Boosting the Energetic Performance of Trinitromethyl-1,2,4-oxadiazole Moiety by Increasing Nitrogen-Oxygen in the Bridge
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. 15N Multinuclear Magnetic Resonance
2.3. Single-Crystal X-ray Analysis
3. Physicochemical and Energetic Properties
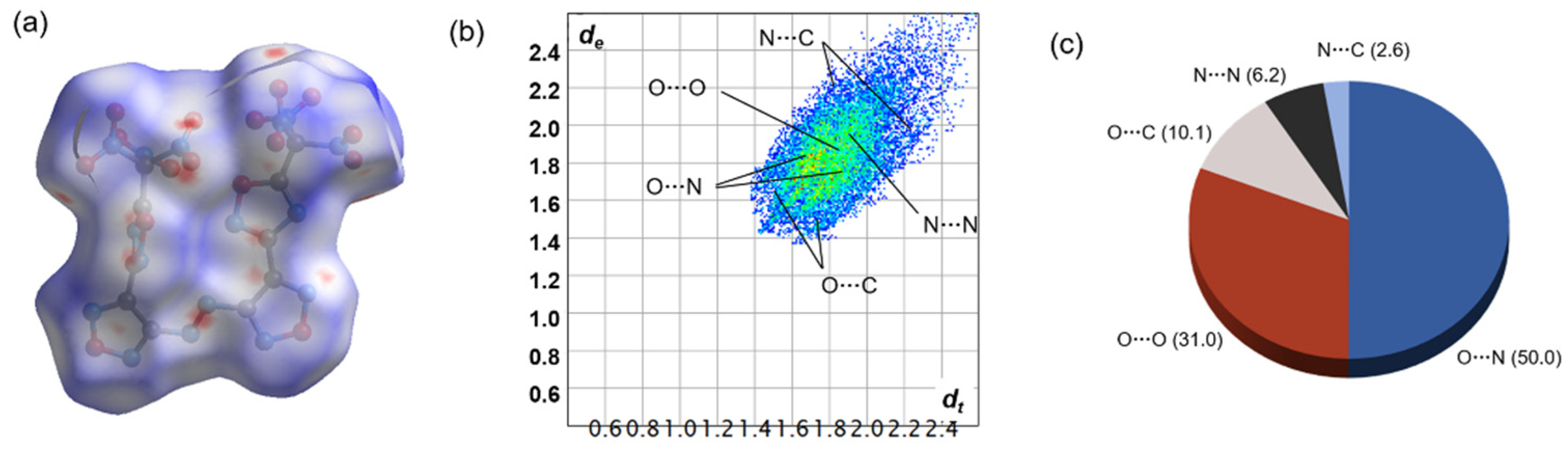
3.1. Hirshfeld Surface
3.2. Density, Differential Scanning Calorimetry, and Oxygen Balance
3.3. Heats of Formation, Sensitivity, and Detonation Performance
4. Experimental Section
4.1. General Methods
4.2. Synthesis of Compound 4,4′-(5-Trinitromethyl-1,2,4-Oxadiazole)-3,3′-Azo-Furazan (2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischer, N.; Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the limits of energetic materials–the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Sun, Q.; Ding, N.; Zhao, C.; Zhang, Q.; Zhang, S.; Li, S.; Pang, S. Full-nitro-nitroamino cooperative action: Climbing the energy peak of benzenes with enhanced chemical stability. Sci. Adv. 2022, 8, 3176. [Google Scholar] [CrossRef] [PubMed]
- Dalinger, I.L.; Suponitsky, K.Y.; Shkineva, T.K.; Lempert, D.B.; Sheremetev, A.B. Bipyrazole bearing ten nitro groups–A novel highly dense oxidizer for forward-looking rocket propulsions. J. Mater. Chem. A 2018, 6, 14780–14786. [Google Scholar] [CrossRef]
- Yu, Q.; Yin, P.; Zhang, J.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Pushing the Limits of Oxygen Balance in 1,3,4-Oxadiazoles. J. Am. Chem. Soc. 2017, 139, 8816–8819. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wang, C.; Wang, G.; Wang, S.; Song, J.; Yin, H.; Fan, G.; Chen, F.X. 1,2,4-Oxadiazole-derived polynitro energetic compounds with sensitivity reduced by a methylene bridge. New J. Chem. 2019, 43, 13330–13333. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Q.; Shreeve, J.M. Dancing with Energetic Nitrogen Atoms: Versatile N-Functionalization Strategies for N-Heterocyclic Frameworks in High Energy Density Materials. Acc. Chem. Res. 2016, 49, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Nitromethane Bridged Bis(1,3,4-oxadiazoles): Trianionic Energetic Salts with Low Sensitivities. Chem. Eur. J. 2017, 23, 17682–17686. [Google Scholar] [CrossRef]
- Yu, Q.; Li, F.; Yin, P.; Pang, S.; Staples, R.J.; Shreeve, J.M. Bridged and fused triazolic energetic frameworks with an azo building block towards thermally stable and applicable propellant ingredients. J. Mater. Chem. A 2021, 9, 24903–24908. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Krumm, B.; Reith, T.; Unger, C.C. Synthetic Routes to a Triazole and Tetrazole with Trinitroalkyl Substitution at Nitrogen. J. Org. Chem. 2018, 83, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, Z.; Yang, H.; Wu, B.; Lin, Q.; Ju, X.; Lu, C.; Cheng, G. N-Trinitroethyl-substituted azoxyfurazan: High detonation performance energetic materials. RSC Adv. 2015, 5, 27305–27312. [Google Scholar] [CrossRef]
