vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef]
- Chiquet, M. Regulation of Extracellular Matrix Gene Expression by Mechanical Stress. Matrix Biol. 1999, 18, 417–426. [Google Scholar] [CrossRef]
- Kessler, D.; Dethlefsen, S.; Haase, I.; Plomann, M.; Hirche, F.; Krieg, T.; Eckes, B. Fibroblasts in Mechanically Stressed Collagen Lattices Assume a “Synthetic” Phenotype. J. Biol. Chem. 2001, 276, 36575–36585. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Ho, C.H. Transforming Growth Factor β Stimulates Fibroblast-Collagen Matrix Contraction by Different Mechanisms in Mechanically Loaded and Unloaded Matrices. Exp. Cell Res. 2002, 273, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Atance, J.; Yost, M.J.; Carver, W. Influence of the Extracellular Matrix on the Regulation of Cardiac Fibroblast Behavior by Mechanical Stretch. J. Cell. Physiol. 2004, 200, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, J.L.; Billiar, K.L. Equibiaxial Cyclic Stretch Stimulates Fibroblasts to Rapidly Remodel Fibrin. J. Biomech. 2006, 39, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.N.; He, J.L.; Zuo, X.Q.; Xie, H.M.; Jia, Z.S. The Therapeutic Effect and Mechanism of Static Progressive Stretching in Different Durations on Traumatic Knee Contracture in Rats. J. Sichuan Univ. 2020, 51, 185–192. (In Chinese) [Google Scholar] [CrossRef]
- Wiig, M.E.; Amiel, D.; Ivarsson, M.; Nagineni, C.N.; Wallace, C.D.; Arfors, K.-E. Type I Procollagen Gene Expression in Normal and Early Healing of the Medial Collateral and Anterior Cruciate Ligaments in Rabbits: An in Situ Hybridization Study. J. Orthop. Res. 1991, 9, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Fosang, A.J.; Last, K.; Knäuper, V.; Murphy, G.; Neame, P.J. Degradation of Cartilage Aggrecan by Collagenase-3 (MMP-13). FEBS Lett. 1996, 380, 17–20. [Google Scholar] [CrossRef]
- Sobolewski, P.; Maślińska, M.; Wieczorek, M.; Łagun, Z.; Malewska, A.; Roszkiewicz, M.; Nitskovich, R.; Szymańska, E.; Walecka, I. Systemic Sclerosis—Multidisciplinary Disease: Clinical Features and Treatment. Reumatologia 2019, 57, 221–233. [Google Scholar] [CrossRef]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Sargent, J.L.; Farina, G.; Tsang, K.; Lafyatis, R.; Glimcher, L.H.; Whitfield, M.L.; Aliprantis, A.O. Interspecies Comparison of Human and Murine Scleroderma Reveals IL-13 and CCL2 as Disease Subset-Specific Targets. Am. J. Pathol. 2012, 180, 1080–1094. [Google Scholar] [CrossRef]
- Sargent, J.L.; Li, Z.; Aliprantis, A.O.; Greenblatt, M.; Lemaire, R.; Wu, M.H.; Wei, J.; Taroni, J.; Harris, A.; Long, K.B.; et al. Identification of Optimal Mouse Models of Systemic Sclerosis by Interspecies Comparative Genomics. Arthritis Rheumatol. 2016, 68, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Guiducci, S.; Romano, E.; Bellando-Randone, S.; Conforti, M.L.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Increased Serum Levels and Tissue Expression of Matrix Metalloproteinase-12 in Patients with Systemic Sclerosis: Correlation with Severity of Skin and Pulmonary Fibrosis and Vascular Damage. Ann. Rheum. Dis. 2012, 71, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R. Transforming Growth Factor β- At the Centre of Systemic Sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J. Exercise for the Patient after Breast Cancer Surgery. Semin. Oncol. Nurs. 2017, 33, 98–105. [Google Scholar] [CrossRef]
- De Groef, A.; Van Kampen, M.; Dieltjens, E.; Christiaens, M.R.; Neven, P.; Geraerts, I.; Devoogdt, N. Effectiveness of Postoperative Physical Therapy for Upper-Limb Impairments after Breast Cancer Treatment: A Systematic Review. Arch. Phys. Med. Rehabil. 2015, 96, 1140–1153. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Khori, V.; Amani Shalamzari, S.; Isanejad, A.; Alizadeh, A.M.; Alizadeh, S.; Khodayari, S.; Khodayari, H.; Shahbazi, S.; Zahedi, A.; Sohanaki, H.; et al. Effects of Exercise Training Together with Tamoxifen in Reducing Mammary Tumor Burden in Mice: Possible Underlying Pathway of MIR-21. Eur. J. Pharmacol. 2015, 765, 179–187. [Google Scholar] [CrossRef]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. J. Natl. Cancer Inst. 2015, 107, djv040. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, X.X.; Huang, M.T.; Liu, Y.; Wagner, G.C.; Lin, Y.; Shih, W.J.; Lee, M.J.; Yang, C.S.; Conney, A.H. Inhibition of Progression of Androgen-Dependent Prostate LNCaP Tumors to Androgen Independence in SCID Mice by Oral Caffeine and Voluntary Exercise. Nutr. Cancer 2012, 64, 1029–1037. [Google Scholar] [CrossRef]
- Berrueta, L.; Bergholz, J.; Munoz, D.; Muskaj, I.; Badger, G.J.; Shukla, A.; Kim, H.J.; Zhao, J.J.; Langevin, H.M. Stretching Reduces Tumor Growth in a Mouse Breast Cancer Model. Sci. Rep. 2018, 8, 7864. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of Interferon-γ During Innate and Adaptive Immune Responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines With Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of Adipose Tissue Inflammation by Interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef]
