Expression of Eosinophilic Subtype Markers in Patients with Kawasaki Disease
Abstract
:1. Background
2. Materials and Methods
mRNA Detected by the Quantitative Reverse Transcriptase-Polymerase Chain Reaction
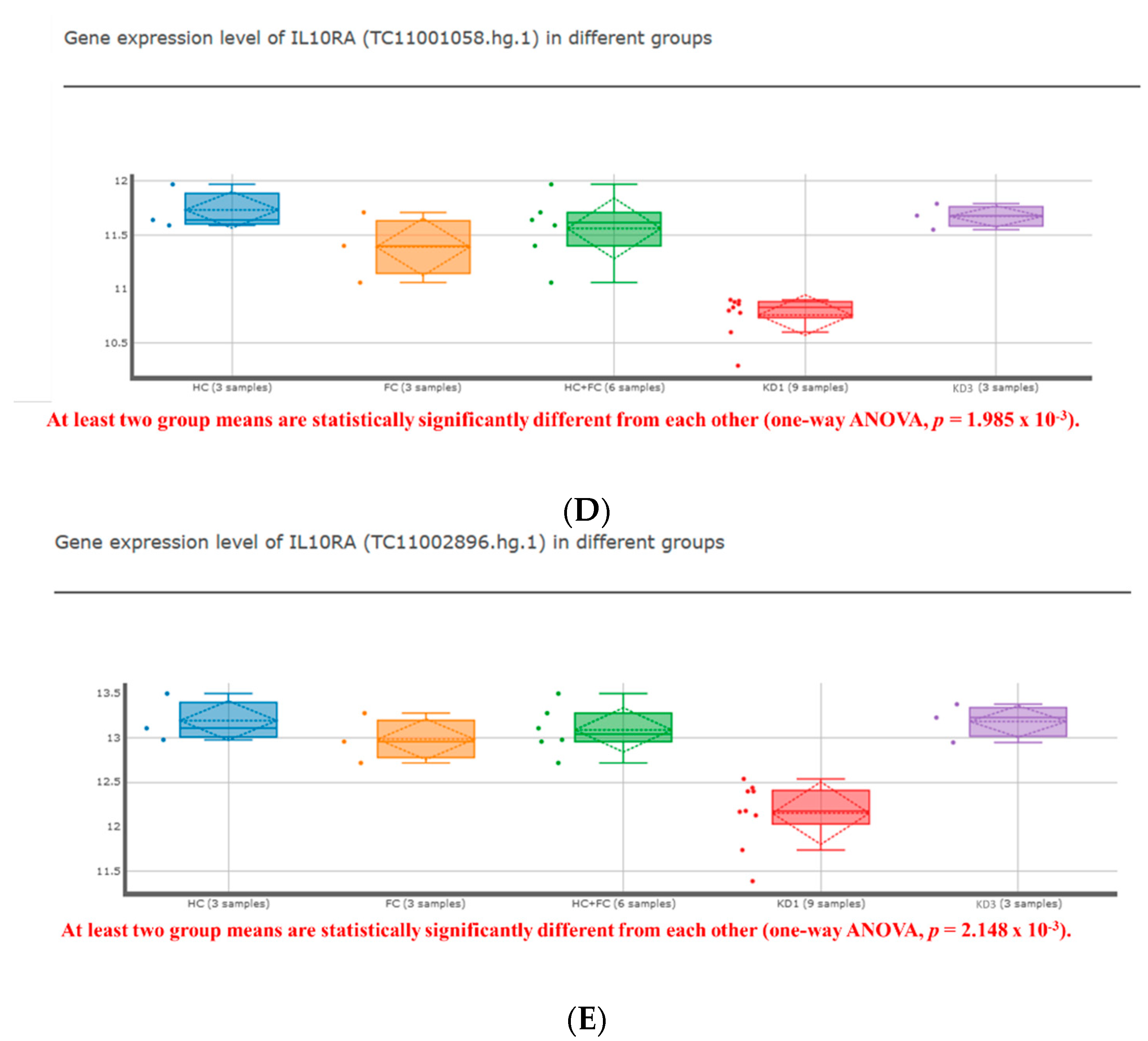
3. Results
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lei, W.T.; Chang, L.S.; Zeng, B.Y.; Tu, Y.K.; Uehara, R.; Matsuoka, Y.J.; Su, K.P.; Lee, P.C.; Cavalcante, J.L.; Stubbs, B.; et al. Pharmacologic interventions for Kawasaki disease in children: A network meta-analysis of 56 randomized controlled trials. EBioMedicine 2022, 78, 103946. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.J.; Carter, K.W.; Warrington, N.M.; Loh, A.M.; Zaloumis, S.; Kuijpers, T.W.; Palmer, L.J.; Burgner, D.P. Hospitalisation with infection, asthma and allergy in Kawasaki disease patients and their families: Genealogical analysis using linked population data. PLoS ONE 2011, 6, e28004. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.-T.; Hsu, C.-W.; Chen, P.-C.; Tseng, P.-T.; Kuo, H.-C.; Guo, M.M.-H.; Tu, Y.-K.; Lin, P.-Y.; Kao, Y.-H.; Chang, L.-S. Increased Risk of Asthma and Allergic Rhinitis in Patients with a Past History of Kawasaki Disease: A Systematic Review and Meta-Analyses. Front. Pediatrics 2021, 9, 746856. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Huang, Y.H.; Guo, M.M.; Chang, L.S.; Kuo, H.C. Kawasaki Disease and Allergic Diseases. Front. Pediatrics 2020, 8, 614386. [Google Scholar] [CrossRef]
- Chang, L.S.; Chen, Y.J.; Huang, P.Y.; Chen, K.D.; Lo, M.H.; Huang, Y.H.; Guo, M.M.; Kuo, H.C. Significantly Lower Immunoglobulin M Levels 6 Months after Disease Onset in Patients with Kawasaki Disease with Coronary Artery Lesions. J. Am. Heart Assoc. 2021, 10, e020505. [Google Scholar] [CrossRef]
- Chang, L.S.; Guo, M.M.; Yan, J.H.; Huang, Y.H.; Lo, M.H.; Kuo, H.C. Low FCMR mRNA expression in leukocytes of patients with Kawasaki disease six months after disease onset. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2020, 31, 554–559. [Google Scholar] [CrossRef]
- Kuo, H.C.; Chang, W.C.; Yang, K.D.; Yu, H.R.; Wang, C.L.; Ho, S.C.; Yang, C.Y. Kawasaki disease and subsequent risk of allergic diseases: A population-based matched cohort study. BMC Pediatrics 2013, 13, 38. [Google Scholar] [CrossRef]
- Kuo, H.C.; Wang, C.L.; Liang, C.D.; Yu, H.R.; Huang, C.F.; Wang, L.; Hwang, K.P.; Yang, K.D. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2009, 20, 266–272. [Google Scholar] [CrossRef]
