Effective Removal of Methylene Blue on EuVO4/g-C3N4 Mesoporous Nanosheets via Coupling Adsorption and Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
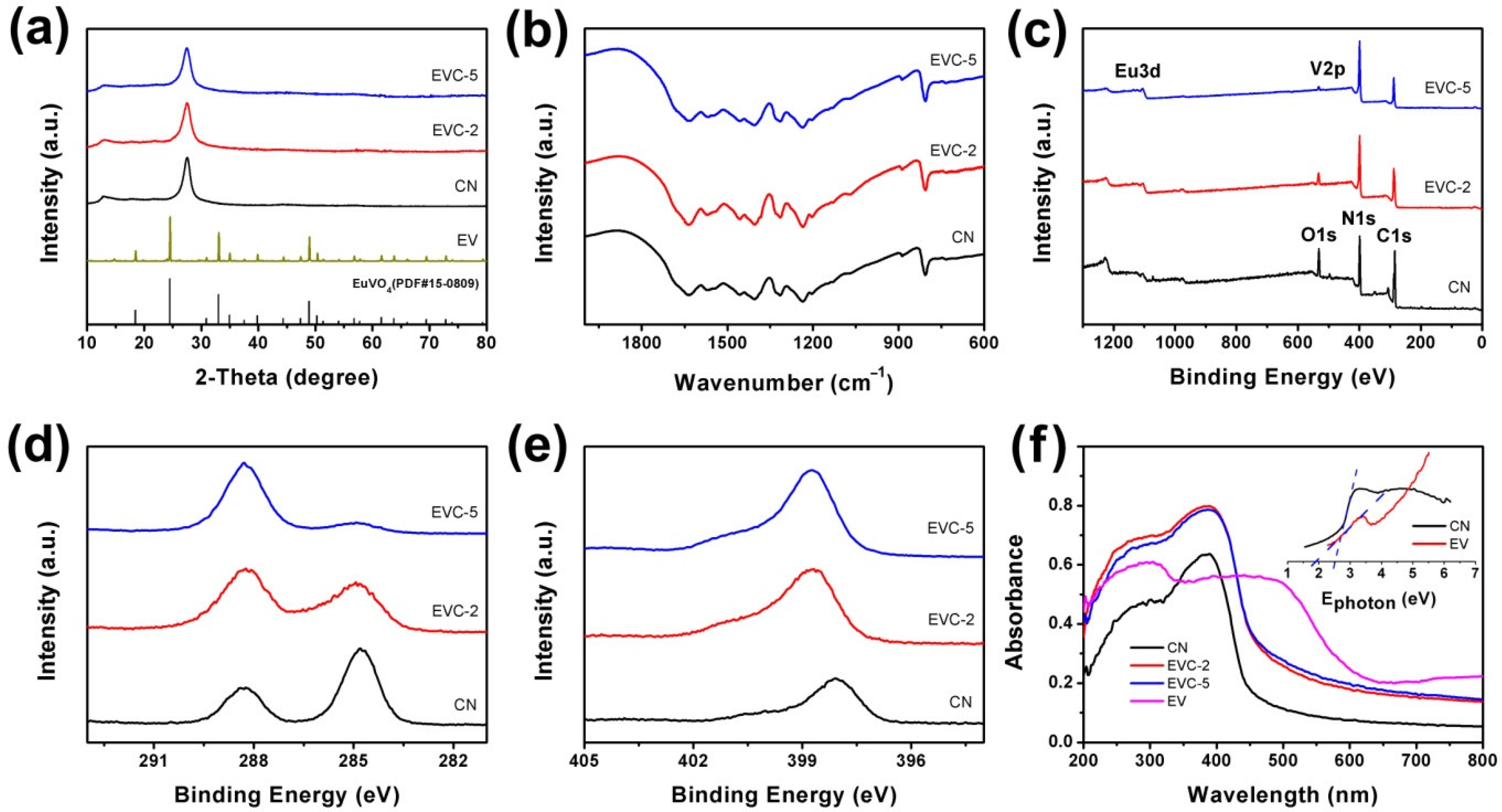
2.1. Characterization of EVCs
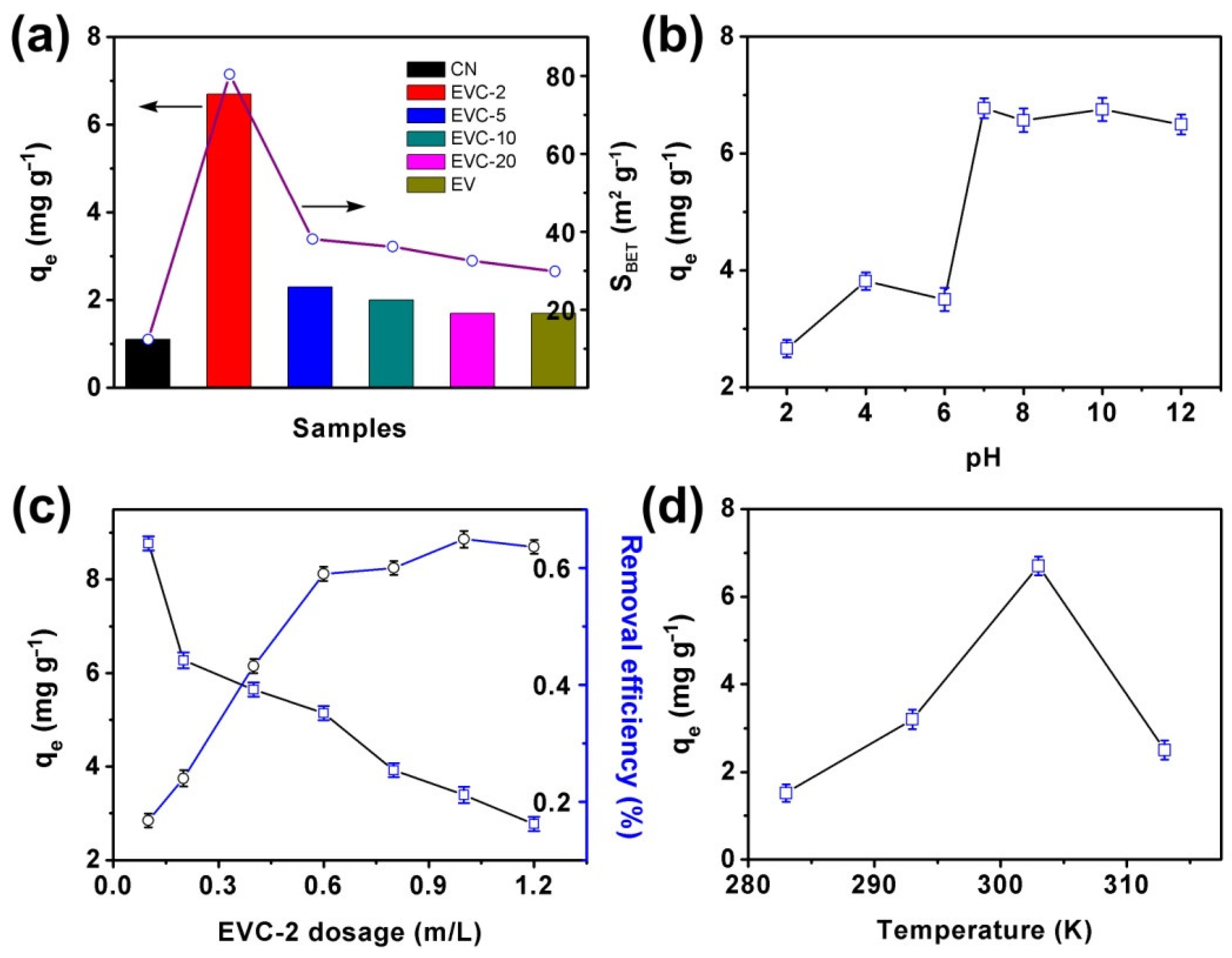
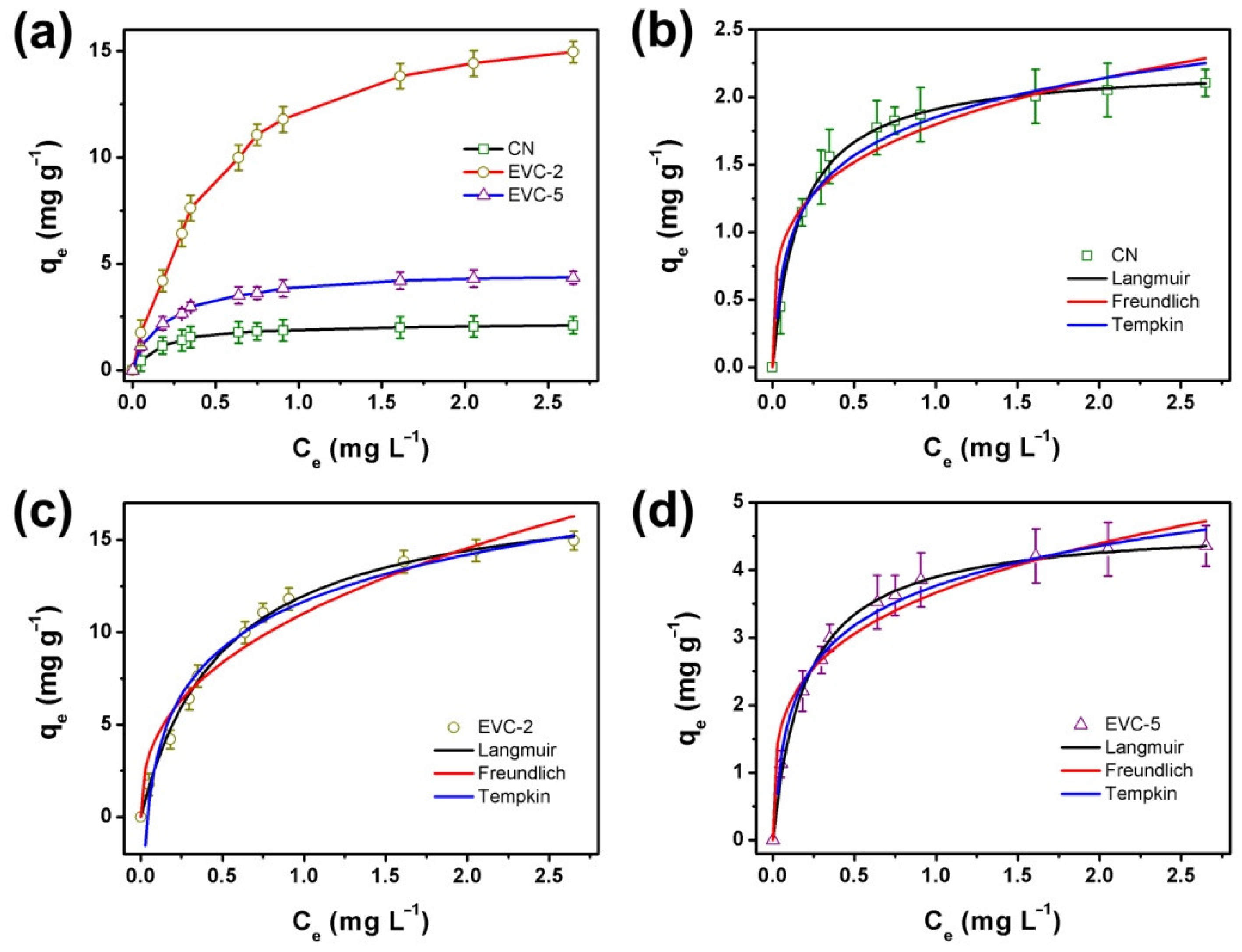
2.2. Adsorption Kinetics and Isotherm of MB
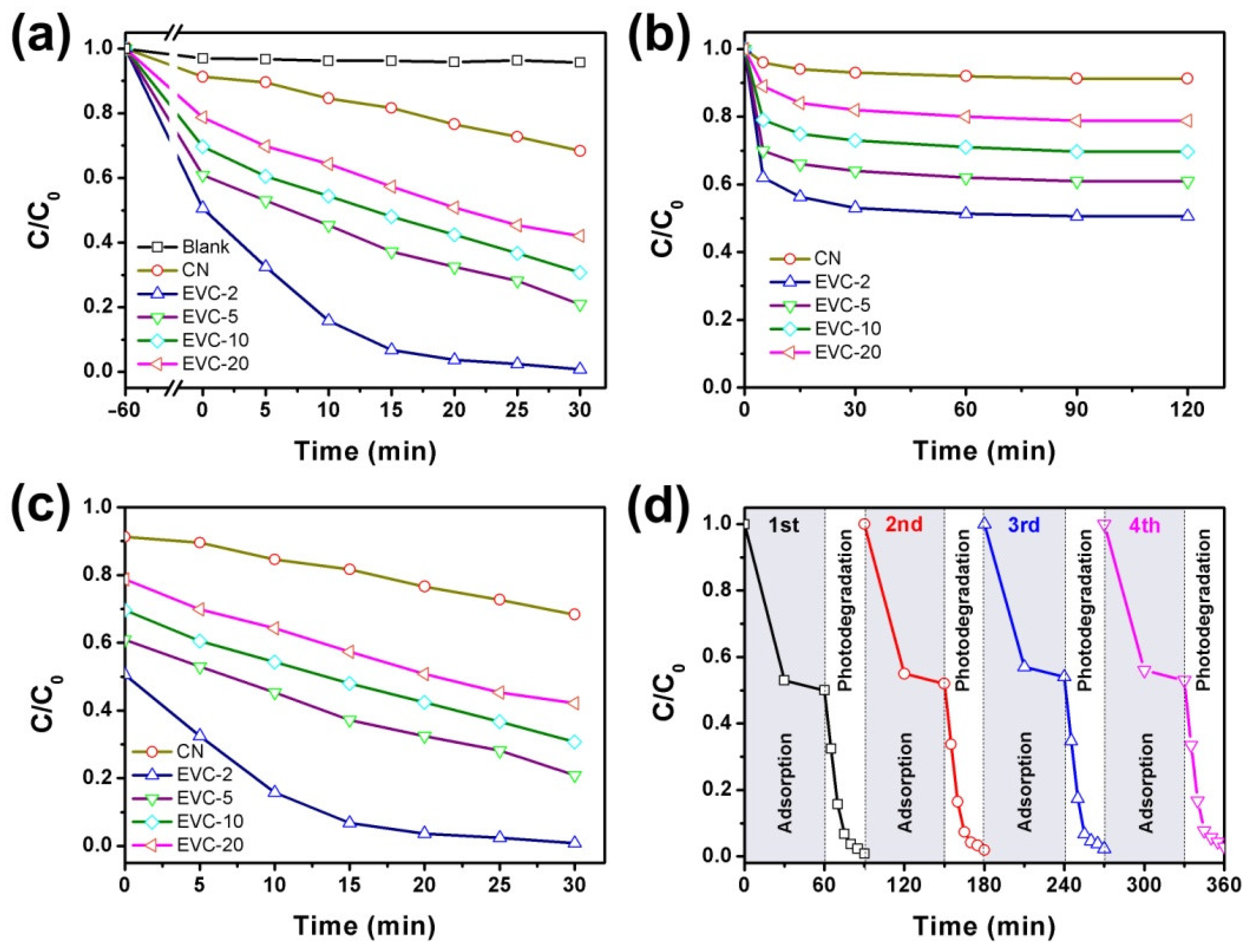
2.3. Adsorption and Photocatalytic Degradation of MB
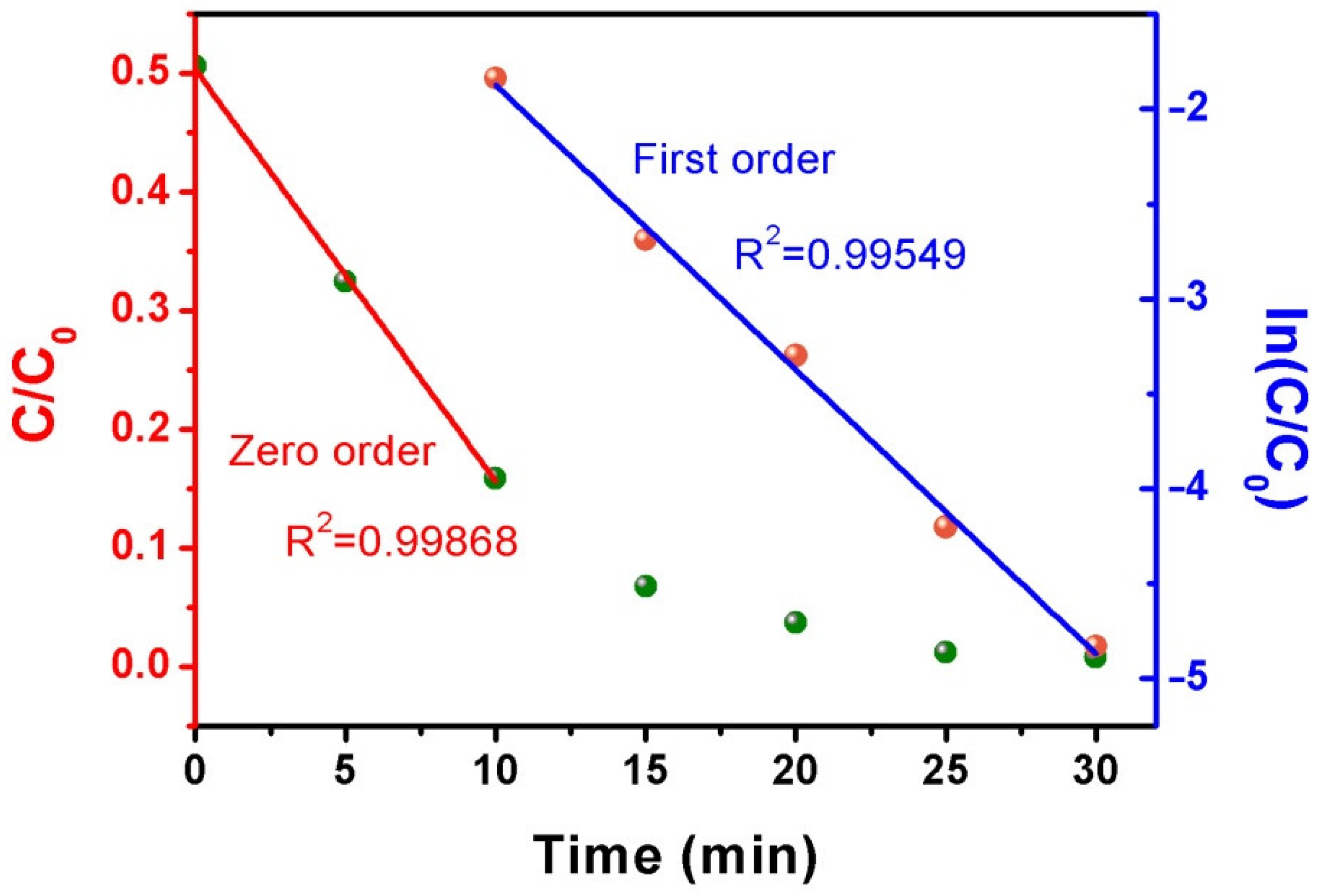
2.4. Photocatalytic Kinetics of MB
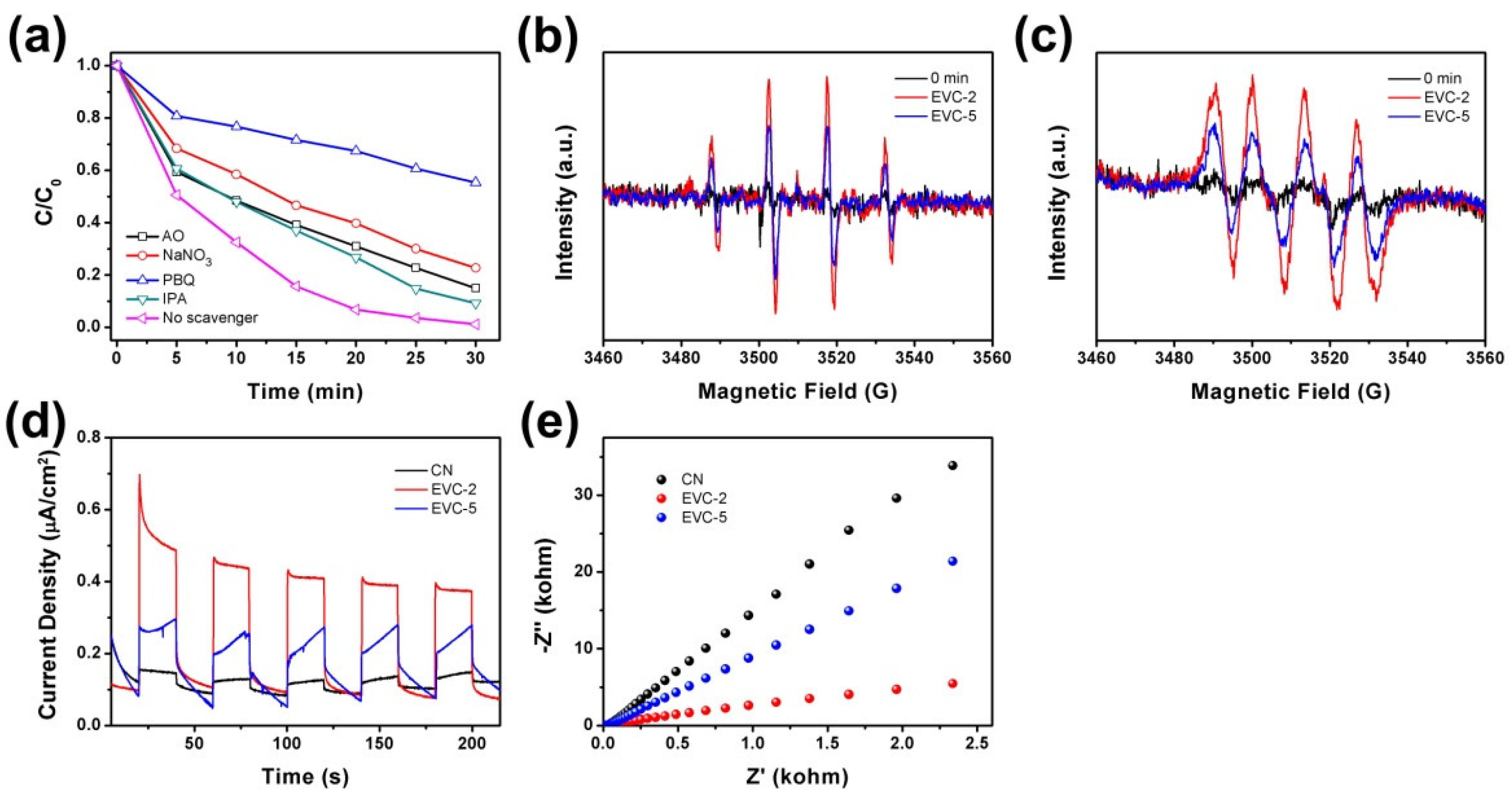
2.5. Active Oxidation Species and Possible Mechanism
3. Materials and Methods
3.1. Synthesis of EVCs
3.2. Catalyst Characterization
3.3. Adsorption of MB
3.4. Photocatalytic Degradation of MB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.Q.; Zhang, F.; Ma, B.; Xu, N.; Junior, L.B.; Yao, B.; Yang, Q.; Liu, D.; Ma, Z. Remarkably enhanced visible-light photocatalytic hydrogen evolution and antibiotic degradation over g-C3N4 nanosheets decorated by using nickel phosphide and gold nanoparticles as cocatalysts. Appl. Surf. Sci. 2020, 517, 146187. [Google Scholar] [CrossRef]
- Ashrafi, H.; Akhond, M.; Absalan, G. Adsorption and photocatalytic degradation of aqueous methylene blue using nanoporous carbon nitride. J. Photochem. Photobiol. A 2020, 396, 112533. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- Fronczak, M. Adsorption performance of graphitic carbon nitride-based materials: Current state of the art. J. Environ. Chem. Eng. 2020, 8, 104411. [Google Scholar] [CrossRef]
