Abstract
The number of grains per panicle significantly contributes to rice yield, but the regulatory mechanism remains largely unknown. Here, we reported a loss-of-function mutant, panicle apical abortion 7 (paa7), which exhibited panicle abortion and degeneration of spikelets on the apical panicles during the late stage of young panicle development in rice. High accumulations of H2O2 in paa7 caused programmed cell death (PCD) accompanied by nuclear DNA fragmentation in the apical spikelets. Map-based cloning revealed that the 3 bp “AGC” insertion and 4 bp “TCTC” deletion mutation of paa7 were located in the 3′-UTR regions of LOC_Os07g47330, which was confirmed through complementary assays and overexpressed lines. Interestingly, LOC_Os07g47330 is known as FRIZZY PANICLE (FZP). Thus, PAA7 could be a novel allele of FZP. Moreover, the severe damage for panicle phenotype in paa7/lax2 double mutant indicated that PAA7 could crosstalk with Lax Panicle 2 (LAX2). These findings suggest that PAA7 regulates the development of apical spikelets and interacts with LAX2 to regulate panicle development in rice.
1. Introduction
The abortion phenomenon of the panicle apical has a negative impact on grain yield, which acts as a severe problem in rice-breeding production. It is mainly induced by environmental and genetic factors. This phenomenon has been widely reported in rice, corn, millet, and other crops [1,2,3]. Many quantitative traits, such as ds-1~ds-9, qMDS3, qDS9, and qPAA8 have been demonstrated to cause panicle apical abortion in rice [4,5,6]. Moreover, several functional genes responsible for panicle apical abortion have been identified, i.e., TUT1 (TUTOU1), OsALMT7 (aluminum-activated malate transporter 7), OsCIPK31 (calcineurin B-like protein-interacting protein kinase 31), SPL6 (squamosa promoter binding protein-like 6), DPS1 (degenerated panicle and partial sterility 1), PAA3 (panicle apical abortion 3), CAX1a (Ca2+/H+ exchanger 1a), SUBSrP1 (subtilisin-like Serine oprotease 1), and TUT2 [3,7,8,9,10,11,12,13]. Among them, mutation of TUT1, which encodes a SCAR/WAVE-like protein, shows defects in the arrangement of actin filaments in the trichome [3], while OsALMT7 encodes an aluminum-activated malate transporter, preferentially expressed in the vascular tissues of developing panicles. The panicle apical portions in OsALMT7 mutant paab1-1 contains less malate than WT, and the injection of malate could alleviate the spikelet degeneration phenotype [13]. DPS1 encodes a mitochondria-localized protein containing a cystathionine b-synthase domain, which plays an important role in regulating ROS homeostasis, anther cuticle formation, and panicle development in rice. DPS1 participates in ROS scavenging by interacting with Trx1 and Trx20 [9]. All the mutations of the above genes lead to unbalanced ROS homeostasis and programmed cell death (PCD) in spikelet cells of apical portions of panicle.
Grain weight, grain number per panicle, and panicle number are the main factors affecting rice yield [14]. Over the past four decades in China, the high production of rice benefits from the ever-increasing grain number per panicle [15], which is determined mainly by panicle branching. Therefore, exploring the molecular mechanisms underlying grain number per panicle is of great value for the improvement of rice yields. Currently, numerous genes that are involved in panicle branch formation have been identified. For instance, the decreased expression of OsCKX2 (Gn1a), which encodes a cytokinin oxidase/dehydrogenase, promotes cytokinin accumulation in the inflorescence meristem and thereby increases the number of spikelets per panicle and rice yield [16]. Aside from genes responsible for enzymes, some transcriptional factors regulate panicle formation as well. The mutation of GRAS family transcription factor MOC1/GNP6 shows defects in panicle branch formation and tiller number [17]. The primary function of FRIZZY PANICLE (FZP), encoding a transcription factor with ERF/AP2 domain, is to negatively regulate the production of axillary meristems and promote the establishment of floret meristems [18]. The 18 bp fragment insertion at 5.3 kb upstream of the FZP promoter inhibited FZP expression, resulting in an increase in number of secondary branches [19]. Overexpression of MOTHER OF FT AND TFL1 (OsMFT1) can inhibit the transcription of FZP, thereby producing more secondary branches [20]. LAX1 encodes a helix-loop-helix (bHLH) transcription factor, determines the initiation and maintenance of axillary meristem, and synergistically regulates sterile lemma development in rice with FZP [21]. The lax1 mutant exhibits reduced tillers and lateral spikelets [22]. In addition, LAX1 interacts with OsPID and participates in the development of rice floral organs by regulating auxin transportation [23]. LAX2/Gnp4 encodes a RAWUL domain protein and regulates the formation of axillary meristems [24]. A similar lax1 phenotype of decreased secondary branches and spikelets per panicle was observed in lax2 mutant [24]. LAX2 can interact with LAX1 to regulate the development of rice axillary meristems, and the lax2/lax1 double mutant shows a more severe reduced-branching phenotype [25].
Here, we identified a new FZP allelic mutant paa7 from a severe panicle mutant abnormal panicle1 (ap1) and presented a novel function of PAA7, which controls panicle apical development. In addition, we further confirmed that PAA7 could interact with LAX2 to co-regulate the identification of the panicle branch. Our findings offer insights into the molecular mechanism underlying rice panicle formation and provide critical genetic resources for improving rice grain yields.
2. Results
2.1. Characterization of the paa7 Mutant
The apical panicle abortion7 (paa7) mutant was obtained from ap1, a severe panicle mutant of an Egyptian rice variety “Giza159”, and showed a stable phenotype of panicle abortion.
There was no significant difference in plant height and tiller number between wild- type (WT) Giza159 and paa7 mutant (Figure 1A,B), but paa7 mutant exhibited remarkably degenerated, whitish spikelets at the top of panicle after heading (Figure 1C). The degeneration appeared more frequently in the primary branches at the top of the panicle, indicating degradation degree was associated with spikelet location (Figure 1D,E). To verify this speculation, we performed statistics on degenerate spikelets of different branches. The results showed that, in the paa7 mutant, approximately 20% of spikelets and 43% of primary branches, mainly located at the top of each panicle, were degenerated (Figure 1F). The degeneration length ranged from 0.6–7 cm, and the mean ratio of degeneration length per bleached primary branches was about 56% (Figure 1F). In addition, the panicle length, grain number per panicle, and 1000-grain weight of paa7 mutant were lower than those of WT (Figure 1G–I).
