Expression Analysis of ZPB2a and Its Regulatory Role in Sperm-Binding in Viviparous Teleost Black Rockfish
Abstract
1. Introduction
2. Results
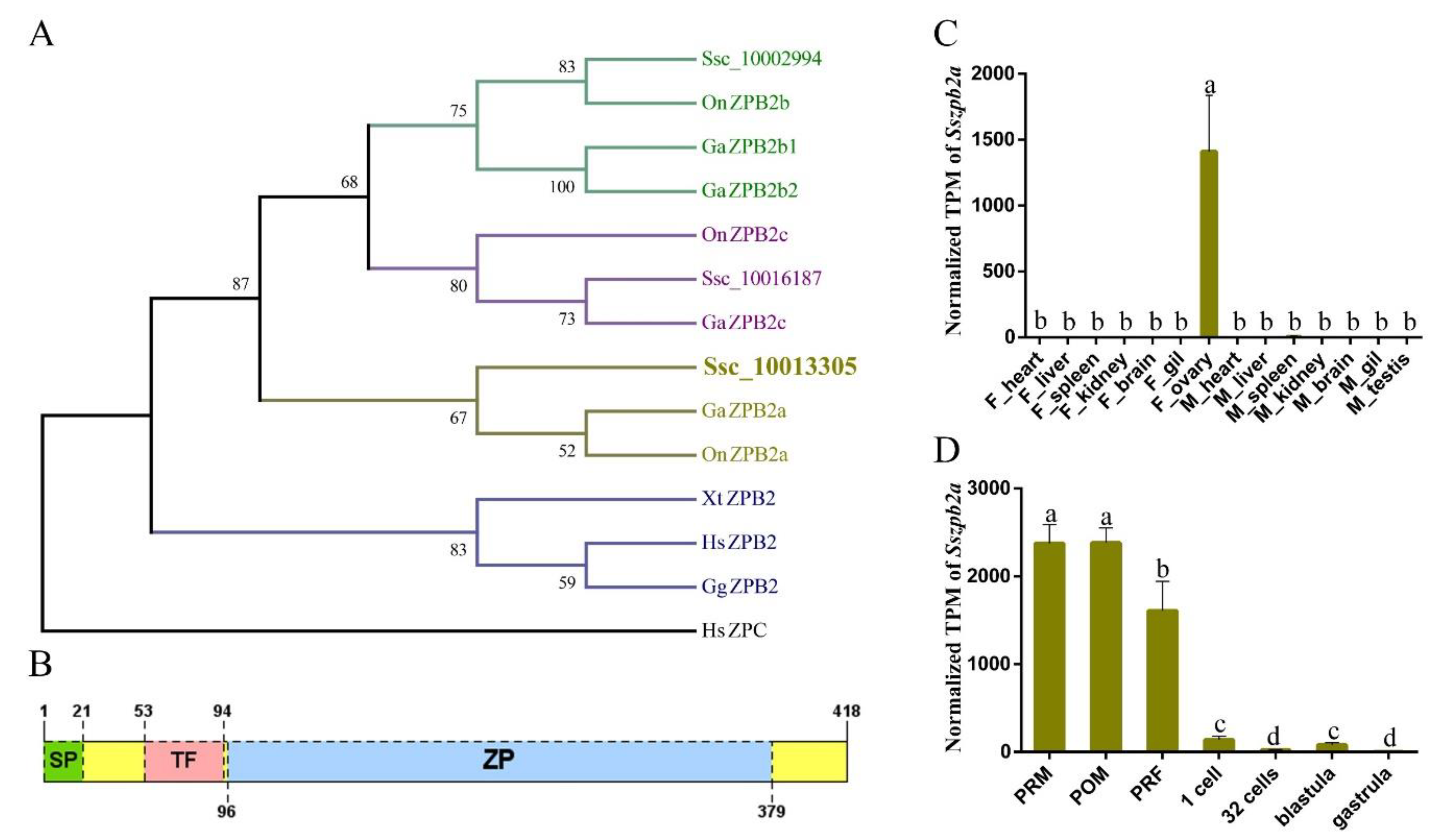
2.1. Identification and Molecular Characteristics of Sszpb2a and Sszpb2c
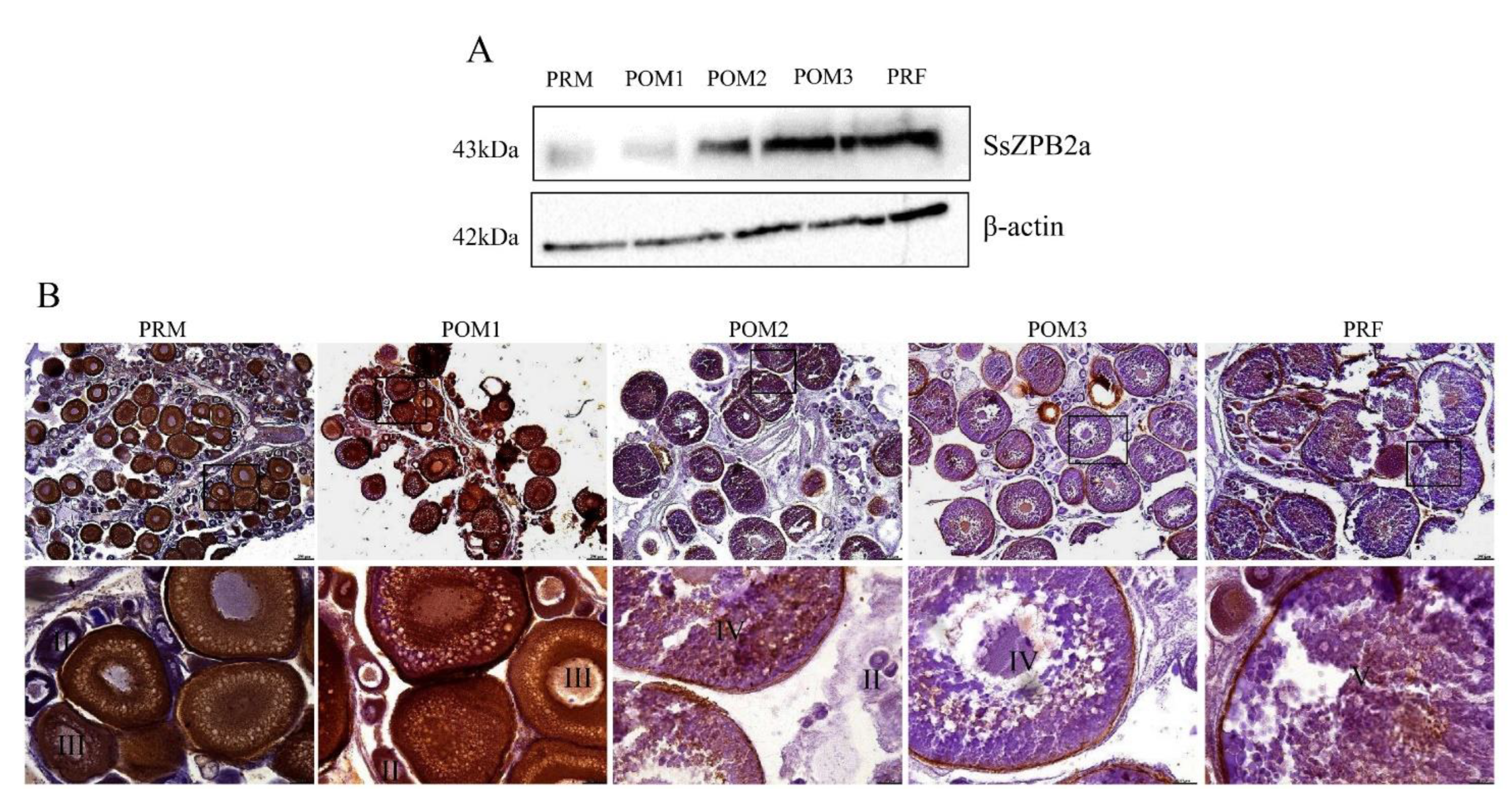
2.2. SsZPB2a Was Up-Regulated in the Oocyte with Ovary Development from PRM to PRF
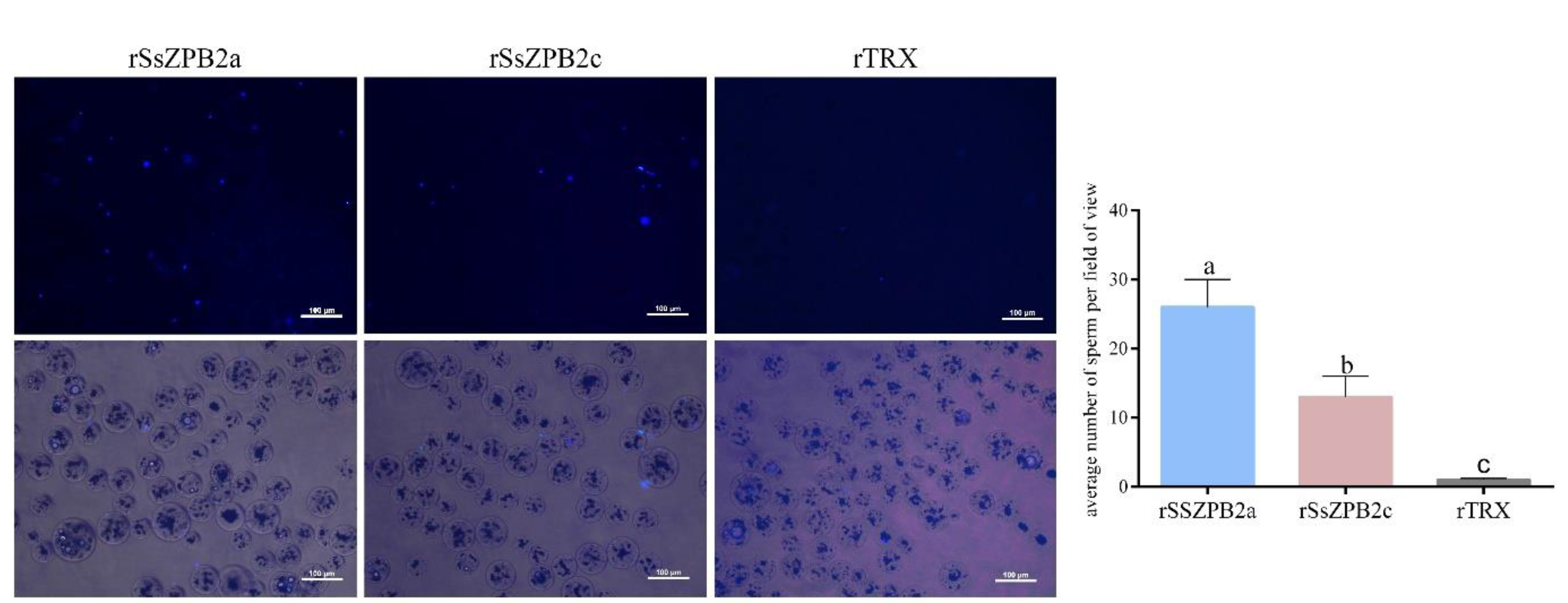
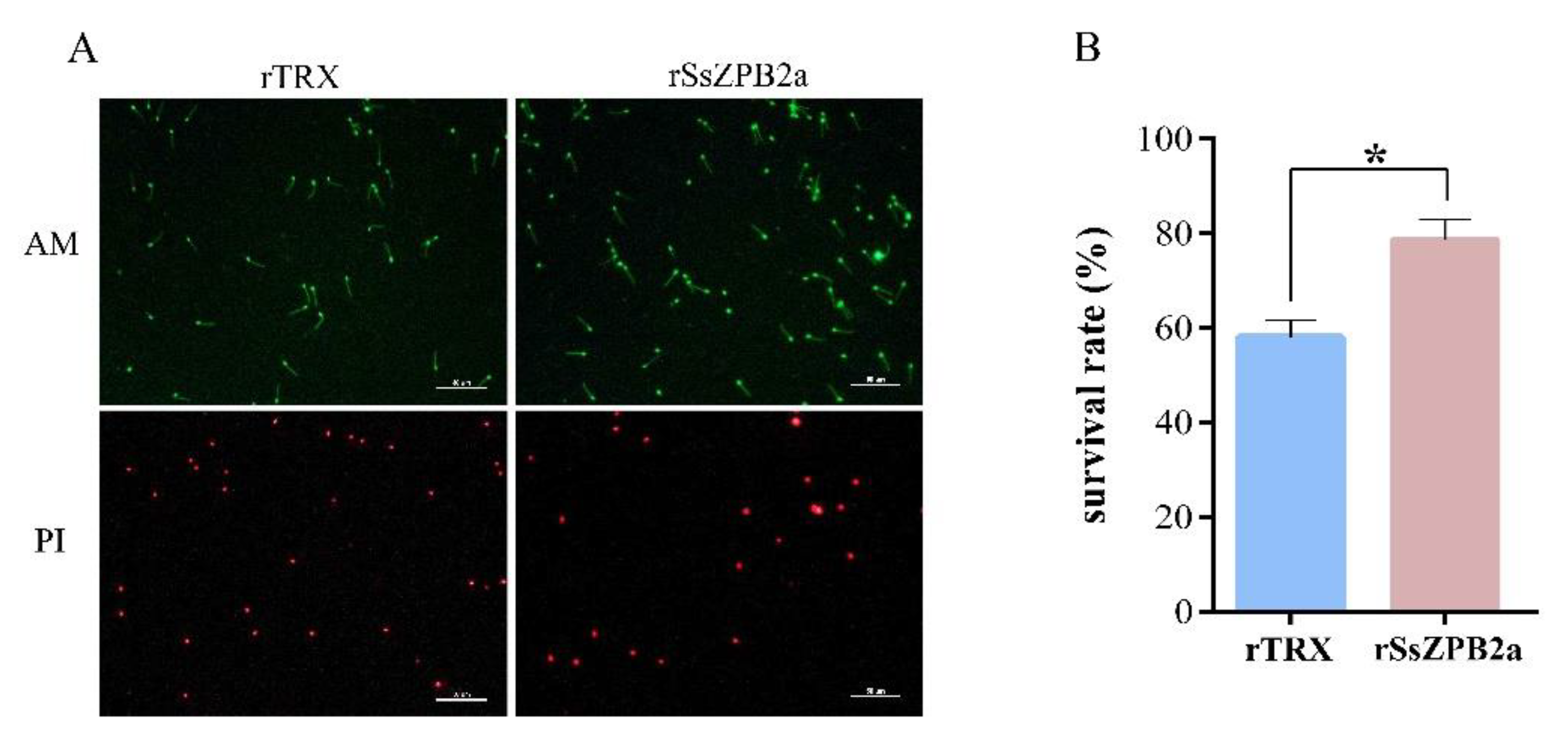
2.3. RSsZPB2a Bound to the Sperm and Affected the Viability of Sperm In Vitro
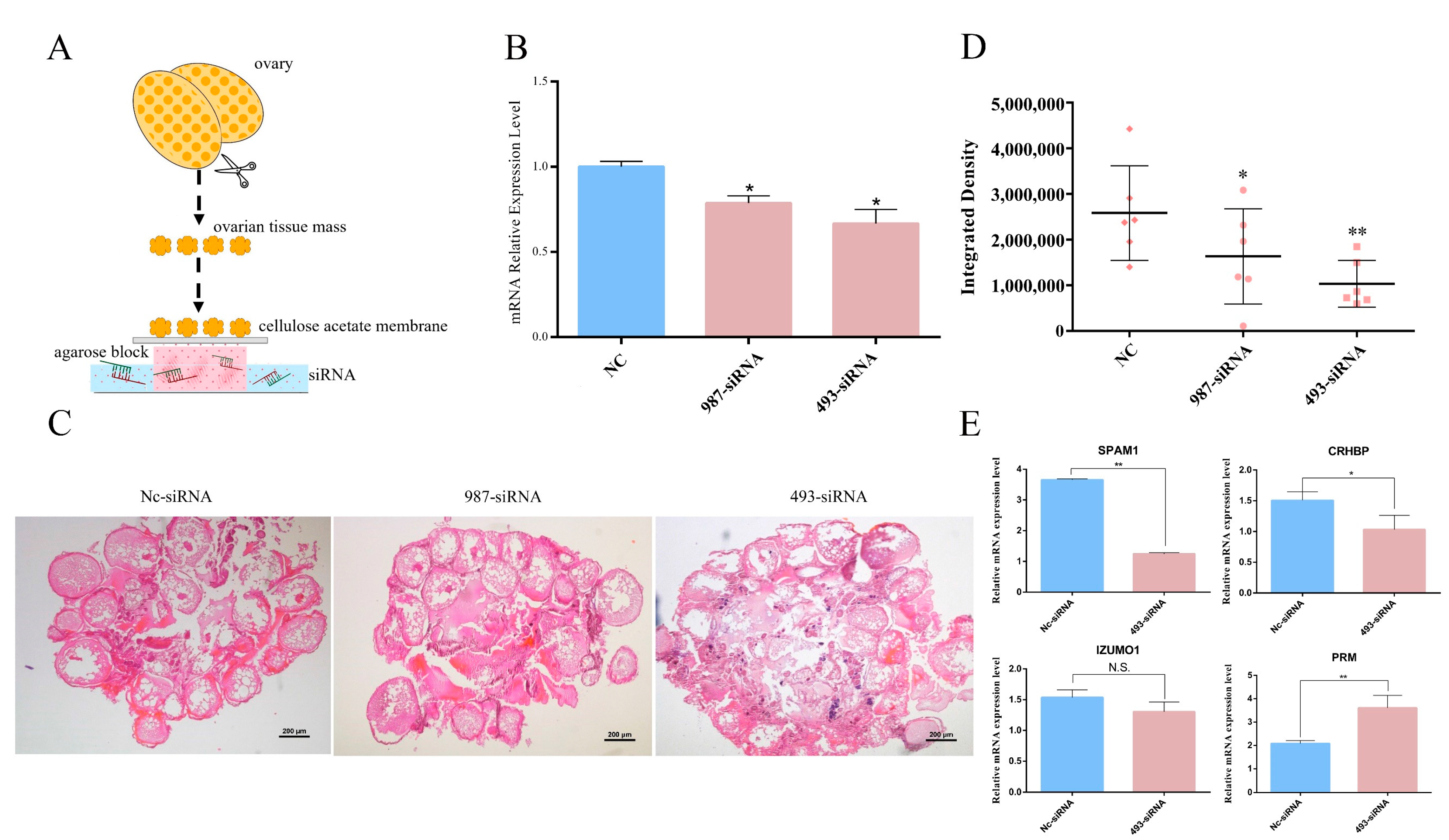
2.4. Knockdown of Sszpb2a Destroyed the Integrity of the Oocyte and Down-Regulated the Expression of Genes Associated with Sperm
3. Discussion
4. Materials and Methods
4.1. Fish and Ovaries Samples
4.2. Phylogenetic Analyses, Domain Prediction of ZPB Genes and Transcript Abundance of Sszpb2a/c Genes
4.3. Expression of Recombinant SsZPB2a (rSsZPB2a) and SsZPB2c (rSsZPB2c) and Preparation of Antiserum Targeting rSsZPB2a
4.4. Western Blot
4.5. Histology and Immunohistochemistry
4.6. Conjugation of rSsZPB2a and rSsZPB2c to Magnetic Beads
4.7. Sperm Preparation and Binding Assay between Sperm and Beads Conjugated with rSsZPB2a or rSsZPB2c
4.8. Knockdown of SsZPB2a in Ovarian Tissue Cultured In Vitro
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claw, K.G.; Swanson, W.J. Evolution of the egg: New findings and challenges. Annu. Rev. Genom. Hum. Genet. 2012, 13, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.; Chen, J.; Cohen, N.; Litscher, E.; Liu, C.; Qi, H.; Williams, Z. Structure and function of the mammalian egg zona pellucida. J. Exp. Zool. 1999, 285, 251–258. [Google Scholar] [CrossRef]