- Yan, L.; Gao, H.; Chen, Y. Na-Doped Graphitic Carbon Nitride for Removal of Aqueous Contaminants via Adsorption and Photodegradation. ACS Appl. Nano Mater. 2021, 4, 7746–7757. [Google Scholar] [CrossRef]
- Shi, J.; Chen, T.; Guo, C.; Liu, Z.; Feng, S.; Li, Y.; Hu, J. The bifunctional composites of AC restrain the stack of g-C3N4 with the excellent adsorption-photocatalytic performance for the removal of RhB. Colloids Surf. A 2019, 580, 123701. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, H.-B.; Choi, J.-H.; Baek, K. Bifunctional iron-modified graphitic carbon nitride (g-C3N4) for simultaneous oxidation and adsorption of arsenic. Environ. Res. 2020, 188, 109832. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Yu, J.; Cao, S.; Jaroniec, M. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Lee, S.; Zhao, X.; Lin, S. Constructing novel graphitic carbon nitride-based nanocomposites—From the perspective of material dimensions and interfacial characteristics. Chemosphere 2022, 302, 134889. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Mahmood, Q.; Ismail, B.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A.; Elkaeed, E.B. Optoelectronic, structural and morphological analysis of Cu3BiS3 sulfosalt thin films. Results Phys. 2022, 36, 105453. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Bahadur, A.; Ismail, B.; Abd-Rabboh, H.S.M.; Hameed, R.; Mahmood, Q.; Ibrar, A.; Nasar, M.S.; et al. Deposition of bismuth sulfide and aluminum doped bismuth sulfide thin films for photovoltaic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 42–53. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Mahmood, Q.; Ismail, B.; Alzhrani, R.M.; Awwad, N.S.; Ibrahium, H.A.; Alam, S.; Yasir, M.; et al. Optoelectronic Analysis of Bismuth Sulfide and Copper-Doped Bismuth Sulfide Thin Films. JOM 2022, 74, 2809–2816. [Google Scholar] [CrossRef]
- Reitz, C.; Smarsly, B.; Brezesinski, T. General Synthesis of Ordered Mesoporous Rare-Earth Orthovanadate Thin Films and Their Use as Photocatalysts and Phosphors for Lighting Applications. ACS Appl. Nano Mater. 2019, 2, 1063–1071. [Google Scholar] [CrossRef]
- Hosseini, S.A. Nanocrystalline EuVO4: Synthesis, characterization, optical and photocatalytic properties. J. Mater. Sci.-Mater. Electron. 2016, 27, 10775–10779. [Google Scholar] [CrossRef]
- Chen, J.; Fang, L.; Li, J.; Tang, Y.; Cheng, K.; Cao, Y. Packing fraction, bond valence and crystal structure of AVO4 (A = Eu, Y) microwave dielectric ceramics with low permittivity. J. Mater. Sci.-Mater. Electron. 2020, 31, 19180–19187. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yoon, S.J.; Parkn, K. Synthesis and photoluminescence properties of EuVO4 red phosphors. Ceram. Int. 2014, 40, 9457–9461. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Zhang, S.; Xie, Z.; Wang, Y.; Liu, Y.; Dai, Y.; Wang, Y.; Zhang, Z.; Liu, Y.; et al. Electrochemical synthesis of EuVO4 for the adsorption of U(VI): Performance and mechanism. Chemosphere 2021, 273, 128569. [Google Scholar] [CrossRef] [PubMed]