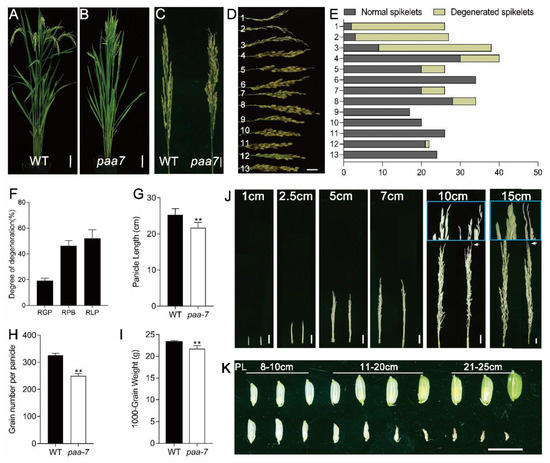
Figure 1.
Phenotypic characterization of paa7. (A,B) Morphology of WT and paa7 plants, bars = 10 cm. (C) Panicle of WT and paa7, bars = 2 cm. (D) Phenotype of primary branches from one panicle; 1–13 indicate primary branches from the top to the bottom of the panicle in paa7, bar = 1 cm. (E) Statistics for the spikelet number correspond to (D). (F–H) Quantification of panicle length, grain number per panicle, 1000-grain weight. (I) Degree of degeneration of paa; 7RGP: ratio of degenerated grains per panicle (%), RPB: ratio of degenerated primary branches (%), RLP: ratio of degenerated length per primary branch (%). Error bars indicate standard error. ** p ≤ 0.01 by Student’s t-test, n = 10. (J) Representative images of WT (left) and paa7 (right) developing panicles; insets show magnified views of the top of panicles, bars = 1 cm. (K) Spikelets of panicles after 8 cm of WT and paa7; the number indicates the length of the panicle from which they were collected, bar = 1 cm.
To differentiate the abortion stage that emerged in paa7, we tracked the development of panicles from 1 cm in length. The results showed that the spikelets in the top panicle of paa7 degenerated during the eighth stage of inflorescence development in the rice (In8) phenotype [26] when the panicle developed to 10–11 cm in length (Figure 1J). The aborted spikelets had no difference from normal spikelets before the 8 cm panicle, while they withered gradually after the In8 stage (Figure 1K).
In addition to the aborted spikelets, other abnormal spikelets, such as ectopic lemma and palea, twisted glume, and shortened lemma, were observed in paa7 (Figure S1A–C). The floral organs, such as anthers, stigmas, and ovary, developed normally in paa7, even in degenerated spikelets (Figure S1D–G). However, vascular bundle number in transverse sections of pedicels was reduced in paa7 (Figure S1H,I), indicating that material transport in the apical spikelet of paa7 mutants could be impaired. Collectively, paa7 is an apical panicle abortion mutant that significantly reduces the number of fertile spikelets and causes a massive loss in grain yield.
2.2. Enhanced Cell Death in paa7 Mutant
The cell death characteristic of degenerated spikelets in 7–8 cm panicle of WT and paa7 mutant were identified through Trypan blue staining and Evans blue staining. The results showed that apical spikelets in paa7 mutant were intensely stained but were hardly stained in WT (Figure 2A,B).
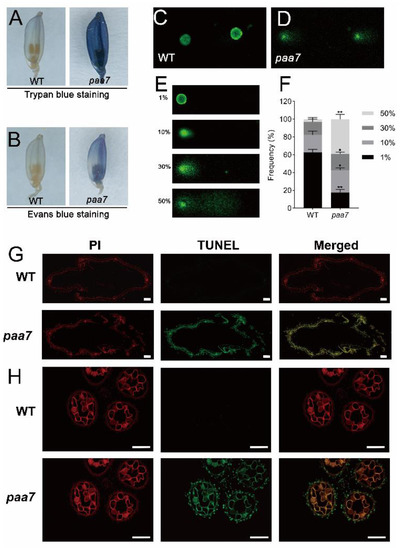
Figure 2.
Cell-death-related events are triggered in paa7. (A,B) Trypan blue and Evans blue staining of apical spikelets. (C,D) Typical comets for DNA damage in nuclei of the apical spikelet cells. (E) Four types of DNA damage extents in each nucleus are indicated by the units 1%, 10%, 30%, or 50%. An increased unit and anthers of WT and paa7 correlated with a larger comet tail. Bars = 100 μm. (F) Frequency distribution of four types of DNA damage extents in WT and paa7, data are means ± SD (n = 3). Asterisks indicate significant difference by Student’s t-test (** p < 0.01 and * p < 0.05). (G,H) TUNEL analysis of the apical spikelet cells of glumes, Bars = 100 μm.
The DNA damages in apical spikelets of 8 cm panicle were further evaluated through comet assay (single-cell gel electrophoresis assay), a simple and effective method for assessing DNA damage in cells [27], and the results indicated significantly increased DNA damage in top spikelets of paa7 compared with WT (Figure 2C–F). In addition, apoptosis in the spikelets of the 8 cm panicle was detected through TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) assay. Compared with WT, the TUNEL-positive signals were much stronger in paa7 (Figure 2G,H). These results suggested that DNA fragmentation occurred at a single-cell level and therewith caused cell death in spikelets and anthers in paa7 mutant.
Excessive ROS accumulation can induce cell apoptosis through the oxidative stress response. Hydrogen peroxide is a signal substance that causes cell death. The cellular ROS levels were monitored with 3,3′-diaminobenzidine (DAB), and the results showed that spikelets of paa7 can be dyed brown, especially in the top spikelets. In contrast, spikelets of WT were hardly stained (Figure 3A), indicating increased ROS levels in degenerated spikelets. The concentration of H2O2 and malondialdehyde (MDA) were significantly increased in apical spikelets of paa7 over those of WT after 8–10 cm panicle in length (Figure 3B–D). All these results indicated that the over-accumulation of oxidant products caused cell death and eventually led to panicle apical abortion in paa7.

Figure 3.
Determination of ROS content in WT and paa7. (A) DAB staining of spikelets in different parts of rice panicle. (B) H2O2 content in spikelets of apical part of the panicle in WT, apical part (ap), middle part (mp), and bottom part (bp) of panicle in paa7; data are means ± SD (n = 3); different letters indicate significant differences by one-way ANOVA and Duncan’s test (p < 0.05). (C) H2O2 content in apical spikelets at different stages of panicle development. (D) MDA content in apical spikelets at different stages of panicle development. Data are means ± SD (n =3); asterisks indicate significant differences by Student’s t-test (** p < 0.01).