- Kuo, H.C.; Yang, K.D.; Liang, C.D.; Bong, C.N.; Yu, H.R.; Wang, L.; Wang, C.L. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2007, 18, 354–359. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, H.J.; Yu, J.J.; Yun, S.W.; Lee, K.Y.; Yoon, K.L.; Kil, H.R.; Kim, G.B.; Han, M.K.; Song, M.S.; et al. IgA Levels Are Associated with Coronary Artery Lesions in Kawasaki Disease. Korean Circ. J. 2021, 51, 267–278. [Google Scholar] [CrossRef]
- Berek, C. Eosinophils: Important players in humoral immunity. Clin. Exp. Immunol. 2016, 183, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Yasutaka, Y.; Van Bui, D.; Suzuki, K.; Sawada, S.; Kobayashi, Y.; Asako, M.; Iwai, H. Multiple Biological Aspects of Eosinophils in Host Defense, Eosinophil-Associated Diseases, Immunoregulation, and Homeostasis: Is Their Role Beneficial, Detrimental, Regulator, or Bystander? Biol. Pharm. Bull. 2020, 43, 20–30. [Google Scholar] [CrossRef]
- Ferrari, D.; Vuerich, M.; Casciano, F.; Longhi, M.S.; Melloni, E.; Secchiero, P.; Zech, A.; Robson, S.C.; Müller, T.; Idzko, M. Eosinophils and Purinergic Signaling in Health and Disease. Front. Immunol. 2020, 11, 1339. [Google Scholar] [CrossRef]
- Arnold, I.C.; Artola-Borán, M.; Tallón de Lara, P.; Kyburz, A.; Taube, C.; Ottemann, K.; van den Broek, M.; Yousefi, S.; Simon, H.U.; Müller, A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 2018, 215, 2055–2072. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Chu, C.H.; Liu, X.; Weng, K.P.; Liu, S.F.; Huang, Y.H.; Kuo, H.C. A novel score system of blood tests for differentiating Kawasaki disease from febrile children. PLoS ONE 2021, 16, e0244721. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Huang, Y.S.; Xia, H.B.; Sun, Y.; Lang, X.L.; Li, Q.Z.; Liu, C.Y.; Kuo, H.C.; Huang, W.D.; Liu, X. A Nomogram Model Identifies Eosinophilic Frequencies to Powerfully Discriminate Kawasaki Disease from Febrile Infections. Front. Pediatrics 2020, 8, 559389. [Google Scholar] [CrossRef] [PubMed]
- Tremoulet, A.H.; Jain, S.; Chandrasekar, D.; Sun, X.; Sato, Y.; Burns, J.C. Evolution of laboratory values in patients with Kawasaki disease. Pediatric Infect. Dis. J. 2011, 30, 1022–1026. [Google Scholar] [CrossRef]
- Chen, Y.J.; Guo, M.M.; Chang, L.S.; Kuo, H.C. The Impact of Onset Age on Eosinophils in Kawasaki Disease. Biomedicines 2022, 10, 835. [Google Scholar] [CrossRef]
- Terai, M.; Yasukawa, K.; Honda, T.; Jibiki, T.; Hirano, K.; Sato, J.; Ishiwada, N.; Seguchi, M.; Ueda, S.; Kohno, Y. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatric Infect. Dis. J. 2002, 21, 777–781. [Google Scholar] [CrossRef]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [Green Version]