- Grandys, M.; Majerczak, J.; Zapart-Bukowska, J.; Duda, K.; Kulpa, J.K.; Zoladz, J.A. Lowered Serum Testosterone Concentration Is Associated With Enhanced Inflammation and Worsened Lipid Profile in Men. Front. Endocrinol. 2021, 12, 735638. [Google Scholar] [CrossRef]
- Chidi-Ogbolu, N.; Baar, K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front. Physiol. 2019, 9, 1834. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ono, Y. Estrogens Maintain Skeletal Muscle and Satellite Cell Functions. J. Endocrinol. 2016, 229, 267–275. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.M.; Davis, J.M.; Wilson, M.A.; Goldsmith, E.C.; Carson, J.A. Estrogen Status and Skeletal Muscle Recovery from Disuse Atrophy. J. Appl. Physiol. 2006, 100, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
| Treatment/Model | Results | References |
|---|---|---|
| Inflammatory Lesion and Tissue Morphology | ||
| Active and passive stretching/carrageenan-induced inflammation | ↓ CD68 expression (macrophages number) ↓ thickness of the inflammatory lesion ↓ lesion mass ↓ number of neutrophil granulocytes and the total number of cells in the inflamed area ↓ neutrophil migration | [47,48,49] |
| Static progressive stretching/post-traumatic knee contracture model | ↓ number of inflammatory cells | [59] |
| MFR/RMS-induced inflammation, fibroblasts | ↓ intercellular distances | [50] |
| Passive stretching/unilateral fascia injury | ↑ fascia thickness from week 8 to 12 | [77] |
| Static tissue stretch/dermatitis followed by fibrosis (systemic sclerosis-like inflammation) | ↓ thickness of the tissue greater relative tissue displacement ↓ fibroblast expansion ex vivo | [71] |
| Proinflammatory Genes and Cytokine Expression and Collagen Metabolism | ||
| Patient studies | ||
| Yoga-based exercises, TCC/human studies | ↓ IL-6 levels in serum ↑ levels of adiponectin in serum | [35,81] |
| Animal studies | ||
| Active stretching/carrageenan-induced inflammation, | ↑ lipoxin A4 and RvD1 [48] ↓ prostaglandin D2 (PGD2) ↑ the ratio of serum LXA4 or RvD1 to PGD ↑ RvD1 | [48,49] |
| Cell culture studies | ||
| IOMT/RMS | ↑ cell proliferation ↓ IL-6 secretion Inhibition of IL-1α, IL-1β, IL-2, IL-3, IL-6, and IL-16 secretion | [90] |
| Equibiaxial strain | ↓ secretion of CCL18 ↓ cell proliferation ↓ secretions of MDC and IL-6 | [93] |
| MFR/RMS | ↓ apoptosis rate ↓ DAPK-2 ↑ CREBS133 ↑ FAK | [50] |
| ALDS/CSDS | ↓ levels of IL-6 and IL-8 | [100] |
| Static isotropic tensile strain, short-term high-frequency cyclic tension, dynamic tensile stretching, | static tensile strain: ↓ COL1A2 ↑ TNF-α,COX-2, IL-6, IL-1ß short-term high-frequency cyclic tension: ↑ IL-6, ↓ IL-1ß dynamic tensile stretching: ↓ COL1A2, TNF-α, IL-6, IL-1ß | [101] |
| Static progressive stretching/post-traumatic knee contracture model | ↓ collagen proliferation | [59] |
| CTS/Il-1β-induced inflammation | Reversion of Il-1β-induced: iNOS and COX-2 expression NO and PGE2 synthesis MMP-9, MMP-13, MMP-1 gene expression ↑ collagen type II production, suppression of IL-1β-dependent collagenase synthesis Reversion of IL-1β-induced down TIMP2 Block of IL-1β-induced inhibition of type-I collagen synthesis inhibition of nuclear translocation of NF-κB ↓ IκBα and IκBβ transcription inhibition of IκBα and IκBβ degradation nuclear translocation of IκBα | [51,111,112,113] |
| DTF | ↓ the mRNA expression of IL-1β inhibition of IL-1β-dependent induction of iNOS Inhibition of the expression of TNF-α and MMP-13 | [115] |
| Static tissue stretch/injury-induced inflammation | ex vivo: ↓ TGF-β1, IL-6 in vivo: no increase in type-1 procollagen observed in stretched group after injury | [122] |
| Dynamic compressive strain/Il-1β-induced inflammation chondrocytes | ↓ nitrate and PGe2 synthesis ↑ DNA synthesis (3H-thymidine incorporation) ↑ sulfate incorporation | [114] |
| CTS and gallic acid/osteoarthritic human articular chondrocytes | ↑ glycosaminoglycan, collagen type II and IX | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, M.; Kupnicka, P.; Bosiacki, M.; Chlubek, D. Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review. Int. J. Mol. Sci. 2022, 23, 10127. https://doi.org/10.3390/ijms231710127
Król M, Kupnicka P, Bosiacki M, Chlubek D. Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review. International Journal of Molecular Sciences. 2022; 23(17):10127. https://doi.org/10.3390/ijms231710127
Chicago/Turabian StyleKról, Małgorzata, Patrycja Kupnicka, Mateusz Bosiacki, and Dariusz Chlubek. 2022. "Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review" International Journal of Molecular Sciences 23, no. 17: 10127. https://doi.org/10.3390/ijms231710127
APA StyleKról, M., Kupnicka, P., Bosiacki, M., & Chlubek, D. (2022). Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review. International Journal of Molecular Sciences, 23(17), 10127. https://doi.org/10.3390/ijms231710127