- Vosoughifar, M. Investigation of the morphologies, optical and magnetic properties of europium vanadate nanoparticles synthesized via a simple method. J. Mater. Sci.-Mater. Electron. 2017, 28, 2227–2232. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Sheng, T.; Wu, X. Photodegradation of acetone by visible light-responsive V2O5/EuVO4 composite. Catal. Today 2010, 158, 209–214. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Guo, H.; Sheng, T.; Wu, X. Visible light photodegradation of organics over VYO composite catalyst. J. Hazard. Mater. 2009, 169, 855–860. [Google Scholar] [CrossRef]
- Monsef, R.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Design of Magnetically Recyclable Ternary Fe2O3/EuVO4/g-C3N4 Nanocomposites for Photocatalytic and Electrochemical Hydrogen Storage. ACS Appl. Energy Mater. 2021, 4, 680–695. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Lim, J.-H.; Jeong, D.Y.; Dewedi, R.P.; Saini, A.K.; Singh, P. Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng. 2018, 93, 528–542. [Google Scholar] [CrossRef]
- Zhang, J.; Mei, J.; Yi, S.; Guan, X. Constructing of Z-scheme 3D g-C3N4-ZnO@graphene aerogel heterojunctions for high-efficient adsorption and photodegradation of organic pollutants. Appl. Surf. Sci. 2019, 492, 808–817. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, H.; Sun, H.; Xiao, K.; Wong, P.K. Enhanced adsorption and photocatalytic activities of ultrathin graphitic carbon nitride nanosheets: Kinetics and mechanism. Chem. Eng. J. 2020, 381, 122760. [Google Scholar] [CrossRef]