- Dolitzky, A.; Shapira, G.; Grisaru-Tal, S.; Hazut, I.; Avlas, S.; Gordon, Y.; Itan, M.; Shomron, N.; Munitz, A. Transcriptional Profiling of Mouse Eosinophils Identifies Distinct Gene Signatures Following Cellular Activation. Front. Immunol. 2021, 12, 802839. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.A.; Bochner, B.S. Eosinophils and eosinophil-associated diseases: An update. J. Allergy Clin. Immunol. 2018, 141, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Mesnil, C.; Bureau, F. Homeostatic Eosinophils: Characteristics and Functions. Front. Med. 2017, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Januskevicius, A.; Jurkeviciute, E.; Janulaityte, I.; Kalinauskaite-Zukauske, V.; Miliauskas, S.; Malakauskas, K. Blood Eosinophils Subtypes and Their Survivability in Asthma Patients. Cells 2020, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Nencini, F.; Maggiore, G.; Chiccoli, F.; Accinno, M.; Vivarelli, E.; Bruno, C.; Locatello, L.G.; Palomba, A.; Nucci, E.; et al. High proportion of inflammatory CD62L(low) eosinophils in blood and nasal polyps of severe asthma patients. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2022. [Google Scholar] [CrossRef]
- Furui, J. Soluble forms of P-, E- and L-selectin in children with Kawasaki disease. Kurume Med. J. 2001, 48, 135–143. [Google Scholar] [CrossRef]
- Tsutsumiuchi, T.; Hoshino, H.; Fujieda, S.; Kobayashi, M. Induction of peripheral lymph node addressin in human nasal mucosa with eosinophilic chronic rhinosinusitis. Pathology 2019, 51, 268–273. [Google Scholar] [CrossRef]
- Chang, L.S.; Ming-Huey Guo, M.; Lo, M.H.; Kuo, H.C. Identification of increased expression of activating Fc receptors and novel findings regarding distinct IgE and IgM receptors in Kawasaki disease. Pediatric Res. 2019, 89, 191–197. [Google Scholar] [CrossRef]
- Wu, W.S.; Yang, T.H.; Chen, K.D.; Lin, P.H.; Chen, G.R.; Kuo, H.C. KDmarkers: A biomarker database for investigating epigenetic methylation and gene expression levels in Kawasaki disease. Comput. Struct. Biotechnol. J. 2022, 20, 1295–1305. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Kobayashi, T.; Saji, T.; Otani, T.; Takeuchi, K.; Nakamura, T.; Arakawa, H.; Kato, T.; Hara, T.; Hamaoka, K.; Ogawa, S.; et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): A randomised, open-label, blinded-endpoints trial. Lancet 2012, 379, 1613–1620. [Google Scholar] [CrossRef]
- Lin, M.T.; Chang, C.H.; Hsieh, W.C.; Chang, C.E.; Chang, Y.M.; Chen, Y.C.; Hsu, J.Y.; Huang, Y.L.; Ma, J.Y.; Sun, L.C.; et al. Coronary Diameters in Taiwanese Children Younger than 6 Years Old: Z-Score Regression Equations Derived from Body Surface Area. Acta Cardiol. Sin. 2014, 30, 266–273. [Google Scholar] [PubMed]