2.3. Mutations in 3′-UTR of PAA7 Responsible for Panicle Apical Abortion
To reveal the genetic features of paa7, we developed two populations derived from the cross of paa7 and 9311, II-32B, respectively. All F1 progenies demonstrated a similar wild-type panicle phenotype. In F2 population, the separation ratio of the phenotype of wild-type to panicle apical abortion corresponded to the separation ratio of 3:1 (Table S1), indicating that the panicle apical abortion trait of paa7 was controlled by a recessive nuclear gene.
The PAA7 gene was initially located between RM1209 and RM1357 on the long arm of chromosome 7 with 284 SSR markers using 349 recessive individuals from the F2 mapping population of paa7 and 9311 (Figure 4A). Subsequently, we delimited PAA7 to a 28 kb interval between the markers I12 and RM22120 using 1580 F2 recessive plants in 2018 (Figure 4B,C).
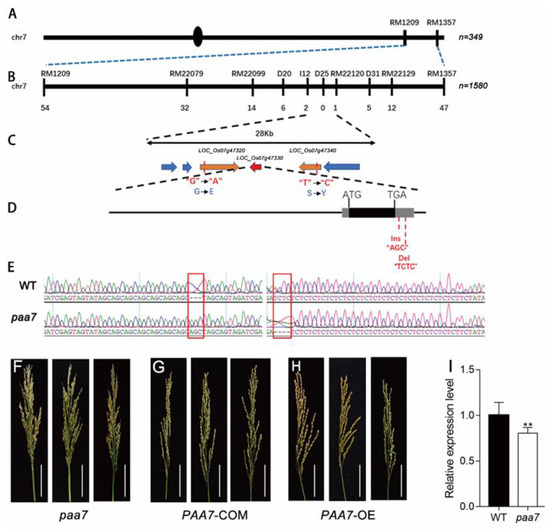
Figure 4.
Mutation of 3′-UTR of PAA7 caused panicle apical abortion. (A) The PAA7 locus was mapped to the long arm of chromosome 7 between the markers RM1209 and RM1357. (B) Mapping of the PAA7 locus between markers I12 and RM22120. ‘n’ represents homozygous recessive individuals derived from an F2 population from the cross paa7 × II-32B, paa7 × 9311. The number of recombinants is indicated below the map. (C) The PAA7 locus was narrowed down to a 28 kb region, including six putative protein-encoding genes. (D) There are 3 bp (AGC) insertion and 4 bp (CTCT) deletion in the 3′UTR in FZP of paa7 mutant. (E) Sequencing results of WT and paa7 gDNA, red box indicated the mutant site. (F–H) Panicles of paa7 mutant plants (F), complementary transgenic plants (G) and overexpressed transgenic plants (H), Bars = 5 cm. (I) Expression of FZP in WT and paa7 mutant, data are means ± SD (n =3), asterisks indicate significant difference by Student’s t-test (** p < 0.01).
There were six predicted coding genes located in this interval region (http://www.gramene.org/, accessed on 1 July 2021) (Figure 4C). To further determine the candidate gene of PAA7, the entire sequences of the six genes were sequenced. Sequence comparison between paa7 and WT revealed that a nucleotide substitution A-G and T-C was found in exon of LOC_Os07g47320 and LOC_Os07g47340, respectively. Further, a 3 bp (AGC) insertion and a 4 bp (TCTC) deletion were found in the 3’UTR of LOC_Os07g47330 (FZP) (Figure 4D,E). No other nucleotide variations were found in the other three genes.
To verify the putative PAA7 gene, LOC_Os07g47320, LOC_Os07g47330 and LOC_Os07g47340 complementation assays were performed in paa7, respectively. The results showed that only transformants with LOC_Os07g47330 complementary vector rescued the paa7 phenotype (Figure 4F,G). Transgenic plants with overexpressed LOC_Os07g47330 also displayed the normal panicle phenotype as expected (Figure 4H). Expression analysis revealed that the expression level of LOC_Os07g47330 was about 20% decreased in paa7 compared with wild-type in 7–8 cm young panicle (Figure 4I).
Taken together, these results suggested that the candidate gene of PAA7 was LOC_Os07g47330, which shared the common locus with the previously reported FZP [18], and the mutations in 3′-UTR of PAA7 were responsible for panicle apical abortion.
2.4. Feeding of Exogenous Auxin Alleviates Apical Spikelet Degeneration in paa7
As the mode of panicle abortion in paa7 is consistent with spatiotemporal features of apical dominance mediated by auxin, we hypothesized that the degenerate phenotype of the top spikelet in paa7 might be related to auxin regulation. The endogenous indole-3-acetic acid (IAA/auxin) concentration was measured in WT and paa7 at different panicle development stages. The results showed that the auxin content of paa7 was slightly lower during the early stage of panicle development compared with wild type; however, it significantly decreased after the panicle developed to 8 cm in length (Figure 5A).
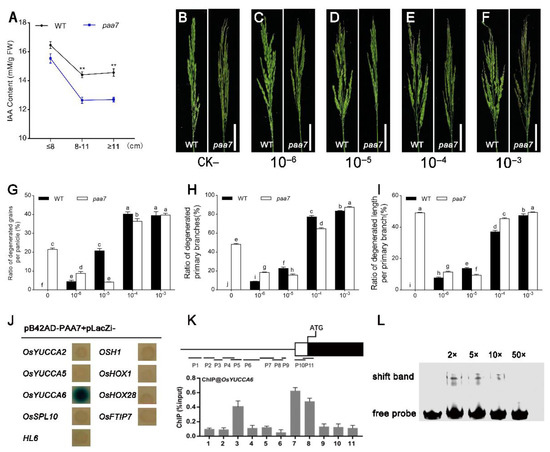
Figure 5.
PAA7 regulates apical panicle degeneration through auxin pathway. (A) Auxin content in panicle of WT and paa7 at different developmental stages. Data are shown as means ± SD from three replicates (** p < 0.01, Student’s t-test). (B–F) Phenotypes of WT and paa7 mutant plants treated with different concentrations of auxin. Bars = 5 cm. (G–I) Ratio of degenerated grains per panicle; ratio of degenerated primary branches; ratio of degenerated length per primary branch (data are shown as means ± SD from 10 replicates. Bars annotated with different letters represent values that are significantly different (p < 0.05) according to a one-way ANOVA). (J) Y1H assay for interactions between FZP and auxin related genes. (K) ChIP-qPCR results show that PAA7 binds to the promoter of OsYUCCA6. ChIP enrichment compared with the input sample was tested using qPCR. Data are shown as means ± SD (n = 3). (L) EMSA assays reveal that PAA7 directly binds to OsYUCCA6 promoter DNA fragments containing “CCGTTG”.