- Bronson, R.A.; McLaren, A. Transfer to the mouse oviduct of eggs with and without the zona pellucida. J. Reprod. Fertil. 1970, 22, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Boja, E.S.; Hoodbhoy, T.; Fales, H.M.; Dean, J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 2003, 278, 34189–34202. [Google Scholar] [CrossRef] [PubMed]
- Topper, E.K.; Kruijt, L.; Calvete, J.; Mann, K.; Töpfer-Petersen, E.; Woelders, H. Identification of bovine zona pellucida glycoproteins. Mol. Reprod. Dev. 1997, 46, 344–350. [Google Scholar] [CrossRef]
- Lefièvre, L.; Conner, S.J.; Salpekar, A.; Olufowobi, O.; Ashton, P.; Pavlovic, B.; Lenton, W.; Afnan, M.; Brewis, I.A.; Monk, M.; et al. Four zona pellucida glycoproteins are expressed in the human. Hum. Reprod. 2004, 19, 1580–1586. [Google Scholar] [CrossRef]
- Spargo, S.C.; Hope, R.M. Evolution and nomenclature of the zona pellucida gene family. Biol. Reprod. 2003, 68, 358–362. [Google Scholar] [CrossRef]
- Goudet, G.; Mugnier, S.; Callebaut, I.; Monget, P. Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biol. Reprod. 2008, 78, 796–806. [Google Scholar] [CrossRef]
- Franken, D.R.; Bastiaan, H.S.; Oehninger, S.C. Physiological induction of the acrosome reaction in human sperm: Validation of a microassay using minimal volumes of solubilized, homologous zona pellucida. J. Assist. Reprod. Genet. 2000, 17, 156–161. [Google Scholar] [CrossRef]