- Chavez, D.E.; Parrish, D.A.; Mitchell, L. Energetic Trinitro- and Fluorodinitroethyl Ethers of 1,2,4,5-Tetrazines. Angew Chem. Int. Ed. 2016, 55, 8666–8669. [Google Scholar] [CrossRef] [PubMed]
- Sheremetev, A.B.; Korolev, V.L.; Potemkin, A.A.; Aleksandrova, N.S.; Palysaeva, N.V.; Hoang, T.H.; Sinditskii, V.P.; Suponitsky, K.Y. Oxygen-Rich 1,2,4-Triazolo[3,4-d]-1,2,4-triazolo[3,4-f]furazano[3,4-b]pyrazines as Energetic Materials. Asian J. Org. Chem 2016, 5, 1388–1397. [Google Scholar] [CrossRef]
- Xiong, H.; Yang, H.; Cheng, G. 3-Trinitromethyl-4-nitro-5-nitramine-1H-pyrazole: A high energy density oxidizer. New J. Chem. 2019, 43, 13827–13831. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hu, J.; Ge, Z.; Sun, C.; Pang, S. Energetic C-trinitromethyl-substituted pyrazoles: Synthesis and characterization. Dalton Trans. 2019, 48, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cheng, G.; Yang, H.; Zhang, J.; Shreeve, J.M. Synthesis and Characterization of 4-(1,2,4-Triazole-5-yl)furazan Derivatives as High-Performance Insensitive Energetic Materials. Chem. Eur. J. 2018, 24, 10488–10497. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiong, H.; Cheng, G.; Yang, H. Incorporating Energetic Moieties into Four Oxadiazole Ring Systems for the Generation of High-Performance Energetic Materials. Chempluschem 2018, 83, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, G.; Gu, H.; Tang, J.; Wang, B.; Yang, H.; Cheng, G. Polynitro-1,2,4-triazole-functionalized azo-furazans as high-performance and insensitive energetic materials. New J. Chem. 2019, 43, 8370–8375. [Google Scholar] [CrossRef]
- Kettner, M.A.; Karaghiosoff, K.; Klapötke, T.M.; Suceska, M.; Wunder, S. 3,3′-Bi(1,2,4-oxadiazoles) featuring the fluorodinitromethyl and trinitromethyl groups. Chem. Eur. J. 2014, 20, 7622–7631. [Google Scholar] [CrossRef] [PubMed]
- Chinnam, A.K.; Staples, R.J.; Shreeve, J.M. 1,2-Bis(5-(trinitromethyl)-1,2,4-oxadiazol-3-yl)diazene: A water stable, high-performing green oxidizer. Dalton Trans. 2021, 50, 16929–16932. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Mayr, N.; Stierstorfer, J.; Weyrauther, M. Maximum compaction of ionic organic explosives: Bis(hydroxylammonium) 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazolate) and its derivatives. Chem. Eur. J. 2014, 20, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Qiu, L.; Yin, P.; He, C.; Pang, S.; Shreeve, J.M. Unraveling the reactivity of the azo bridge in 3,3′-(5-dinitromethyl-1,2,4-oxadiazolyl)-4,4'-azofurazanate in the synthesis of energetic compounds. Chem. Commun. 2022, 58, 2874–2877. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; Parrish, D.A.; Shreeve, J.M. Tetranitroacetimidic Acid: A High Oxygen Oxidizer and Potential Replacement for Ammonium Perchlorate. J. Am. Chem. Soc. 2014, 136, 11934–11937. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Sućeska, M. EXPLO5 6.05; Brodarski Institute: Zagreb, Croatia, 2020. [Google Scholar]
- He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Energetic salts of 4-nitramino-3-(5-dinitromethyl-1,2,4-oxadiazolyl)-furazan: Powerful alliance towards good thermal stability and high performance. J. Mater. Chem. A 2018, 6, 16833–16837. [Google Scholar] [CrossRef]



| Compd | Tm a /°C | Td b /°C | ρ c /g cm−3 | ΔHf d /kJ mol−1 | D e /m s−1 | P f /GPa | IS g /J | FS h /N | OB i /% |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 58.1 | 115.9 | 1.897 | 984.766 | 9351 | 37.46 | 3 | 40 | −10.66 |
| A | - | 125 | 1.850 | 461.4 | 8722 | 33.15 | 5 | 80 | +6.9 |
| B | - | 124 | 1.936 | 61.9 | 8814 | 34.50 | 10 | 80 | +7.3 |
| RDX j | - | 204 | 1.80 | 70.3 | 8795 | 34.9 | 7.5 | 120 | −21.62 |
| HMX j | - | 287 | 1.91 | 74.8 | 9144 | 39.2 | 7.4 | 120 | −21.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Dou, H.; He, C.; Pang, S. Boosting the Energetic Performance of Trinitromethyl-1,2,4-oxadiazole Moiety by Increasing Nitrogen-Oxygen in the Bridge. Int. J. Mol. Sci. 2022, 23, 10002. https://doi.org/10.3390/ijms231710002
Chen P, Dou H, He C, Pang S. Boosting the Energetic Performance of Trinitromethyl-1,2,4-oxadiazole Moiety by Increasing Nitrogen-Oxygen in the Bridge. International Journal of Molecular Sciences. 2022; 23(17):10002. https://doi.org/10.3390/ijms231710002
Chicago/Turabian StyleChen, Peng, Hui Dou, Chunlin He, and Siping Pang. 2022. "Boosting the Energetic Performance of Trinitromethyl-1,2,4-oxadiazole Moiety by Increasing Nitrogen-Oxygen in the Bridge" International Journal of Molecular Sciences 23, no. 17: 10002. https://doi.org/10.3390/ijms231710002
APA StyleChen, P., Dou, H., He, C., & Pang, S. (2022). Boosting the Energetic Performance of Trinitromethyl-1,2,4-oxadiazole Moiety by Increasing Nitrogen-Oxygen in the Bridge. International Journal of Molecular Sciences, 23(17), 10002. https://doi.org/10.3390/ijms231710002