To confirm that decreased auxin might be responsible for apical panicle abortion, 10−6–10−3 M concentration of exogenous auxin were sprayed every day since the In1 stage (Figure 5B–F). We found that the degenerate phenotype occurred in panicles of WT plants with all concentrations of IAA treatment, and the degeneration was positively correlated with IAA concentration. However, the apical spikelet abortion phenotype in paa7 was alleviated under 10−6 M and 10−5 M exogenous auxin treatment (Figure 5B–D,G–I), while more severe panicle apical abortion was displayed under 10−4 M and 10−3 M exogenous auxin treatment (Figure 5E–I). These results suggested that insufficient synthesis of auxin may be responsible for the panicle apical abortion phenotype in paa7.
2.5. PAA7 Activates the Expression of OsYUCCA6 to Regulate the Auxin Content in Young Panicle
In order to detect the effect of PAA7 on the regulation of auxin-, the expression level of auxin synthesis-associated genes was detected in apical spikelets tissue of panicle 6 cm–8 cm in length. A total of 22 genes related to auxin synthesis were selected for RT-qPCR analysis. The results showed that the expression level of 9 genes, including OsYUCCA2, OsYUCCA5, OsYUCCA6, OsSPL10, HL6, OSH1, OsHOX1, OsHOX28, and OsFTIP7, were significantly changed in paa7 (Figure S2).
Yeast one-hybrid assay was further carried out to verify the interaction between PAA7 and the promoter of these nine genes. The results indicated that only the promoter regions of OsYUCCA6 could interact with PAA7 in yeast (Figure 5J). Chromatin immunoprecipitation (ChIP)-qPCR showed that PAA7 was significantly enriched in OsYUCCA6-7 promoter regions (Figure 5K) in vivo, which contained MYB recognition site (CCGTTG) through cis-element analysis. In addition, EMSA analysis was performed to verify that PAA7 can bind directly to the 59-bp fragment containing the MYB recognition site of OsYUCCA6 in vitro (Figure 5L). Taken together, PAA7 activates auxin biosynthesis by directly binding to the MYB recognition site in the promoter region of OsYUCCA6.
2.6. PAA7 Synergistically Works with LAX2 during Panicle Development
Both paa7 (Figure 6A) and previously reported lax2-4 (Figure 6B) mutants [28] were derived from a same natural panicle mutant ap1 (Figure 6C–E), which shows frizzy panicle phenotype and is similar to severe fzp allelic mutant. We speculate that LAX2-4 and PAA7 may synergistically regulate rice panicle development. To further elucidate this speculation, reciprocal crosses were performed between lax2-4 and paa7. All F1 progenies showed a wild-type panicle phenotype. However, in F2 population, the separation ratio of the phenotype of wild-type, panicle apical abortion (paa7), lax panicle (lax2-4) and frizzy panicle (ap1) basically corresponded to the segregation ratio of 9:3:3:1 (Table S2). Interestingly, the phenotype of the original ap1 mutant, which exhibited curled panicle branches and almost no spikelets (Figure 6C,D), was separated in F2 population. The spikelets were replaced by higher-order vermiculate branches in lax2-4/paa7 double mutant (Figure 6E), similarly with fzp strong mutant’s phenotype reported previously [18]. These results suggested a possible genetic interaction existed between LAX2-4 and PAA7.

Figure 6.
Phenotypic characterization of paa7/lax2−4 double mutants. Panicle of paa7 (A), lax2-4 (B), paa7/lax2−4 (C−E), bars = 2 cm. (F) Sequencing results of targeted regions of in cr−L2−paa7#1−#4 tran−genic plants. Panicle of cr−L2−paa7#1 (G), cr−L2−paa7#2 (H), cr−L2−paa7#3 (I), and cr−L2−paa7#4 (J), bars = 2 cm.
In addition, a CRISPR/Cas9-LAX2 knockout vector was introduced into the paa7 mutant and four T1 independent homozygous lines were obtained (Figure 6F). All these four lines showed a similar panicle phenotype with ap1 Figure 6G–J and Figure S3A–D), indicated that the mutation of LAX2 will aggravate paa7 phenotype. Thus, we speculated that LAX2 and PAA7 work synergistically in regulating the transition from branch meristem to spikelet meristem during panicle development.
To explore the relationship between LAX2 and PAA7, yeast two-hybrid assay was performed and the results showed that LAX2 could interact with PAA7 in yeast (Figure 7A). Further pull-down, co-immunoprecipitation and bimolecular fluorescent complimentary assay demonstrated that LAX2 could directly interact with PAA7 (Figure 7B–D). These results suggested that PAA7 interacts with LAX2 to co-regulate panicle development.

Figure 7.
PAA7 interacts with LAX2. (A) Yeast two−hybrid assays showed the interaction between PAA7 and LAX2 proteins; pGBKT7−53 and pGADT7−T pair was used as a positive control, the pGBKT7−Lam and pGADT7−T pair was used as negative control, and the pGBKT7−LAX2 and pGADT7 pair was the self-activation test. (B) PAA7 binds LAX2 in vitro. MBP−PAA7 were incubated with GST−LAX2 and pulled down by GST−LAX2 and detected by immunoblot with anti−MBP ant-body. (C) Co−IP assay confirmation of PAA7 and LAX2 interaction. (D) Bimolecular fluorecence complementation (BiFC) assay showed that PAA7 and LAX2 proteins interact in the nucleus of rice protoplasts. Bar = 10 μm.
3. Discussion
3.1. paa7 Is a Novel Allele of fzp
FZP encodes an AP2 family transcription factor and plays essential roles in rice panicle development [18]. To date, 18 fzp mutants have been reported in rice [18,19,21,29,30,31], with most due to mutation in the coding or 5’UTR region of FZP, causing multiple phenotypes [31,32]. Weak fzp mutants like sgdp7 or qSrn7 have smaller but more spikelets per panicle [19,30]; fzp-12 and fzp-13 display defects in the sterile lemma identity [29]. However, severe fzp mutants like fzp-1 show a frizzy panicle phenotype, similar to stick panicles, and the spikelets are replaced with masses of higher-order branches rather than glumes and florets [18].