- Töpfer-Petersen, E.; Petrounkina, A.M.; Ekhlasi-Hundrieser, M. Oocyte-sperm interactions. Anim. Reprod. Sci. 2000, 60, 653–662. [Google Scholar] [CrossRef]
- Chakravarty, S.; Kadunganattil, S.; Bansal, P.; Sharma, R.K.; Gupta, S.K. Relevance of glycosylation of human zona pellucida glycoproteins for their binding to capacitated human spermatozoa and subsequent induction of acrosomal exocytosis. Mol. Reprod. Dev. Inc. Gamete Res. 2008, 75, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, H.H.; Refai, F.H.; Avella, M.A. The molecular mechanisms mediating mammalian fertilization. Development 2019, 146, dev176966. [Google Scholar] [CrossRef]
- Rankin, T.; Talbot, P.; Lee, E.; Dean, J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 1999, 126, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.A.; Baibakov, B.; Dean, J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J. Cell Biol. 2014, 205, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Gahlay, G.; Gauthier, L.; Baibakov, B.; Epifano, O.; Dean, J. Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science 2010, 329, 216–219. [Google Scholar] [CrossRef]
- Baibakov, B.; Boggs, N.A.; Yauger, B.; Baibakov, G.; Dean, J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J. Cell Biol. 2012, 197, 897–905. [Google Scholar] [CrossRef]
- Morin, G.; Sullivan, R.; Laflamme, I.; Robert, C.; Leclerc, P. SPAM1 isoforms from two tissue origins are differentially localized within ejaculated bull sperm membranes and have different roles during fertilization. Biol. Reprod. 2010, 82, 271–281. [Google Scholar] [CrossRef]