- Guo, M.M.; Chang, L.S.; Huang, Y.H.; Wang, F.S.; Kuo, H.C. Epigenetic Regulation of Macrophage Marker Expression Profiles in Kawasaki Disease. Front. Pediatrics 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Um, H.N.; Jang, J.; Bae, Y.A.; Park, W.J.; Kim, H.J.; Yoon, M.S.; Chung, I.Y.; Jung, Y. Eosinophil Activation by Toll-Like Receptor 4 Ligands Regulates Macrophage Polarization. Front. Cell Dev. Biol. 2019, 7, 329. [Google Scholar] [CrossRef]
- Polukort, S.H.; Rovatti, J.; Carlson, L.; Thompson, C.; Ser-Dolansky, J.; Kinney, S.R.; Schneider, S.S.; Mathias, C.B. IL-10 Enhances IgE-Mediated Mast Cell Responses and Is Essential for the Development of Experimental Food Allergy in IL-10-Deficient Mice. J. Immunol. 2016, 196, 4865–4876. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, Y.H.; Hyun, M.C.; Kim, Y.H.; Kim, H.S.; Lee, Y.H. T-Helper Cytokine Profiles in Patients with Kawasaki Disease. Korean Circ. J. 2015, 45, 516–521. [Google Scholar] [CrossRef]
- Cui, H.D.; Qi, Z.M.; Yang, L.L.; Qi, L.; Zhang, N.; Zhang, X.L.; Du, S.Y.; Jiang, Y. Interleukin-10 receptor expression and signalling were down-regulated in CD4⁺ T cells of lupus nephritis patients. Clin. Exp. Immunol. 2011, 165, 163–171. [Google Scholar] [CrossRef]
- Hellmark, T.; Ohlsson, S.; Pettersson, Å.; Hansson, M.; Johansson, Å.C.M. Eosinophils in anti-neutrophil cytoplasmic antibody associated vasculitis. BMC Rheumatol. 2019, 3, 9. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kimura, H.; Okada, Y.; Inoue, Y.; Kobayashi, T.; Shinohara, M.; Morikawa, A. Increased CD11b expression on polymorphonuclear leucocytes and cytokine profiles in patients with Kawasaki disease. Clin. Exp. Immunol. 2007, 148, 112–118. [Google Scholar] [CrossRef]
- Geng, Z.; Tao, Y.; Zheng, F.; Wu, L.; Wang, Y.; Wang, Y.; Sun, Y.; Fu, S.; Wang, W.; Xie, C.; et al. Altered Monocyte Subsets in Kawasaki Disease Revealed by Single-cell RNA-Sequencing. J. Inflamm. Res. 2021, 14, 885–896. [Google Scholar] [CrossRef]
- Hou, L.; Yuki, K. SerpinB1 expression in Th17 cells depends on hypoxia-inducible factor 1-alpha. Int. Immunopharmacol. 2020, 87, 106826. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Li, C.; Wang, G.; Yang, J.; Zu, Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin. Exp. Immunol. 2010, 162, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Su, Y.; Huo, Y. Forkhead box protein O1 (FoxO1)/SERPINB1 ameliorates ROS production in diabetic nephropathy. Food Sci. Nutr. 2021, 9, 44–51. [Google Scholar] [CrossRef]