In this study, we characterized a new allelic mutant of FZP, paa7, displaying typical panicle apical abortion (Figure 1C), which has never been reported in previous FZP allelic mutants. The variations located in 3′-UTR of PAA7 were responsible for the phenotype of panicle apical abortion in paa7 (Figure 4); 3′-UTR is unique in eukaryotes, which contains various cis-acting elements and involves post-transcriptional regulation by binding with trans-acting factors or microRNAs [33]. In this study, the expression level of PAA7 was reduced by 20% in paa7 mutant because of the 3 bp “AGC” insertion and 4 bp “TCTC” deletion in 3′-UTR of PAA7 (Figure 4I). The complementary assays and overexpressed lines confirmed that the mutations in 3′-UTR of PAA7 were responsible for panicle apical abortion (Figure 4F–H).
Collectively, paa7 is a novel allele of fzp, with a new phenotype of panicle apical abortion and new functional variations in 3′-UTR.
3.2. Panicle Abortion in paa7 Possibly Be Caused by Down-Rugulation of OsYUCCA6
Panicle apical abortion in rice can lead to a decrease in grain yield. To date, nine panicle apical abortion genes have been identified. These genes are involved in many biological processes, such as maleic acid transport (ALMT7), endoplasmic reticulum stress (SPL6), and redox balance (DPS1) [8,9,13], etc. However, it remains unclear whether auxin synthesis accounts for panicle apical abortion.
In this study, the development of apical spikelets in paa7 was hindered, which may be ascribed to the impaired material transportation in the panicle. Compared with WT, the content of IAA/auxin of the apical panicles, the vascular bundles’ number in the pedicel’s transverse section, and the number of sclerenchyma cells were reduced significantly in paa7 mutant (Figure S1H,I), which caused weakened branch transport capacity. We hypothesized that auxin deficiency leads to failure of vascular bundle development and further collapse of material transport and accumulation of intracellular ROS. The content of endogenous auxin and response to appropriate concentration (10−5–10−6) of exogenous auxin treatment in paa7 confirmed this speculation (Figure 5B–D). However, excessive auxin (10−3–10−4) had opposing effects, in that it aggravated the degeneration phenotype in paa7 (Figure 5E,F), which corresponds to previous studies [7]. Thus, the panicle apical abortion of paa7 was associated with auxin.
YUCCAs, which are involved in auxin synthesis through the Trp-dependent pathway, can convert tryptamine (TAM) to N-hydroxytryptamine (NHT) in vitro [34]. OsYUCCA6 is preferentially expressed in the top of coleoptiles and is involved in IAA biosynthesis in rice [35]. The decreased expression levels of OsYUCCA6 may lead to lower auxin content in young panicles of paa7 mutant. We speculate that the down-regulation of OsYUCCA6 led to auxin insufficient in young panicles of paa7. However, why auxin deficiency leads to apical abortion in rice still needs to be further explored.
3.3. LAX2 and PAA7 Interact to Regulate Panicle Development in Rice
LAX2-4/LAX2 and PAA7/FZP play important roles in rice panicle development and are involved in the regulation of the transition from branch meristem to spikelet meristem with opposite effects [24,31]. Previous research suggests that both LAX2 and FZP could interact with LAX1, which was also known in rice panicle and floret development [21,36]. Tillers, secondary branches, and lateral spikelets were incapable of development in lax1/lax2 double mutants [36], while the branches developed normally but failed to produce spikelets in lax1/fzp double mutant. While LAX1 and FZP could synergistically control sterile lemma development [21], the interaction between LAX2 and FZP remains unclear.
As mentioned above, both paa7 mutant and lax2-4 mutant were derived from the same panicle defective ap1 mutant. The phenotype of paa7/lax2-4 double mutant was consistent with ap1 mutant (Figure 6C–E), and the loss of functional LAX2-4 aggravated the panicle defect of paa7 (Figure 6F–J). In lax2-4/paa7 double mutants, branch meristems could not be transformed into spikelet meristems normally, but reversely showed the characteristics of axillary meristem and continuously produced new high-order branches (Figure 6C–E). The protein interaction experiments also verified the interaction between LAX2-4 and PAA7/FZP (Figure 7). Thus, LAX2-4 and PAA7/FZP work synergistically to regulate the differentiation and meristem identity transformation of branch meristems during young panicle development.
One model was proposed to explain the interaction between LAX2 and PAA7/FZP (Figure 8). In WT, PAA7/FZP and its related genes are expressed normally to produce healthy panicles. However, the expression of PAA7/FZP and its downstream auxin-related genes are suppressed and finally caused panicle apical abortion in paa7. In addition, the adverse panicle of paa7 was enhanced by the loss of functional LAX2 (Figure 8).

Figure 8.
A working model for the FZP in the regulation of panicle development. In WT, PAA7/FZP could interact with LAX2 and activates OsYUCCA6 to control the development of rice panicle. In the paa7 mutant, lower expression led to downregulation of OsYUCCA6 and auxin deficiency in top spikelets of young panicle, finally resulting in panicle apical abortion; the loss of function of LAX2 aggravates the phenotype of paa7 mutant, which presented as ap1 (fizzy panicle).
4. Materials and Methods
4.1. Plant Materials and Growth Condition
The paa7 mutant was obtained from a severe panicle mutant of an Egyptian rice variety, “Giza159”, after trait isolation and phenotypic stabilization.
Rice plants were grown in the paddy field at China National Rice Research Institute (CNRRI) regularly. The transgenic plants were grown in the net house with a 14 h light (30 °C)/10 h dark (28 °C) light cycle at CNRRI. All plants were grown following standard agricultural field management.
4.2. Histological Analysis and TUNEL Assay
For histological observation, paraffin section was performed. Briefly, fresh spikelets and branches from wild type and paa7 were placed in 50% formalin–acetic-acid–alcohol mixed solution and treated as previously described [37]. Then, the paraffin sections were stained with toluidine blue and observed through microscope (Nikon ECLIPSE E100 Nikon Imaging (Shanghai, China) Sales Co., Ltd.).
For TUNEL assay, apical spikelets of 7–8 cm panicle in length of WT and paa7 were fixed in the FAA fixation solution overnight at 4 °C. The subsequent steps were taken according to the previously described method [13].
4.3. Trypan Blue and Evans Blue Staining
For trypan blue staining, apical spikelets of 7–8 cm panicle in length of WT and paa7 were incubated with trypan blue solution (0.81% NaCl, 0.06% KH2PO4 and 0.4% trypan blue) for 10 min at 100 °C. After incubation, 2.5 mg/mL of chloral hydrate solution was used to decolorize.