- Xu, K.; Yang, X.; Ruan, L.; Qi, S.; Liu, J.; Liu, K.; Pan, S.; Feng, G.; Dai, Z.; Yang, X.; et al. Superior Adsorption and Photocatalytic Degradation Capability of Mesoporous LaFeO3/g-C3N4 for Removal of Oxytetracycline. Catalysts 2020, 10, 301. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, S.; Jiang, P.; Xu, Z. Graphitic C3N4 modified by Ni2P cocatalyst: An efficient, robust and low cost photocatalyst for visible-light-driven H2 evolution from water. Chem. Eng. J. 2017, 315, 296–303. [Google Scholar] [CrossRef]
- Xu, K.; Feng, J. Superior photocatalytic performance of LaFeO3/g-C3N4 heterojunction nanocomposites under visible light irradiation. RSC Adv. 2017, 7, 45369–45376. [Google Scholar] [CrossRef]
- Chu, Z.; Li, J.; Lan, Y.-P.; Chen, C.; Yang, J.; Ning, D.; Xia, X.; Mao, X. KCl-LiCl molten salt synthesis of LaOCl/CeO2-g-C3N4 with excellent photocatalytic-adsorbed removal performance for organic dye pollutant. Ceram. Int. 2022, 48, 15439–15450. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, Y.; Cai, P. Sugarcane cellulose-based composite hydrogel enhanced by g-C3N4 nanosheet for selective removal of organic dyes from water. Int. J. Biol. Macromol. 2022, 205, 37–48. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-containing carbon nitride compounds: A new functional organic-metal hybrid material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Wang, T.; Huang, M.; Liu, X.; Zhang, Z.; Liu, Y.; Tang, W.; Bao, S.; Fang, T. Facile one-step hydrothermal synthesis of α-Fe2O3/g-C3N4 composites for the synergistic adsorption and photodegradation of dyes. RSC Adv. 2019, 9, 29109–29119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, W.; Zhao, Z.; Zhong, H.; Zhu, Z.; Chen, W. In