- Kumari, S.; Arora, M.; Singh, J.; Chauhan, S.S.; Kumar, S.; Chopra, A. L-Selectin expression is associated with inflammatory microenvironment and favourable prognosis in breast cancer. 3 Biotech 2021, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef]



| Non-Febrile Controls | Febrile Controls | KD before IVIG | KD after IVIG | |
|---|---|---|---|---|
| (n = 18) | (n = 18) | (n = 50) | (n =1 8) | |
| Age (years) | 2.46 ± 0.35 | 1.97 ± 0.26 | 1.36 ± 0.14 | 1.90 ± 0.32 |
| Male/female ratio | 1 | 0.8 | 1.17 | 1 |
| (A) | ||||||||
| Gene Symbol | RefSeq | Column ID | Fold-Change (KD1 vs. HC) | p-Value (KD1 vs. HC) | Fold-Change (KD1 vs. FC) | p-Value (KD1 vs. FC) | Fold-Change (KD3 vs. KD1) | p-Value (KD3 vs. KD1) |
| PDE2A | NM_001143839 | TC11002059.hg.1 | 1.133 | 0.223 | 1.074 | 0.474 | 1.040 | 0.743 |
| LAIR1 | NM_002287 | TC19001844.hg.1 | 1.386 | 0.001 ** | 1.130 | 0.108 | −1.421 | <0.001 ** |
| SELL | NM_000655 | TC01003500.hg.1 | 1.355 | 0.005 ** | 1.312 | 0.009 ** | −1.484 | 0.001 ** |
| PRG3 | NM_006093 | TC11001790.hg.1 | 1.035 | 0.718 | −1.046 | 0.634 | 1.032 | 0.739 |
| RUNX3 | NM_001031680 | TC01002370.hg.1 | −1.274 | 0.004 ** | −1.224 | 0.011 * | 1.245 | 0.007 ** |
| P2RX4 | NM_002560 | TC12000956.hg.1 | 1.010 | 0.864 | −1.148 | 0.033 * | 1.025 | 0.656 |
| IL12RB2 | NM_001559 | TC01000741.hg.1 | −1.046 | 0.537 | −1.247 | 0.013 * | −1.116 | 0.154 |
| MATERNAL | NM_001004431 | TC17000959.hg.1 | −1.026 | 0.715 | 1.024 | 0.739 | 1.019 | 0.788 |
| PON2 | NM_000305 | TC07001617.hg.1 | −1.076 | 0.395 | −1.081 | 0.369 | 1.057 | 0.519 |
| NEDD4 | NM_006154 | TC15001471.hg.1 | 1.452 | 0.031 * | 1.424 | 0.038 * | −1.441 | 0.033 * |
| ANXA1 | NM_000700 | TC09000335.hg.1 | 1.415 | 0.092 | 1.709 | 0.018* | −1.525 | 0.049 * |
| LDLR | NM_000527 | TC19000191.hg.1 | 1.322 | 0.017 * | 1.016 | 0.870 | −1.418 | 0.005* |
| MYC | NM_002467 | TC08000749.hg.1 | −1.061 | 0.334 | −1.191 | 0.016 * | 1.116 | 0.093 |
| S1PR2 | NM_004230 | TC19001160.hg.1 | −1.099 | 0.489 | −1.218 | 0.168 | 1.117 | 0.417 |
| SERPINB1 | NM_030666 | TC06001217.hg.1 | 1.955 | 0.001 ** | 1.736 | 0.004 ** | −2.036 | <0.001 ** |
| Rnase1 | NM_002933 | TC14000902.hg.1 | 1.088 | 0.367 | −1.099 | 0.316 | −1.046 | 0.624 |
| Rnase3 | NM_002935 | TC14000072.hg.1 | 2.016 | 0.002 * | 1.475 | 0.039 * | −1.836 | 0.005 |
| Rnase12 | NM_001024822 | TC14000890.hg.1 | −1.008 | 0.854 | −1.045 | 0.310 | 1.014 | 0.745 |
| (B) | ||||||||
| Gene Symbol | RefSeq | Column ID | Fold-Change (KD1 vs. HC) | p-Value (KD1 vs. HC) | Fold-Change (KD1 vs. FC) | p-Value (KD1 vs. FC) | Fold−Change (KD3 vs. KD1) | p-Value (KD3 vs. KD1) |