For Evans blue staining, apical spikelets of 8 cm panicle in length of WT and paa7 were incubated with Evans blue solution (0.25%, w/v) overnight at room temperature. After incubation, absolute alcohol was used to decolorize. Photographs were taken with a digital camera (D810, Nikon Imaging (Shanghai, China) Sales Co., Ltd.).
4.4. Comet Assay
The comet assay was performed with the Single Cell Gel Electrophoresis Assay Kit (Trevigen, Gaithersburg, MD, USA) according to the manufacturer’s instructions. The comet nuclei were visualized by confocal microscopy (LSM 780; Zeiss) at 480 nm; 30 comet nuclei in each slide were analyzed by Comet Assay Software Project (CASP, http://www.casp.of.pl/).
4.5. Determination of H2O2 and Malondialdehyde (MDA) Contents
The H2O2 and MDA contents were determined with spikelets of 7–8 cm panicle using H2O2 content assay kit (Comin, Suzhou, China) and MDA content assay kit (Comin, China), respectively, according to the manufacturer’s instructions.
For 3,3-diaminobenzidine (DAB) staining, after incubation in 1 mg/mL DAB solution overnight at room temperature and decolorized with absolute alcohol, spikelets of WT and paa7 samples were observed and photographed with a digital camera (D810, Nikon).
4.6. Positional Cloning of the PAA7 Gene
Two F2 populations derived from the cross between paa7 mutant and indica cultivar 9311, II-32B were constructed for genetic analysis and physical mapping.
F2 plants with recessive paa7 phenotype were used for genetic mapping, while 46 InDel and SNP markers were developed by comparing the genomic sequences of Nipponbare and 9311 between the markers RM1209 and RM1307 for fine mapping.
All the markers used in this study are listed in Table S3.
4.7. RNA Extraction and Quantitative Real-Time PCR Analysis
Total RNA was extracted from young panicle tissues using the Total RNA Miniprep kit (Axygene, Shanghai, China); cDNA was synthesized with 1 μg RNA using the ReverTra Ace qPCR-RT kit (Toyobo (Shanghai, China) BIOTECH Co., Ltd.). RT-qPCR was performed using PowerUp™ SYBR™ Green mix (Thermo Fisher Scientific, Waltham, MA, USA) in an Applied Biosystems 7900HT instrument. Rice Ubiquitin (LOC_Os03g13170) was used as an endogenous control. The primers used for RT-qPCR are listed in Table S3. Data were analyzed following the relative quantification method [38].
4.8. Vector Construction and Transformation
For the complementary assay, full-length gDNA of LOC_Os07g47320, LOC_Os07g47330, and LOC_Os07g47340 were cloned into pCAMBIA1300 BamH I site using ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China), respectively. For the over-expression vector, full-length cDNA of LOC_Os07g47330 was cloned into pUBI-Flag-GFP Kpn I site under the control of Maize Ubiquitin promoter. CRISPR/Cas9 vectors were constructed according to a previous report [39].
All the vectors were transformed by A. tumefaciens-mediated transformation as previously described [40].
4.9. Yeast Two-Hybrid (Y2H) Assay
The CDS of LAX2 and PAA7 amplified from Nipponbare cDNA were cloned into the pGBKT7 or pGADT7 vector, respectively. Yeast two-hybrid analysis was performed with Matchmaker Gold Yeast Two-Hybrid System (Clontech, Beijing, China) according to the manufacturer’s instructions. The primers used are listed in Table S3.
4.10. Pull-Down Assay
The purified recombinant protein was incubated in pull-down buffer (50 mM Tris-HCl (pH 7.5), 5% glycerol, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 0.01% Nonidet P-40, 150 mM KCl) for 2 h at 4 °C with 20 μL His-beads per tube. After incubation, beads were washed 6 times with pull-down buffer. The sample was denatured at 100 °C for 5 min in 50 μL SDS loading buffer and analyzed by SDS-PAGE; anti-MBP and anti-His were used to detect the samples, respectively.
4.11. Bimolecular Fluorescence Complementation (BiFC) Assay
For BiFC assays, full-length CDS of LAX2 and PAA7 were cloned into1300-YN vector or 2300-YC vector, respectively. The constructs were co-expressed in rice protoplasts. YFP signals were observed about 24 h after expression. The primers used are listed in Table S3.
4.12. Coimmunoprecipitation (Co-IP) Assay
Both the p35S-PAA7-GFP and p35S-LAX2-HA vectors were co-expressed in rice protoplasts for 24 h, and nucleoprotein was extracted with protein extraction buffer. Then, 25 µL of anti-HA beads were added after equilibrium to the extracted protein and incubated at room temperature for 0.5 h. After incubation, the beads were washed five times with 5X TBST buffer; then, 100 µL 50 mM NaOH was added, and the samples were boiled for 5 min and centrifuged. The supernatant was separated by 10% SDS–PAGE. Anti-GFP antibody (Abmart, M20008; 1:5000) and anti-HA antibody (Abmart, M20008; 1:5000) were used for further study.
4.13. Auxin Content Determination
Determination of IAA content was carried out using the enzyme-linked immunosorbent assay (ELISA) method as previously described [41]. Briefly, 0.1 g panicle tissue was ground to a fine powder in liquid nitrogen and homogenized with 900 μL PBS buffer (PH 7.2–7.4). After centrifuging at 3000× g for 20 min at 4 °C, the supernatant was used to determine the content of auxin. The subsequent steps follow the manufacturer’s instructions of plant indole acetic acid (IAA) ELISA kit (Dogesce, Beijing, China).
4.14. Yeast One-Hybrid Assay
The promoter sequence (1000 bp upstream of the transcriptional start site) of OsYUCCA2, OsYUCCA5, OsYUCCA6, OsSPL10, HL6, OSH1, OsHOX1, OsHOX28 and OsFTIP7 was amplified and cloned in the pLacZi vector. The PAA7 full-length cDNA sequence was inserted into the pB42AD vector. Plasmids were transformed into yeast strain EGY48. Transformed yeast strains were grown on SD/-Ura/-Trp plates. The interaction was confirmed by blue colonies on the SD/-Trp/-Ura medium containing X-gal.
4.15. Chromatin Immunoprecipitation-Quantitative PCR (ChIP-qPCR)
Chromatin was isolated from 2–4 g leaves of OE-FZP plants according to the previous method [42]. The following steps were performed as previously described [42,43]. Gene-specific primers were selected in the 1500 bp promoter region of OsYUCCA6. The input and immunoprecipitated DNA were used for qRT-PCR with gene-specific primers, respectively. Each sample was repeated three times. The qPCR results were analyzed according to the manual of the Magna ChIP HiSens kit (Millipore).