situ stable growth of β-FeOOH on g-C3N4 for deep oxidation of emerging contaminants by photocatalytic activation of peroxymonosulfate under solar irradiation. Chem. Eng. J. 2020, 400, 125872. [Google Scholar] [CrossRef]
- Zhu, T.; Song, Y.; Ji, H.; Xu, Y.; Song, Y.; Xia, J.; Yin, S.; Li, Y.; Xu, H.; Zhang, Q.; et al. Synthesis of g-C3N4/Ag3VO4 composites with enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2015, 271, 96–105. [Google Scholar] [CrossRef]
- Liu, X.; Jin, A.; Jia, Y.; Xia, T.; Deng, C.; Zhu, M.; Chen, C.; Chen, X. Synergy of adsorption and visible-light photocatalytic degradation of methylene blue by a bifunctional Z-scheme heterojunction of WO3/g-C3N4. Appl. Surf. Sci. 2017, 405, 359–371. [Google Scholar] [CrossRef]
- Li, H.; Budarin, V.L.; Clark, J.H.; North, M.; Wu, X. Rapid and efficient adsorption of methylene blue dye from aqueous solution by hierarchically porous, activated starbons®: Mechanism and porosity dependence. J. Hazard. Mater. 2022, 436, 129174. [Google Scholar] [CrossRef]
- Ghereghlou, M.; Esmaeili, A.A.; Darroudi, M. Adsorptive Removal of Methylene Blue from Aqueous Solutions Using Magnetic Fe3O4@C-dots: Removal and kinetic studies. Sep. Sci. Technol. 2022, 57, 2005–2023. [Google Scholar] [CrossRef]
- Azeez, L.; Adebisi, S.A.; Adejumo, A.L.; Busari, H.K.; Aremu, H.K.; Olabode, O.A.; Awolola, O. Adsorptive properties of rod-shaped silver nanoparticles-functionalized biogenic hydroxyapatite for remediating methylene blue and congo red. Inorg. Chem. Commun. 2022, 142, 109655. [Google Scholar] [CrossRef]