- Yoon, S.; Chang, K.T.; Cho, H.; Moon, J.; Kim, J.S.; Min, S.H.; Koo, D.B.; Lee, S.R.; Kim, S.H.; Park, K.E.; et al. Characterization of pig sperm hyaluronidase and improvement of the digestibility of cumulus cell mass by recombinant pSPAM1 hyaluronidase in an in vitro fertilization assay. Anim. Reprod. Sci. 2014, 150, 107–114. [Google Scholar] [CrossRef]
- Conner, S.J.; Hughes, D.C. Analysis of fish ZP1/ZPB homologous genes--evidence for both genome duplication and species-specific amplification models of evolution. Reproduction 2003, 126, 347–352. [Google Scholar] [CrossRef]
- Kobayashi, H.; Iwamatsu, T. Fine structure of the storage micropocket of spermatozoa in the ovary of the guppy Poecilia reticulata. Zool. Sci. 2002, 19, 545–555. [Google Scholar] [CrossRef]
- Wu, T.; Cheng, Y.; Liu, Z.; Tao, W.; Zheng, S.; Wang, D. Bioinformatic analyses of zona pellucida genes in vertebrates and their expression in Nile tilapia. Fish Physiol. Biochem. 2018, 44, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, Q.; Wu, Z.; Cao, D.D.; Ma, Z.; Xu, Q.; Hu, P.; Fu, Y.; Shen, Y.; Chan, J.; et al. Neofunctionalization of zona pellucida proteins enhances freeze-prevention in the eggs of Antarctic notothenioids. Nat. Commun. 2016, 7, 12987. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chang, Y.; Bao, L.; Yu, M.; Li, R.; Niu, J.; Fan, G.; Song, W.; Seim, I.; Qin, Y.; et al. A chromosome-level genome of black rockfish, Sebastes schlegelii, provides insights into the evolution of live birth. Mol. Ecol. Resour. 