Structural Design and Finite Element Simulation Analysis of Grade 3 Graded Porous Titanium Implant
Abstract
:1. Introduction
2. Results and Discussion
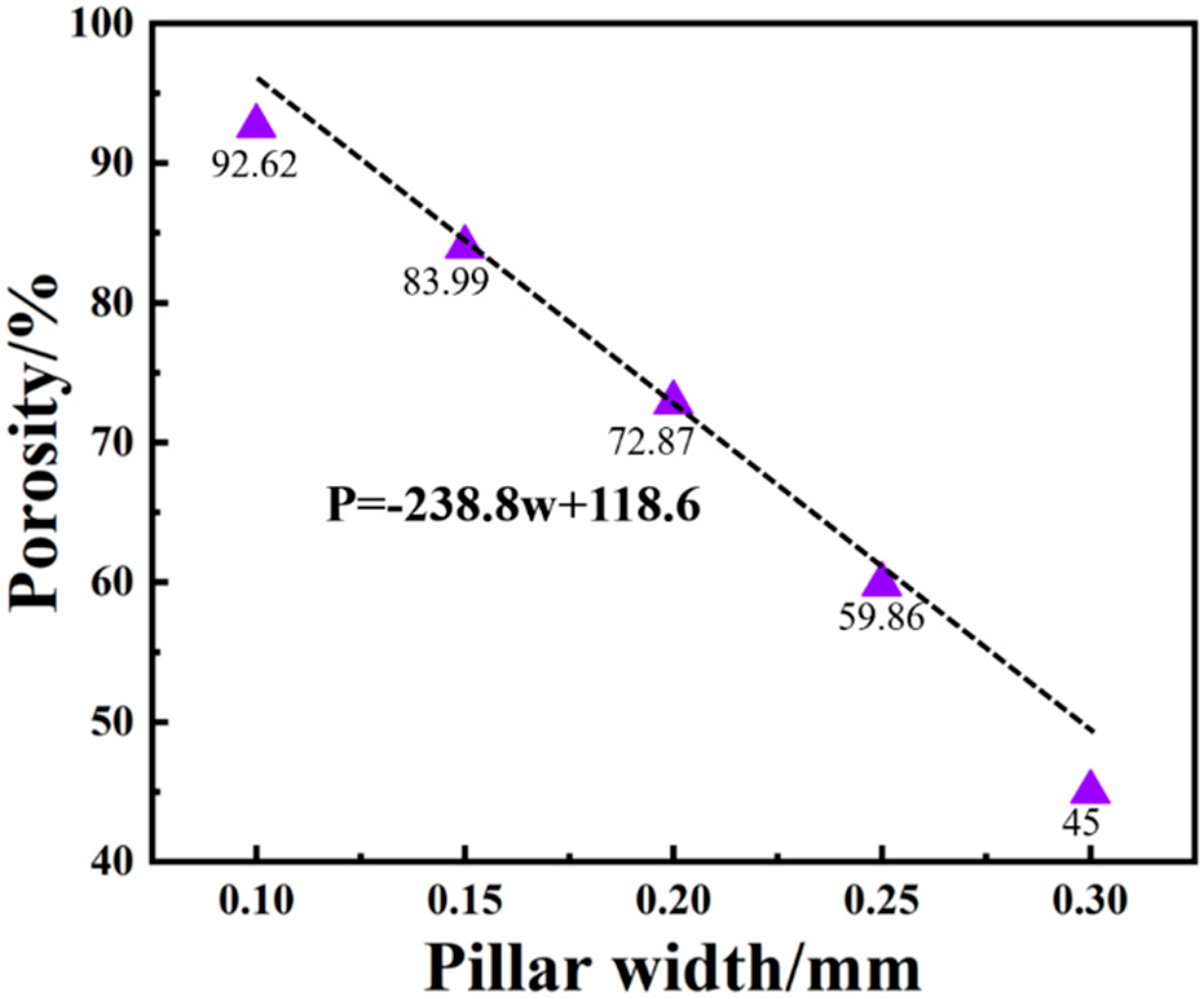
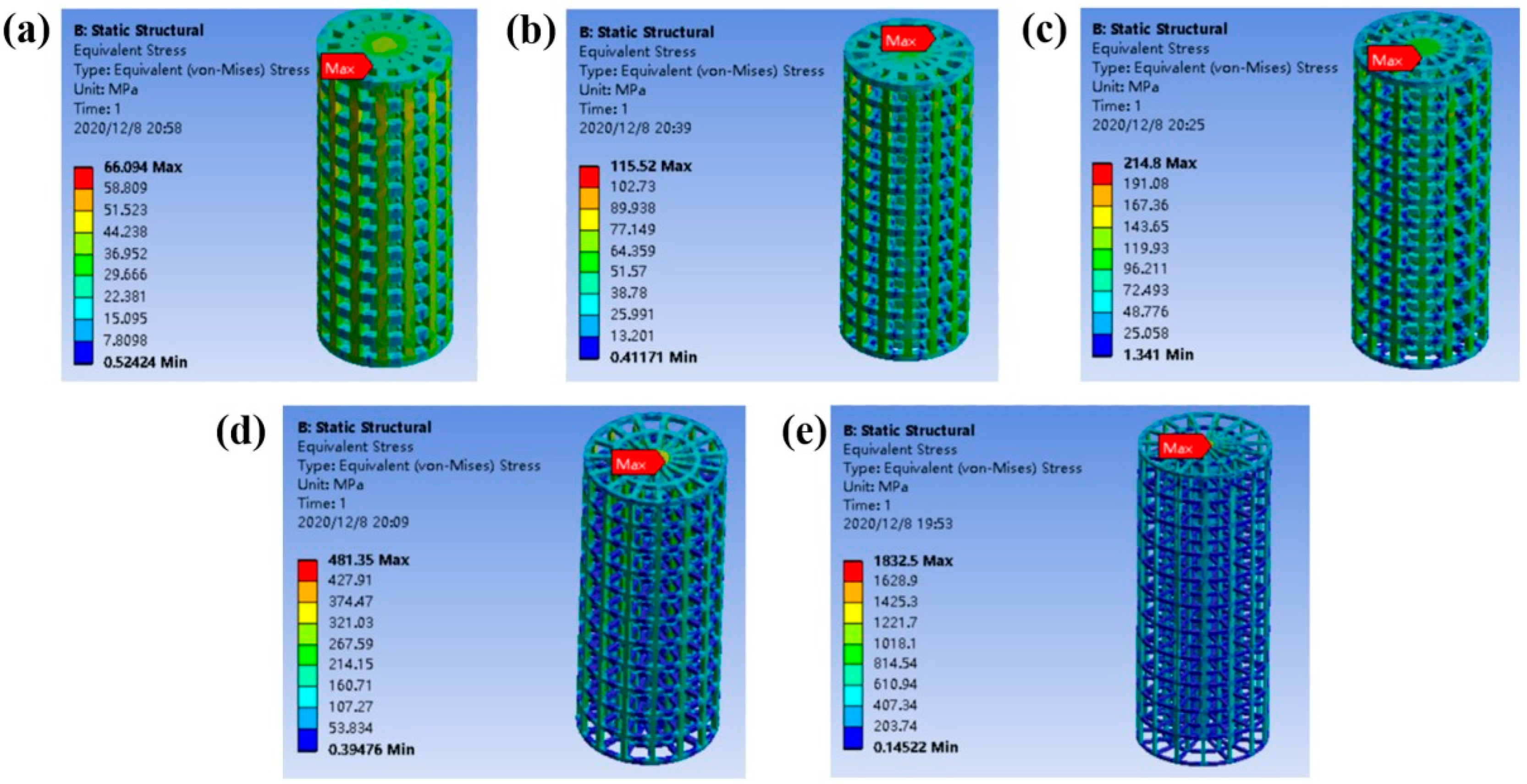
2.1. Mechanical Properties of Columns with Different Gradient Porous Structures
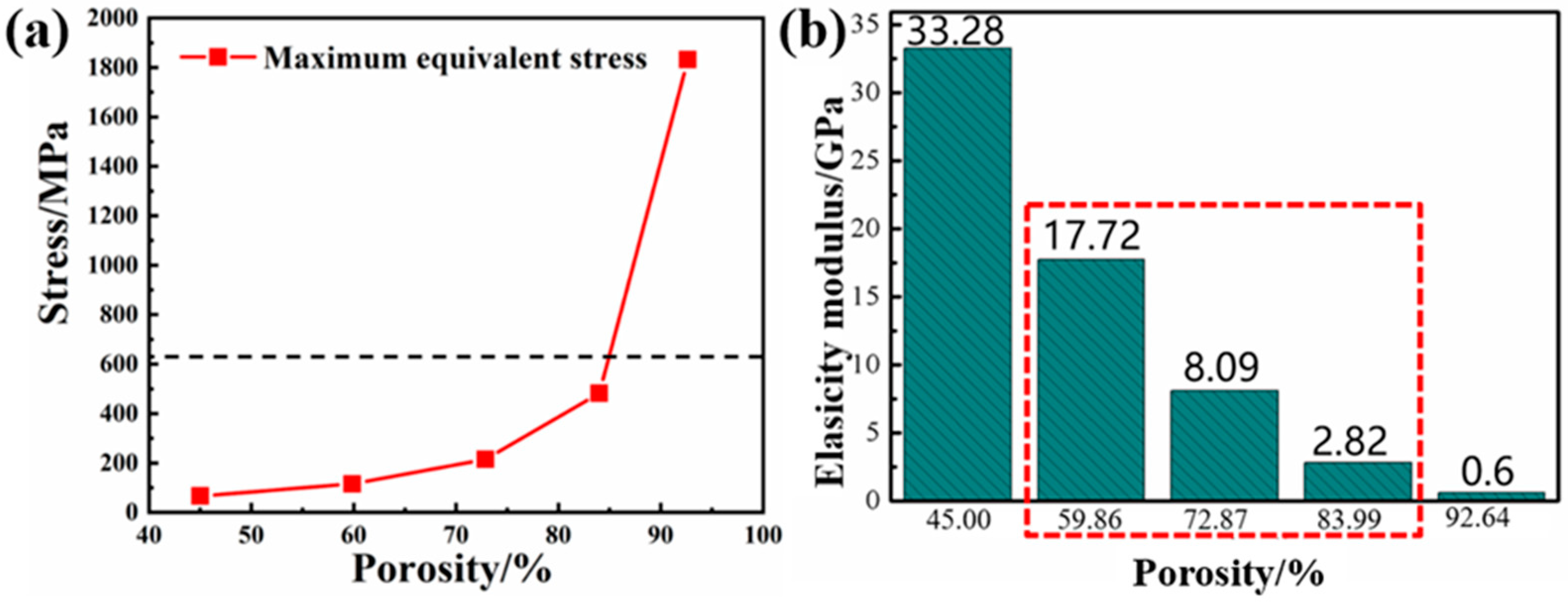
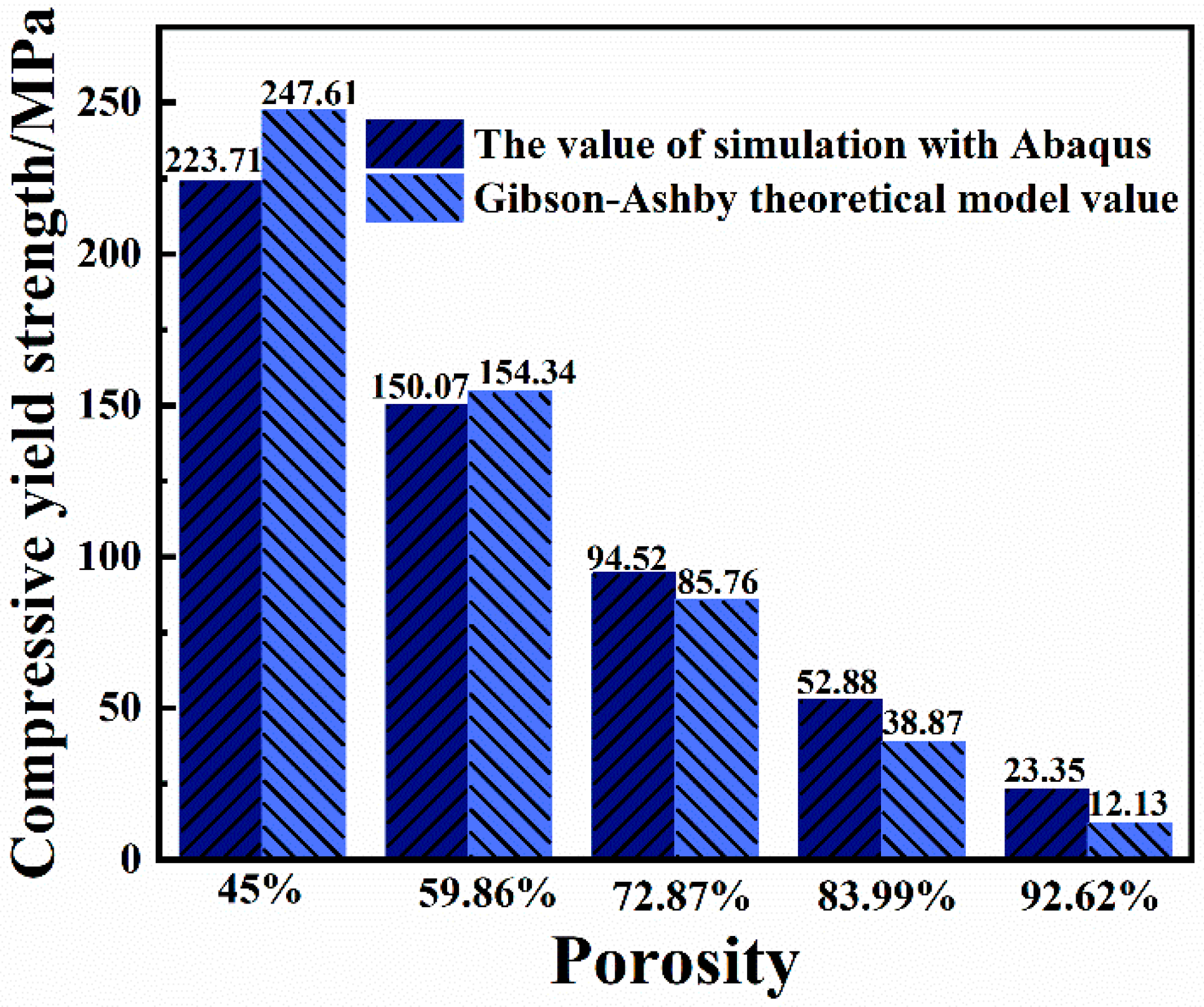
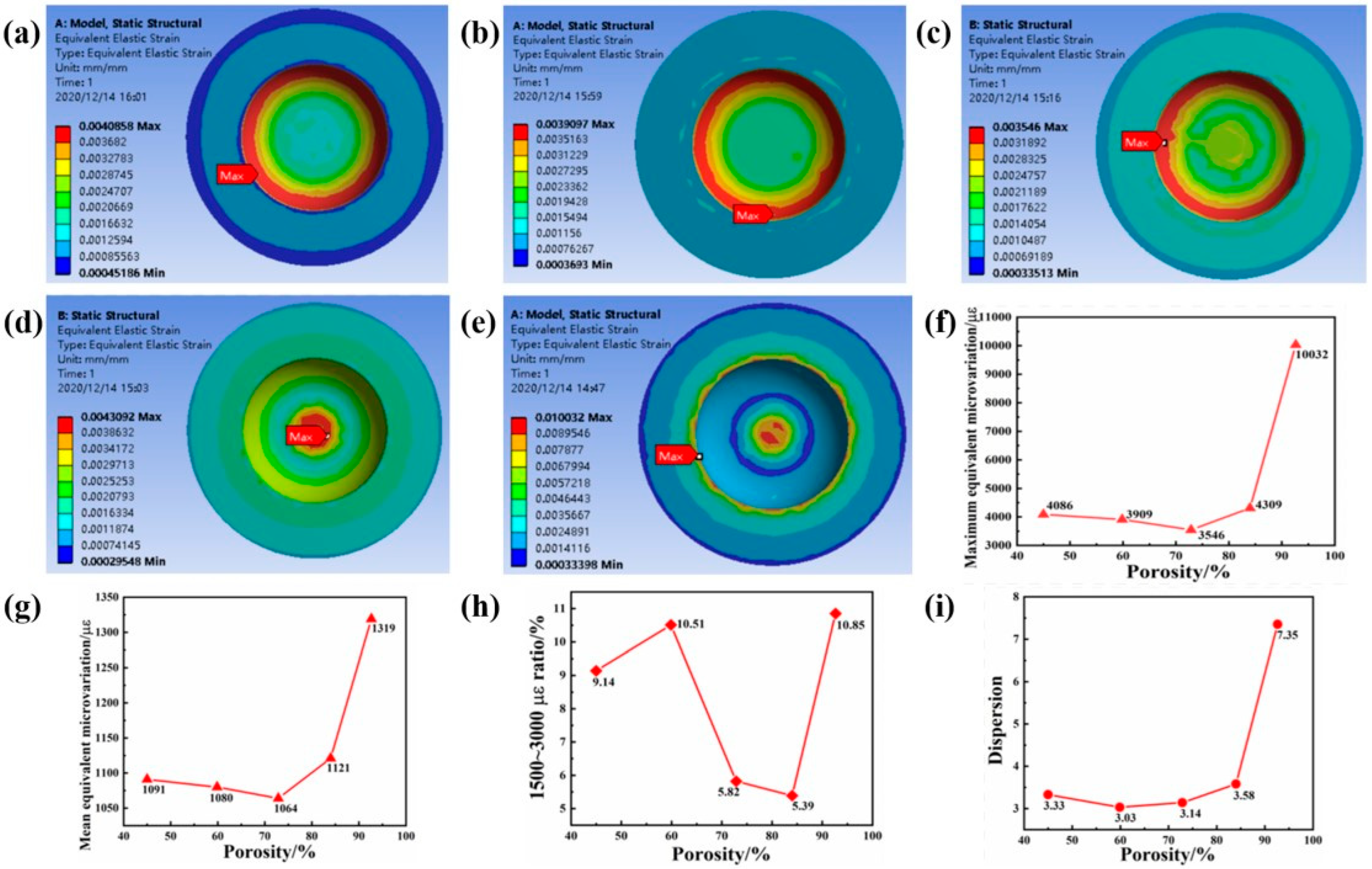
2.2. The Influence of Porosity on the Mechanical Properties of Implants
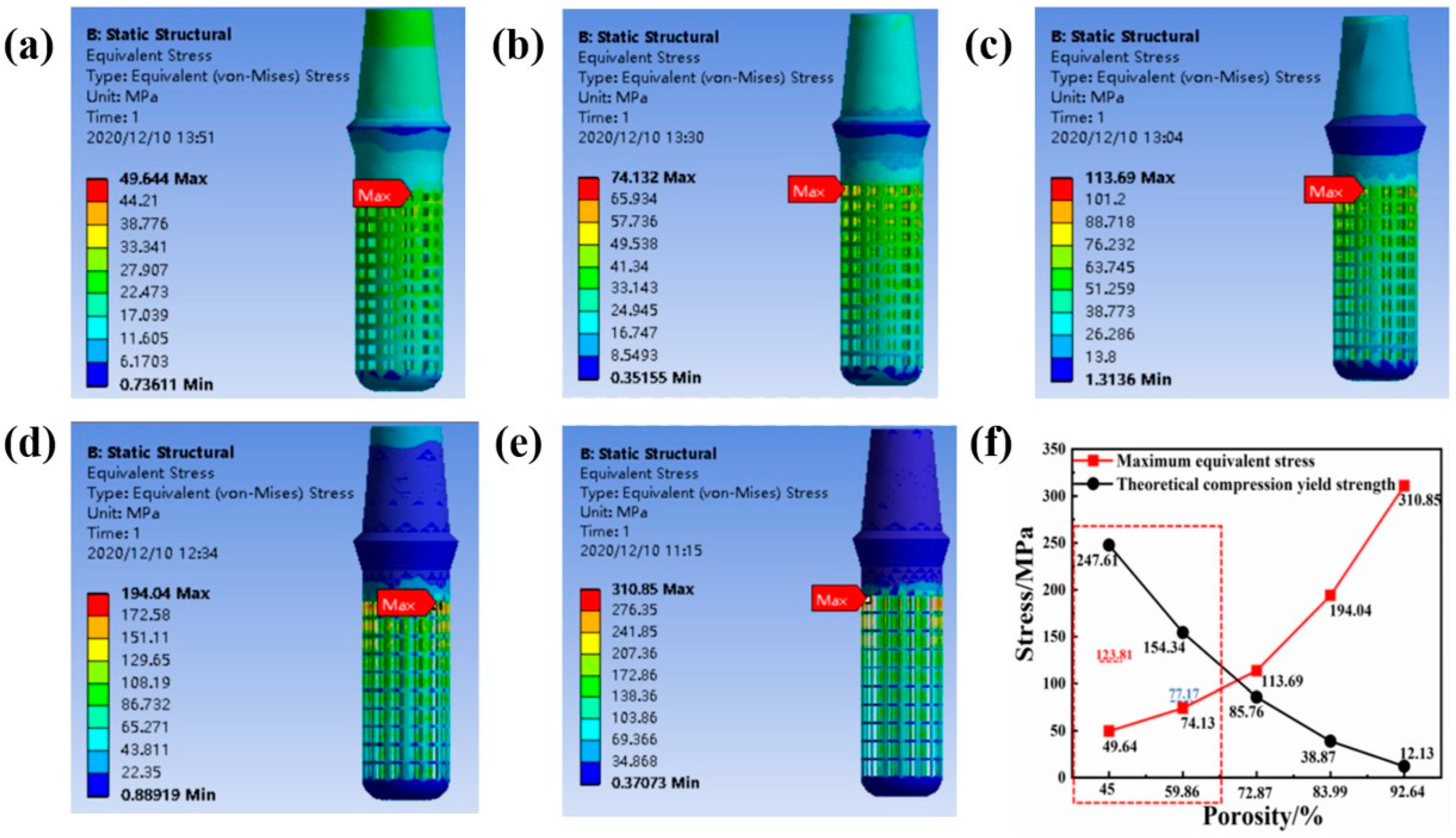
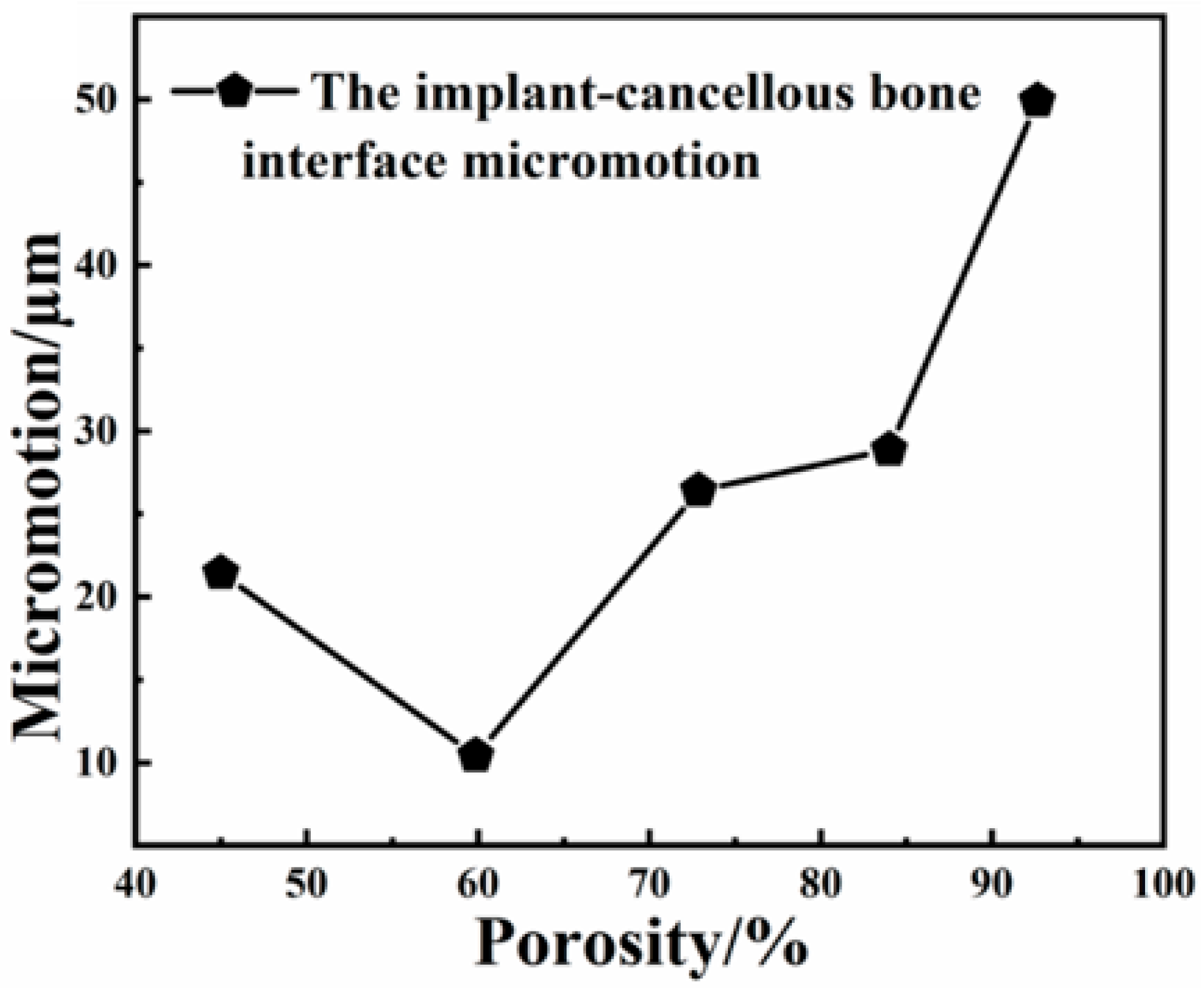
2.3. The Effect of Porosity on the Biological Fit of Implants
3. Materials and Methods
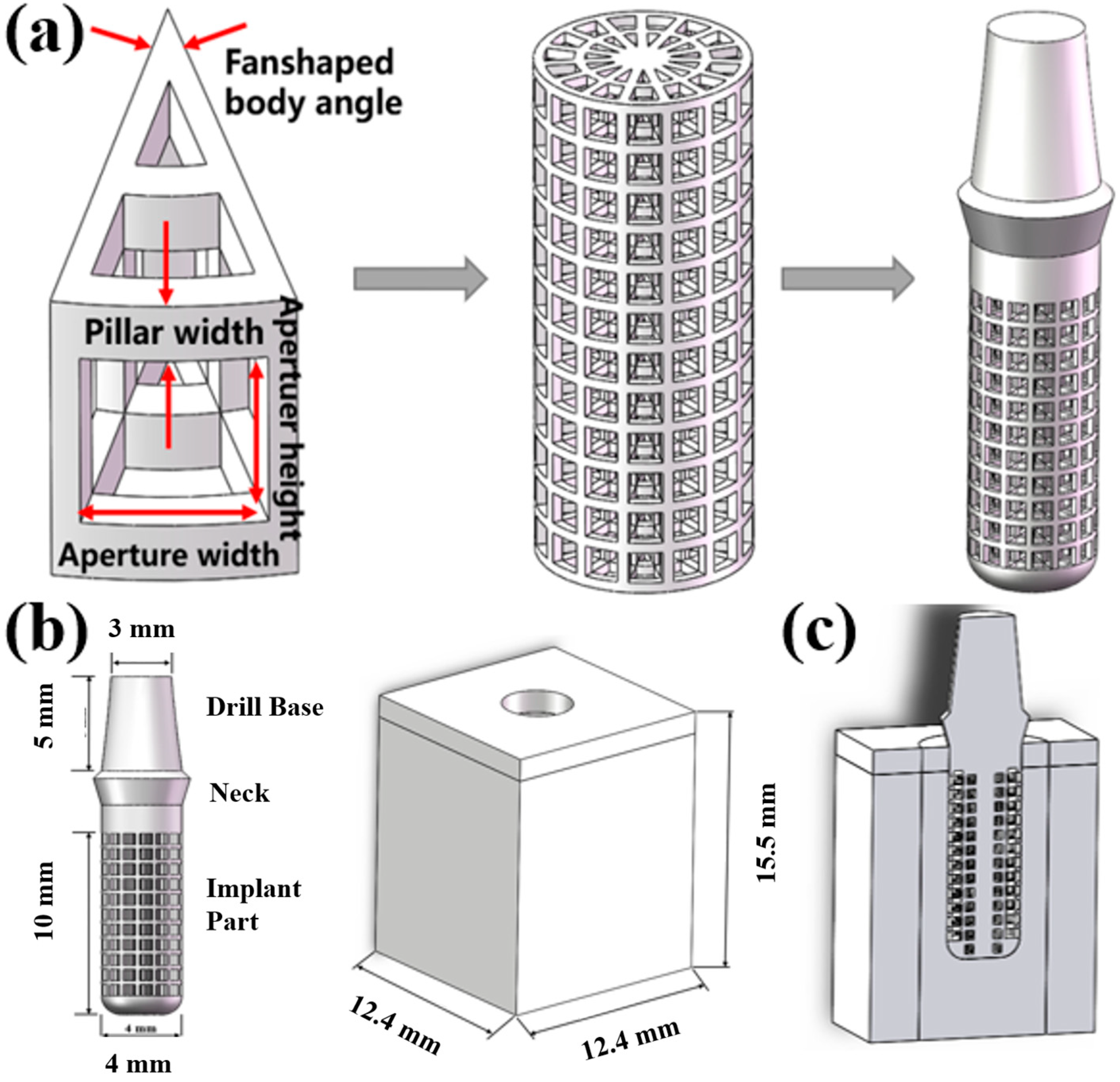
3.1. Built Model
3.2. Statics Simulation
3.2.1. Material Parameter Setting
3.2.2. Meshing
3.2.3. Contact and Constraint Setting
3.2.4. Loading Method
3.2.5. Evaluation of Calculation Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wally, Z.J.; Haque, A.M.; Feteira, A.