4.16. Electrophoresis Mobility Shift Assay (EMSA)
Full-length CDS of PAA7 were cloned into the pMAL-C2X vector by fusing the MBP tag to their N termini. The MBP-PAA7 fusion protein was purified with the PurKine™ MBP-Tag Protein Purification Kit (Dextrin) (Abbkine, USA). The EMSA probes in a length of 59nt in the OsYUCCA6 promoter were synthesized and labeled with Cy5.5 by Tsingke Biotechnology Co., Ltd. (Beijing, China). The reaction was performed as described by [43]. The fluorescence signal was visualized using an Odyssey CLx infrared fluorescence imaging system (LI-COR). The primers used in this experiment are listed in Table S3.
5. Conclusions
In this study, we reported a panicle apical abortion mutant paa7. The mutation in the 3’-UTR regions of LOC_Os07g47330 disrupted the normal transcript of PAA7 and caused panicle apical abortion in rice. Moreover, PAA7 could crosstalk with LAX2 to co-regulate the panicle development in rice. In summary, our results emphasize the importance of PAA7 in the young panicle regulation network and provide insights into the mechanism of panicle and spikelet development in rice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23169487/s1.
Author Contributions
D.D., H.Z. and L.M. designed the research; D.D., L.H., C.D. and M.L. performed the experiments; D.D., L.H., M.Z. and H.W. analyzed the data; D.D. wrote the manuscript; H.Z., J.C., H.W. and L.M. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Central Public-interest Scientific Institution Basal Research Fund (No. CPSIBRF-CNRRI-202203), the Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2016-CNRRI), the Chinese 863 Program (2014AA10A604-15), National Program of National Natural Science Foundation of China (31501288, 31701398) and Open Project Program of State Key Laboratory of Rice Biology (20210207).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Ruiqing Li (Anhui Agricultural University) for his critical comments to help improving the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.; Tang, S.; Zhi, H.; Xing, L.; Zhang, H.; Tang, C.; Wang, E.; Zhao, M.; Jia, G.; Feng, B.; et al. The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet. Crop J. 2021, 10, 342–353. [Google Scholar] [CrossRef]
- Pei, Y.; Deng, Y.; Zhang, H.; Zhang, Z.; Liu, J.; Chen, Z.; Cai, D.; Li, K.; Du, Y.; Zang, J.; et al. Ear apical degeneration 1 regulates maize ear development by maintaining malate supply for apical inflorescence. Plant Cell 2022, 34, 2222–2241. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhu, X.; Wang, Q.; Zhang, J.; Chen, H.; Dong, G.; Zhu, L.; Zheng, H.; Xie, Q.; Nian, J.; et al. Rice tutou 1 encodes a suppressor of camp receptor-like protein that is important for actin organization and panicle development. Plant Physiol. 2015, 169, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.J.; Sun, Y.J.; Xu, H.S.; Yu, S.B. Identification of quantitative trait locus and epistatic interaction for degenerated spikelets on the top of panicle in rice. Plant Breed. 2011, 130, 177–184. [Google Scholar] [CrossRef]
- Cheng, Z.-J.; Mao, B.-G.; Gao, S.-W.; Zhang, L.; Wang, J.-L.; Lei, C.-L.; Zhang, X.; Wu, F.-Q.; Guo, X.-P.; Wan, J. Fine mapping of qPAA8, a gene controlling panicle apical development in rice. J. Integr. Plant Biol. 2011, 53, 710–718. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Zhou, H.; Yu, S. Development and characterization of contiguous segment substitution lines with background of an elite restorer line. Acta Agron. Sin. 2007, 33, 979–986. [Google Scholar]
- Peng, Y.; Hou, F.; Bai, Q.; Xu, P.; Liao, Y.; Zhang, H.; Gu, C.; Deng, X.; Wu, T.; Chen, X.; et al. Rice calcineurin B-Like protein-interacting protein kinase 31 (OsCIPK31) is involved in the development of panicle apical spikelets. Front. Plant Sci. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Wang, Q.L.; Sun, A.-Z.; Chen, S.-T.; Chen, L.-S.; Guo, F.-Q. SPL6 represses signalling outputs of ER stress in control of panicle cell death in rice. Nat. Plants 2018, 4, 280–288. [Google Scholar] [CrossRef]
- Zafar, S.A.; Patil, S.B.; Uzair, M.; Fang, J.; Zhao, J.; Guo, T.; Yuan, S.; Uzair, M.; Luo, Q.; Shi, J.; et al. Degenerated panicle and partial sterility 1 (DPS 1) encodes a cystathionine β-synthase domain containing protein required for anther cuticle and panicle development in rice. New Phytol. 2020, 225, 356–375. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Xiong, M.; Huang, M.; Li, Z.; Wang, Z.; Zhu, H.; Chen, R.; Lu, L.; Cheng, Q.; Wang, Y.; et al. Panicle apical abortion 3 controls panicle development and seed size in rice. Rice 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Ali, A.; Wu, T.; Zhang, H.; Xu, P.; Zafar, S.A.; Liao, Y.; Chen, X.; Zhou, H.; Liu, Y.; Wang, W.; et al. A putative Subtilisin-like serine protease 1 (SUBSrP1) regulates anther cuticle biosynthesis and panicle development in rice. J. Adv. Res. 2022. [Google Scholar] [CrossRef]
- Zhu, Z.-C.; Luo, S.; Lei, B.; Li, X.-Y.; Cheng, Z.-J. Locus tutou 2 determines the panicle apical abortion phenotype of rice (Oryza sativa L.) in tutou 2 mutant. J. Integr. Agric. 2022, 21, 621–630. [Google Scholar] [CrossRef]
- Heng, Y.; Wu, C.; Long, Y.; Luo, S.; Ma, J.; Chen, J.; Liu, J.; Zhang, H.; Ren, Y.; Wang, M.; et al. Osalmt 7 maintains panicle size and grain yield in rice by mediating malate transport. Plant Cell 2018, 30, 889–906. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Zhang, Q. Genetic and molecular bases of rice yield. In Annual Review of Plant Biology; Merchant, S., Briggs, W.R., Ort, D., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 61, pp. 421–442. [Google Scholar]