2019, 19, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Xiao, Y.; Zhao, H.; Xu, S.; Wang, Y.; Wu, L.; Zhou, L.; Du, T.; Lv, X.; et al. Sequencing of the black rockfish chromosomal genome provides insight into sperm storage in the female ovary. DNA Res. 2019, 26, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Tomita, K.; Sano, K.; Kaneko, T. Molecular events in adaptive evolution of the hatching strategy of ovoviviparous fishes. J. Exp. Zool. Part B Mol. Dev. Evol. 2015, 324, 41–50. [Google Scholar] [CrossRef]
- Chen, H.; Griffiths, G.; Galileo, D.S.; Martin-DeLeon, P.A. Epididymal SPAM1 is a marker for sperm maturation in the mouse. Biol. Reprod. 2006, 74, 923–930. [Google Scholar] [CrossRef][Green Version]
- Gupta, S.K.; Bansal, P.; Ganguly, A.; Bhandari, B.; Chakrabarti, K. Human zona pellucida glycoproteins: Functional relevance during fertilization. J. Reprod. Immunol. 2009, 83, 50–55. [Google Scholar] [CrossRef]
- Fahrenkamp, E.; Algarra, B.; Jovine, L. Mammalian egg coat modifications and the block to polyspermy. Mol. Reprod. Dev. 2020, 87, 326–340. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, Y.; Beall, S.; Sadusky, A.B.; Dean, J. A Unique Egg Cortical Granule Localization Motif Is Required for Ovastacin Sequestration to Prevent Premature ZP2 Cleavage and Ensure Female Fertility in Mice. PLoS Genet. 2017, 13, e1006580. [Google Scholar] [CrossRef]