| CD34 | NM_001025109 | TC01003777.hg.1 | 1.005 | 0.949 | −1.010 | 0.905 | 1.052 | 0.537 |
| CD101 | NM_001256106 | TC01001025.hg.1 | 1.183 | 0.097 | 1.006 | 0.946 | −1.202 | 0.074 |
| RIPK2 | NM_003821 | TC08000545.hg.1 | −1.010 | 0.961 | 1.523 | 0.071 | −1.056 | 0.793 |
| HCAR2 | NM_177551 | TC12002078.hg.1 | 2.185 | 0.025 * | 1.328 | 0.345 | −2.924 | 0.005 ** |
| ITGA1 | NM_181501 | TC05003439.hg.1 | 1.100 | 0.334 | 1.083 | 0.413 | −1.283 | 0.027 * |
| ITGAX | NM_000887 | TC16000375.hg.1 | 1.774 | 0.009 ** | 1.578 | 0.026 * | −1.712 | 0.012 * |
| TLR4 | NM_003266 | TC09000601.hg.1 | 1.858 | 0.019 * | 1.658 | 0.044* | −2.145 | 0.007 ** |
| BCL2A1 | NM_001114735 | TC15001719.hg.1 | 1.242 | 0.144 | 1.615 | 0.007 ** | −1.348 | 0.057 |
| IL13RA1 | NM_001560 | TC0X000575.hg.1 | 1.294 | 0.185 | 1.380 | 0.108 | −1.426 | 0.081 |
| IL1RN | NM_173843 | TC02000720.hg.1 | 1.465 | 0.054 | 1.222 | 0.271 | −1.657 | 0.018 * |
| SLC3A2 | NM_001012662 | TC11000567.hg.1 | −1.160 | 0.057 | −1.281 | 0.006 ** | 1.182 | 0.036 * |
| TREM1 | NM_001242589 | TC06001717.hg.1 | −1.027 | 0.888 | 1.250 | 0.252 | −1.047 | 0.807 |
| C3AR1 | NM_004054 | TC12001171.hg.1 | 3.061 | 0.003 ** | 1.172 | 0.577 | −2.732 | 0.006 ** |
| H1F0 | NM_005318 | TC22000288.hg.1 | 1.103 | 0.293 | −1.055 | 0.555 | −1.089 | 0.354 |
| CD300C (AF251705) | NM_006678 | TC17001858.hg.1 | 1.205 | 0.176 | −1.079 | 0.562 | −1.195 | 0.193 |
| MIF | NM_002415 | TC22001456.hg.1 | −1.119 | 0.402 | −1.182 | 0.225 | 1.018 | 0.892 |
| OLFM4 | NM_006418 | TC13000228.hg.1 | 2.604 | 0.023 * | 1.253 | 0.527 | −2.573 | 0.024 * |
| GRN | NM_002087 | TC17000570.hg.1 | 1.515 | 0.041 * | −1.057 | 0.752 | −1.662 | 0.018 * |
| LPIN1 | NM_145693 | TC02000078.hg.1 | −1.082 | 0.037 * | −1.095 | 0.021 * | 1.077 | 0.046 * |
| CD33 | NM_001772 | TC19000775.hg.1 | 1.197 | 0.012 * | 1.042 | 0.480 | −1.167 | 0.024 * |
| IL10RA | NM_001558 | TC11001058.hg.1 | −1.351 | <0.001 ** | −1.262 | 0.002 ** | 1.300 | 0.001 ** |
| RETN | NM_020415 | TC19000133.hg.1 | 1.365 | 0.042 * | −1.004 | 0.974 | −1.453 | 0.020 * |
| ACP5 | NM_001111034 | TC19001188.hg.1 | −1.023 | 0.731 | −1.111 | 0.133 | 1.007 | 0.914 |
| CXCR2 | NM_001168298 | TC02001292.hg.1 | 1.531 | 0.066 | 1.306 | 0.219 | −1.894 | 0.013 * |
| IL6 | NM_000600 | TC07000137.hg.1 | −1.071 | 0.337 | 1.077 | 0.296 | −1.004 | 0.960 |
| ADORA2A | NM_000675 | TC22001457.hg.1 | −1.025 | 0.658 | −1.053 | 0.375 | 1.121 | 0.069 |
| BCL6 | NM_001130845 | TC03002096.hg.1 | 2.047 | 0.005 ** | 1.675 | 0.024 * | −2.072 | 0.004 ** |
| PGF | NM_001207012 | TC14001313.hg.1 | −1.007 | 0.951 | −1.018 | 0.882 | 1.094 | 0.465 |
| (A) | ||||||||