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Selective Laser Melting processed Ti6Al4V lattices with graded porosities for dental applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Karolewska, K.; Ligaj, B.; Wirwicki, M.; Szala, G. Strength analysis of Ti6Al4V titanium alloy produced by the use of additive manufacturing method under static load conditions. J. Mater. Res. Technol. 2019, 9, 1365–1379. [Google Scholar] [CrossRef]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Heinegård, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tian, J.; Liu, Z.; Lu, X.; Hayat, M.D.; Yan, Y.; Li, Z.; Qu, X.; Wen, C. Novel porous Ti35Zr28Nb scaffolds fabricated by powder metallurgy with excellent 77osteointegration ability for bone-tissue engineering applications. Mater. Sci. Eng C Mater. Biol. Appl. 2019, 105, 110015. [Google Scholar] [CrossRef]
- Lu, T.; Wen, J.; Qian, S.; Cao, H.; Ning, C.; Pan, X.; Jiang, X.; Liu, X.; Chu, P.K. Enhanced osteointegration on tantalum-implanted polyetherether-ketone surface with bone-like elastic modulus. Biomaterials 2015, 51, 173–183. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Kelly, C.N.; Francovich, J.; Julmi, S.; Safranski, D.; Guldberg, R.E.; Maier, H.J.; Gall, K. Fatigue bchavior of As-built selective laser melted tita-nium scaffolds with sheet-based gyroid microarchitecture for bone tissue engineering. Acta Biomater. 2019, 94, 610–626. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Munir, K.; Lin, J.; Brandt, M.; Atrens, A.; Xiao, Y.; Kanwar, J.R.; Wen, C. Novel B-Ti35Zr28Nb alloy scaffolds manufactured using selective laser melting for bone implant ap-plications. Acta Biomater. 2019, 87, 273–284. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Brandt, M.; Wen, C. Ultrahigh-strength titanium gyroid scaffolds manufactured by selective laser melting (SLM) for bone implant applications. Acta Mater. 2018, 158, 354–368. [Google Scholar] [CrossRef]
- Cho, H.H.; Cho, Y.; Han, H.N. Finite element analysis for mechanical response of Ti foams with regular structure obtained by selective laser melting. Acta Mater. 2015, 97, 199–206. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Fox, P.; Jones, E.; Ngo, C.; Sutcliffe, C.J. Selective laser melting: A unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applica-tions. II. Randomized structures. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 89, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.M.; Liu, Y.F.; Jiang, X.F.; Dong, X.T.; Jun, J.; Baur, D.A.; Xu, J.J.; Pan, H.; Xu, X. Bionic mechanical design and 3D printing of novel porous Ti6Al4V implants for biomedical applications. J. Zhejiang Univ.-Sci. B 2019, 20, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, Q.; Wang, C.; Dong, J.; He, G. Fabrication of graded porous titanium–magnesium composite for load-bearing biomedical applications. Mater. Des. 2015, 67, 354–359. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fang, G.; Leeflang, S.; Zadpoor, A.A.; Zhou, J. Topological design, permeability and mechanical behavior of additively manufactured functionally graded porous metallic biomaterials. Acta Biomater. 2019, 84, 437–452. [Google Scholar] [CrossRef]
- Van Hooreweder, B.; Apers, Y.; Lietaert, K.; Kruth, J.P. Improving the fatigue performance of porous metallic biomaterials produced by Selective Laser Melting. Acta Biomater. 2017, 47, 193–202. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wang, Q.; Wen, S.; Wei, Q.; Yan, C.; Hao, L.; Liu, J.; Shi, Y. Continuous functionally graded porous titanium scaffolds manufactured by selective laser melting for bone implants. J. Mech. Behav. Biomed. Mater. 2018, 80, 119–127. [Google Scholar] [CrossRef]
- Roy, S.; Dey, S.; Khutia, N.; Chowdhury, A.R.; Datta, S. Design of patient specific dental implant using FE analysis and computational intelligence techniques. Appl. Soft Comput. 2018, 65, 272–279. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Arzukanyan, A.V.; Spagnuolo, G.; Campagna, P.; Cicciù, M. Application of bioengineering devices for stress evaluation in dentistry: The last 10 years FEM parametric analysis of outcomes and current trends. Minerva Stomatol. 2020, 69, 55–62. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: London, UK, 1997; pp. 85–114. [Google Scholar]