- Liu, W.; Li, K.; Bai, D.; Yin, J.; Tang, Y.; Chi, F.; Zhang, L.; Wang, Y.; Pan, J.; Liang, S.; et al. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum. Genet. 2017, 136, 975–985. [Google Scholar] [CrossRef]
- Boisen, I.M.; Rehfeld, A.; Mos, I.; Poulsen, N.N.; Nielsen, J.E.; Schwarz, P.; Rejnmark, L.; Dissing, S.; Bach-Mortensen, P.; Juul, A.; et al. The Calcium-Sensing Receptor Is Essential for Calcium and Bicarbonate Sensitivity in Human Spermatozoa. J. Clin. Endocrinol. Metab. 2021, 106, e1775–e1792. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, H. Respiratory behaviour of sea-urchin spermatozoa. I. Effect of pH and egg water on the respiratory rate. J. Exp. Zool. 1976, 198, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sofikitis, N.; Giotitsas, N.; Tsounapi, P.; Baltogiannis, D.; Giannakis, D.; Pardalidis, N. Hormonal regulation of spermatogenesis and spermiogenesis. J. Steroid Biochem. Mol. Biol. 2008, 109, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Sie, B.S.; Liu, M.L.; Agresta, F.; Baker, H.W. Relationship between seminal plasma zinc concentration and spermatozoa-zona pellucida binding and the ZP-induced acrosome reaction in subfertile men. Asian J. Androl. 2009, 11, 499–507. [Google Scholar] [CrossRef]
- Gupta, S.K. Human Zona Pellucida Glycoproteins: Binding Characteristics with Human Spermatozoa and Induction of Acrosome Reaction. Front. Cell Dev. Biol. 2021, 9, 619868. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Page, C.; Franken, D.R. The zona pellucida-induced acrosome reaction of human spermatozoa involves extracellular signal-regulated kinase activation. Andrologia 2001, 33, 337–342. [Google Scholar] [CrossRef]