| Febrile Controls | KD | p-Value | ||||||
| (n = 24) | (n = 94) | |||||||
| Age (years) | 2.14 ± 0.26 | 1.84 ± 0.17 | 0.088 | |||||
| male | 10 | 60 | 0.042 | |||||
| female | 14 | 34 | ||||||
| (B) | ||||||||
| Age (years) | SELL | |||||||
| KD1 | CAL(−) | CAL(+) | p Value | CAL(−) | CAL(+) | p Value | ||
| (n = 55) | (n = 39) | (n = 55) | (n = 39) | |||||
| 1.89 ± 0.19 | 1.78 ± 0.31 | 0.118 | 0.87 ± 0.12 | 1.33 ± 0.18 | 0.012 * | |||
| KD2 | CAL(−) | CAL(+) | p Value | |||||
| (n = 12) | (n = 12) | |||||||
| 1.06 ± 0.15 | 0.94 ± 0.13 | 0.525 | 1.18 ± 0.15 | 1.45 ± 0.20 | 0.326 | |||
| KD3 | CAL(−) | CAL(+) | p Value | |||||
| (n = 16) | (n = 6) | |||||||
| 1.74 ± 0.33 | 1.20 ± 0.23 | 0.417 | 1.59 ± 0.23 | 1.46 ± 0.33 | 0.883 | |||
| KD4 | CAL(−) | CAL(+) | p Value | |||||
| (n = 14) | (n = 6) | |||||||
| 2.53± 0.38 | 1.55 ± 0.18 | 0.070 | 1.84 ± 0.19 | 1.99 ± 0.46 | 1.000 | |||
| (A) | ||||||
| Febrile Controls | KD | p Value | ||||
| (n = 32) | (n = 43) | |||||
| Age (years) | 2.53 ± 0.24 | 2.16 ± 0.29 | 0.055 | |||
| male | 20 | 34 | 0.114 | |||
| female | 12 | 9 | ||||
| (B) | ||||||
| Age (years) | IL10RA | |||||
| KD1 | CAL(−) | CAL(+) | p-Value | CAL(−) | CAL(+) | p-Value |
| (n = 26) | (n = 17) | (n = 26) | (n = 17) | |||
| 2.16 ± 0.33 | 2.17 ± 0.54 | 0.535 | 2.96 ± 0.4 | 0.96 ± 0.19 | <0.001 * | |
| KD2 | CAL(−) | CAL(+) | p-Value | |||
| (n = 26) | (n = 6) | |||||
| 1.97 ±0.30 | 1.32 ± 0.36 | 0.530 | 2.56 ± 0.62 | 0.79 ± 0.32 | 0.043 * | |
| KD4 | CAL(−) | CAL(+) | p-Value | |||
| (n = 24) | (n = 6) | |||||
| 2.41± 0.29 | 2.30 ± 0.54 | 0.876 | 0.77 ± 0.61 | 1.83 ± 0.75 | 0.464 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-S.; Chen, K.-D.; Huang, Y.-H.; Kuo, H.-C. Expression of Eosinophilic Subtype Markers in Patients with Kawasaki Disease. Int. J. Mol. Sci. 2022, 23, 10093. https://doi.org/10.3390/ijms231710093
Chang L-S, Chen K-D, Huang Y-H, Kuo H-C. Expression of Eosinophilic Subtype Markers in Patients with Kawasaki Disease. International Journal of Molecular Sciences. 2022; 23(17):10093. https://doi.org/10.3390/ijms231710093
Chicago/Turabian StyleChang, Ling-Sai, Kuang-Den Chen, Ying-Hsien Huang, and Ho-Chang Kuo. 2022. "Expression of Eosinophilic Subtype Markers in Patients with Kawasaki Disease" International Journal of Molecular Sciences 23, no. 17: 10093. https://doi.org/10.3390/ijms231710093
APA StyleChang, L.-S., Chen, K.-D., Huang, Y.-H., & Kuo, H.-C. (2022). Expression of Eosinophilic Subtype Markers in Patients with Kawasaki Disease. International Journal of Molecular Sciences, 23(17), 10093. https://doi.org/10.3390/ijms231710093