- Frost, H.M. A 2003 Update of Bone Physiology and Wolff’s Law for Clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [PubMed]
- Brunski, J.B.; Puleo, D.A.; Nanci, A. Biomaterials and biomechanics of oral and maxillofacial im-plants: Current status and future developments. Int. J. Oral Maxillofac Implant. 2000, 15, 15–46. [Google Scholar]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implant. Res. 2009, 20, 467–471. [Google Scholar] [CrossRef]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacu-umdiffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- de Mello Santos, L.S.; Rossi, A.C.; Freire, A.R.; Matoso, R.I.; Caria, P.H.F.; Prado, F.B. Finite Element Analysis of Three Situa-tions of Trauma in Human Edentulous Mandible. J. Oral Maxillofac. Surg. 2014, 73, 683–691. [Google Scholar] [CrossRef]
- Sugiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Tsutsumi, S.; Kirita, T. The effects of bone density and crestal cortical bone thickness on micromotion and peri-implant bone strain distribution in an immediately loaded implant: A nonlinear finite element analysis. J. Periodontal Implant. Sci. 2016, 46, 152–165. [Google Scholar] [CrossRef]
- Sugiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Kirita, T. Micromotion analysis of different implant configuration, bone density, and crestal cortical bone thickness in immediately loaded mandibular full-arch implant restorations: A nonlinear finite element study. Clin. Implant Dent. Relat. Res. 2017, 20, 43–49. [Google Scholar] [CrossRef]


| Pillar Width/mm | Fan-Shaped Body Angle/° | Aperture Height/mm | Aperture Width/mm |
|---|---|---|---|
| 0.1 | 24 | 0.8 | 0.73 |
| 0.15 | 24 | 0.7 | 0.68 |
| 0.2 | 24 | 0.6 | 0.63 |
| 0.25 | 24 | 0.5 | 0.59 |
| 0.3 | 24 | 0.4 | 0.54 |
| Material | Elastic Modulus/GPa | Poisson’s Ratio |
|---|---|---|
| Titanium | 110 | 0.35 |
| Cortical bone | 13.7 | 0.3 |
| Cancellous bone | 1.37 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Xu, W.; Chen, M.; Chen, D.; Sun, G.; Zhang, C.; Pan, Y.; Lu, J.; Guo, E.; Lu, X. Structural Design and Finite Element Simulation Analysis of Grade 3 Graded Porous Titanium Implant. Int. J. Mol. Sci. 2022, 23, 10090. https://doi.org/10.3390/ijms231710090
Liu B, Xu W, Chen M, Chen D, Sun G, Zhang C, Pan Y, Lu J, Guo E, Lu X. Structural Design and Finite Element Simulation Analysis of Grade 3 Graded Porous Titanium Implant. International Journal of Molecular Sciences. 2022; 23(17):10090. https://doi.org/10.3390/ijms231710090
Chicago/Turabian StyleLiu, Bowen, Wei Xu, Mingying Chen, Dongdong Chen, Guyu Sun, Ce Zhang, Yu Pan, Jinchao Lu, Enbo Guo, and Xin Lu. 2022. "Structural Design and Finite Element Simulation Analysis of Grade 3 Graded Porous Titanium Implant" International Journal of Molecular Sciences 23, no. 17: 10090. https://doi.org/10.3390/ijms231710090
APA StyleLiu, B., Xu, W., Chen, M., Chen, D., Sun, G., Zhang, C., Pan, Y., Lu, J., Guo, E., & Lu, X. (2022). Structural Design and Finite Element Simulation Analysis of Grade 3 Graded Porous Titanium Implant. International Journal of Molecular Sciences, 23(17), 10090. https://doi.org/10.3390/ijms231710090