- Li, G.; Zhang, H.; Li, J.; Zhang, Z.; Li, Z. Genetic control of panicle architecture in rice. Crop J. 2021, 9, 590–597. [Google Scholar] [CrossRef]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, X.; Ma, X.; Xu, B.; Zhao, Y.; Ma, Z.; Li, G.; Khan, N.U.; Pan, Y.; Liang, Y.; et al. GNP6, a novel allele of moc 1, regulates panicle and tiller development in rice. Crop J. 2020, 9, 57–67. [Google Scholar] [CrossRef]
- Komatsu, M.; Chujo, A.; Nagato, Y.; Shimamoto, K.; Kyozuka, J. Frizzy panicle is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 2003, 130, 3841–3850. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Huang, Y.; Hu, Y.; Liu, H.; Zhang, B.; Smaczniak, C.; Hu, G.; Han, Z.; Xing, Y. Duplication of an upstream silencer of FZP increases grain yield in rice. Nat. Plants 2017, 3, 885–893. [Google Scholar] [CrossRef]
- Song, S.; Wang, G.; Hu, Y.; Liu, H.; Bai, X.; Qin, R.; Xing, Y. Osmft 1 increases spikelets per panicle and delays heading date in rice by suppressing EHD1, FZP and sepallata-like genes. J. Exp. Bot. 2018, 69, 4283–4293. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; He, Y.; Yan, L.; Wang, R.; Zhao, Y. Synergistic roles of LAX1 and FZP in the development of rice sterile lemma. Crop J. 2020, 8, 16–25. [Google Scholar] [CrossRef]
- Komatsu, K.; Maekawa, M.; Ujiie, S.; Satake, Y.; Furutani, I.; Okamoto, H.; Shimamoto, K.; Kyozuka, J. LAX and SPA: Major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. USA 2003, 100, 11765–11770. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Xie, D.; Tang, Z.; Shi, D.; Yang, W. Pinoid regulates floral organ development by modulating auxin transport and interacts with mads16 in rice. Plant Biotechnol. J. 2020, 18, 1778–1795. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, J.; Tang, Z.; Sun, X.; Zhang, H.; Yu, J.; Yao, G.; Li, G.; Guo, H.; Li, J.; et al. Gnp4/LAX2, a rawul protein, interferes with the Osiaa3–Osarf25 interaction to regulate grain length via the auxin signaling pathway in rice. J. Exp. Bot. 2018, 69, 4723–4737. [Google Scholar] [CrossRef]
- Tabuchi, H.; Zhang, Y.; Hattori, S.; Omae, M.; Shimizu-Sato, S.; Oikawa, T.; Qian, Q.; Nishimura, M.; Kitano, H.; Xie, H.; et al. Lax panicle2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell 2011, 23, 3276–3287. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Sunohara, H.; Nagato, Y. Developmental course of inflorescence and spikelet in rice. Breed. Sci. 2004, 54, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Speit, G.; Rothfuss, A. The Comet Assay: A Sensitive Genotoxicity Test for the Detection of DNA Damage and Repair; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Dai, D.; Chen, J.; Du, C.; Liang, M.; Wu, M.; Mou, T.; Zhang, H.; Ma, L. A 2-MB chromosome inversion interrupted transcription of lax2-4 and generated pleiotropic phenotypes in rice. J. Plant Growth Regul. 2021, 41, 2328–2337. [Google Scholar] [CrossRef]
- Ren, D.; Hu, J.; Xu, Q.; Cui, Y.; Zhang, Y.; Zhou, T.; Rao, Y.; Xue, D.; Zeng, D.; Zhang, G.; et al. FZP determines grain size and sterile lemma fate in rice. J. Exp. Bot. 2018, 69, 4853–4866. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Agata, A.; Ota, S.; Ishihara, R.; Takeda, Y.; Kunishima, T.; Ikeda, M.; Kyozuka, J.; Hobo, T.; Kitano, H. Comprehensive panicle phenotyping reveals that qSrn7/FZP influences higher-order branching. Sci. Rep. 2018, 8, 12511. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, S.; Fu, Y.; Sun, H.; Ma, X.; Tan, L.; Liu, F.; Sun, X.; Sun, H.; Gu, P.; et al. Variation in the regulatory region of FZP causes increases in secondary inflorescence branching and grain yield in rice domestication. Plant J. 2018, 96, 716–733. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.-H.; Hoque, M.S.; Dennis, E.S.; Upadhyaya, N.M. Ds tagging of branched floretless 1 (BFL1) that mediates the transition from spikelet to floret meristem in rice (Oryza sativa L). BMC Plant Biol. 2003, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Mignone, F.; Gissi, C.; Liuni, S.; Pesole, G. Untranslated regions of mRNAs. Genome Biol. 2002, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the yucca genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-Y.; Li, J.-J.; Yao, G.-X.; Zhang, H.-L.; Dou, H.-J.; Shi, H.-L.; Sun, X.-M.; Li, Z.-C. Fine mapping and cloning of the grain number per-panicle gene (Gnp4) on chromosome 4 in rice (Oryza sativa L.). Agric. Sci. China 2011, 10, 1825–1833. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Ma, L.; Sang, X.; Ling, Y.; Wang, Y.; Yu, P.; Zhuang, H.; Huang, J.; Wang, N.; et al. Lateral floret 1 induced the three-florets spikelet in rice. Proc. Natl. Acad. Sci. USA 2017, 114, 9984–9989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Zhang, S.; Qiu, Z.; Zhao, J.; Nie, W.; Lin, H.; Zhu, Z.; Zeng, D.; Qian, Q.; Zhu, L. Fructokinase-like protein 1 interacts with TRXz to regulate chloroplast development in rice. J. Integr. Plant Biol. 2018, 60, 94–111. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, C.; Fu, Y.; Liu, Q.; Jiao, X.; Wang, K. Expanding the range of crispr/cas9 genome editing in rice. Mol. Plant 2016, 9, 943–945. [Google Scholar] [CrossRef] [Green Version]
- Toki, S.; Hara, N.; Ono, K.; Onodera, H.; Tagiri, A.; Oka, S.; Tanaka, H. Early infection of scutellum tissue with agrobacterium allows high-speed transformation of rice. Plant J. 2006, 47, 969–976. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Wang, L.; Liu, L.; Li, L.; Sun, L.; Rao, Q.; Zhang, J.; Huang, S. JMJ704 positively regulates rice defense response against xanthomonas oryzae pv. oryzae infection via reducing H3K4me2/3 associated with negative disease resistance regulators. BMC Plant Biol. 2015, 15, 286. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Tong, X.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).