- Fan, H.; Li, F.; Huang, H.; Yang, J.; Zeng, D.; Liu, J.; Mou, H. pH graded lignin obtained from the by-product of extraction xylan as an adsorbent. Ind. Crops Prod. 2022, 184, 114967. [Google Scholar] [CrossRef]
- Liang, C.; Shi, Q.; Feng, J.; Yao, J.; Huang, H.; Xie, X. Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors. Nanomaterials 2022, 12, 1814. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Fazal, A.; Hamid, S.; Arfan, M.; Khan, I.; Usman, M.; Shafiee, A.; Ashiq, M.N. CoFe2O4 decorated g-C3N4 nanosheets: New insights into superoxide anion mediated photomineralization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104556. [Google Scholar] [CrossRef]
- Ismail, A.A.; Faisal, M.; Harraz, F.A.; Al-Hajry, A.; Al-Sehemi, A.G. Synthesis of mesoporous sulfur-doped Ta2O5 nanocomposites and their photocatalytic activities. J. Colloid Interface Sci. 2016, 471, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.W.; An, L.L.; Chen, J.S.; Bae, J.H.; Kim, Y.S. Preparation of aminefunctionalizedlignins for the selective adsorption of Methylene blue and Congo red. Chemosphere 2022, 295, 133815. [Google Scholar] [CrossRef]
- Venkatesha, T.C.; Viswanatha, R.; Arthoba Nayaka, Y.; Chethana, B.K. Kinetics and thermodynamics of reactive and vat dyes adsorption on MgO nanoparticles. Chem. Eng. J. 2012, 198–199, 1–10. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B-Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Li, X.; Liu, C.; Wang, H.; Huo, P.; Yan, Y. Molecularly imprinted Ag/Ag3VO4/g-C3N4 Z-scheme photocatalysts for enhanced preferential removal of tetracycline. J. Colloid Interface Sci. 2019, 552, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced Photoresponsive Ultrathin Graphitic-Phase C3N4 Nanosheets for Bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21+. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, X.; Wang, L.; Xiao, B.; Lei, L.; Zhu, J.; Liu, Z.; Xi, X.; Feng, G.; Li, R.; Feng, J. Effective Removal of Methylene Blue on EuVO4/g-C3N4 Mesoporous Nanosheets via Coupling Adsorption and Photocatalysis. Int. J. Mol. Sci. 2022, 23, 10003. https://doi.org/10.3390/ijms231710003
Ran X, Wang L, Xiao B, Lei L, Zhu J, Liu Z, Xi X, Feng G, Li R, Feng J. Effective Removal of Methylene Blue on EuVO4/g-C3N4 Mesoporous Nanosheets via Coupling Adsorption and Photocatalysis. International Journal of Molecular Sciences. 2022; 23(17):10003. https://doi.org/10.3390/ijms231710003
Chicago/Turabian StyleRan, Xia, Li Wang, Bo Xiao, Li Lei, Jinming Zhu, Zuoji Liu, Xiaolan Xi, Guangwei Feng, Rong Li, and Jian Feng. 2022. "Effective Removal of Methylene Blue on EuVO4/g-C3N4 Mesoporous Nanosheets via Coupling Adsorption and Photocatalysis" International Journal of Molecular Sciences 23, no. 17: 10003. https://doi.org/10.3390/ijms231710003
APA StyleRan, X., Wang, L., Xiao, B., Lei, L., Zhu, J., Liu, Z., Xi, X., Feng, G., Li, R., & Feng, J. (2022). Effective Removal of Methylene Blue on EuVO4/g-C3N4 Mesoporous Nanosheets via Coupling Adsorption and Photocatalysis. International Journal of Molecular Sciences, 23(17), 10003. https://doi.org/10.3390/ijms231710003