- Qu, B.; Yang, S.; Ma, Z.; Gao, Z.; Zhang, S. A new LDLa domain-containing C-type lectin with bacterial agglutinating and binding activity in amphioxus. Gene 2016, 594, 220–228. [Google Scholar] [CrossRef]
- Ganguly, A.; Bukovsky, A.; Sharma, R.K.; Bansal, P.; Bhandari, B.; Gupta, S.K. In humans, zona pellucida glycoprotein-1 binds to spermatozoa and induces acrosomal exocytosis. Hum. Reprod. 2010, 25, 1643–1656. [Google Scholar] [CrossRef]
- Zhu, Q.; Huo, H.; Fu, Q.; Yang, N.; Xue, T.; Zhuang, C.; Liu, X.; Wang, B.; Su, B.; Li, C. Identification and characterization of a C-type lectin in turbot (Scophthalmus maximus) which functioning as a pattern recognition receptor that binds and agglutinates various bacteria. Fish Shellfish Immunol. 2021, 115, 104–111. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, P.; Wang, S.; Wang, Y.; Feng, S.; Zhang, S.; Li, H. The hepatic lectin of zebrafish binds a wide range of bacteria and participates in immune defense. Fish Shellfish Immunol. 2018, 82, 267–278. [Google Scholar] [CrossRef]
- Hamze, J.G.; Canha-Gouveia, A.; Algarra, B.; Gómez-Torres, M.J.; Olivares, M.C.; Romar, R.; Jiménez-Movilla, M. Mammalian spermatozoa and cumulus cells bind to a 3D model generated by recombinant zona pellucida protein-coated beads. Sci. Rep. 2019, 9, 17989. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lv, X.; Hu, B.; Shao, Z.; Wang, B.; Ma, K.; Lin, H.; Cui, M. RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death. Apoptosis 2017, 22, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Kortner, T.M.; Rocha, E.; Arukwe, A. Androgenic modulation of early growth of Atlantic cod (Gadus morhua L.) previtellogenic oocytes and zona radiata-related genes. J. Toxicol. Environ. Health Part A 2009, 72, 184–195. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Qu, J.; Huang, D.; He, Y.; Niu, J.; Qi, J. Expression Analysis of ZPB2a and Its Regulatory Role in Sperm-Binding in Viviparous Teleost Black Rockfish. Int. J. Mol. Sci. 2022, 23, 9498. https://doi.org/10.3390/ijms23169498
Li R, Qu J, Huang D, He Y, Niu J, Qi J. Expression Analysis of ZPB2a and Its Regulatory Role in Sperm-Binding in Viviparous Teleost Black Rockfish. International Journal of Molecular Sciences. 2022; 23(16):9498. https://doi.org/10.3390/ijms23169498
Chicago/Turabian StyleLi, Rui, Jiangbo Qu, Dan Huang, Yan He, Jingjing Niu, and Jie Qi. 2022. "Expression Analysis of ZPB2a and Its Regulatory Role in Sperm-Binding in Viviparous Teleost Black Rockfish" International Journal of Molecular Sciences 23, no. 16: 9498. https://doi.org/10.3390/ijms23169498
APA StyleLi, R., Qu, J., Huang, D., He, Y., Niu, J., & Qi, J. (2022). Expression Analysis of ZPB2a and Its Regulatory Role in Sperm-Binding in Viviparous Teleost Black Rockfish. International Journal of Molecular Sciences, 23(16), 9498. https://doi.org/10.3390/ijms23169498





