Selection, Preparation and Application of Quantum Dots in Perovskite Solar Cells
Abstract
:1. Introduction
2. Quantum Dot Materials
3. Quantum Dots as Additives in Perovskite Solar Cells
3.1. Quantum Dots as Additives in Electron Transport Layers
- (1)
- Proper alignment of energy levels to allow for the efficient extraction of electrons.
- (2)
- High electron mobility to transfer the photogenerated electrons to the external circuits faster. However, electron transport and extraction in the ETL of PSCs are not ideal, showing stability and hysteresis issues. QDs doping into ETL presented an effective method for optimizing ETM. Table 1 lists the details of the devices [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
3.1.1. Carbon Quantum Dots as Additives in Electron Transport Layers
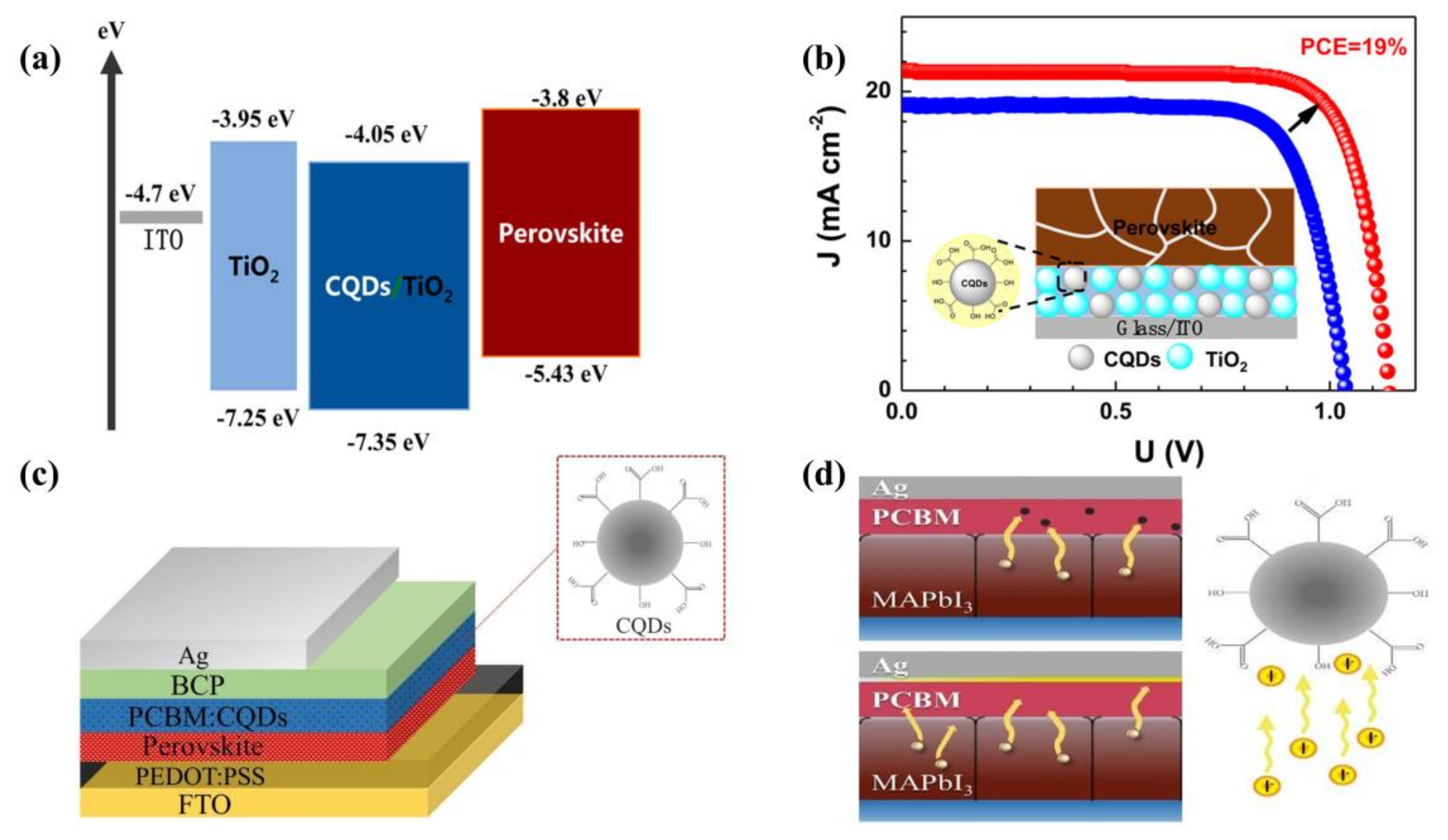
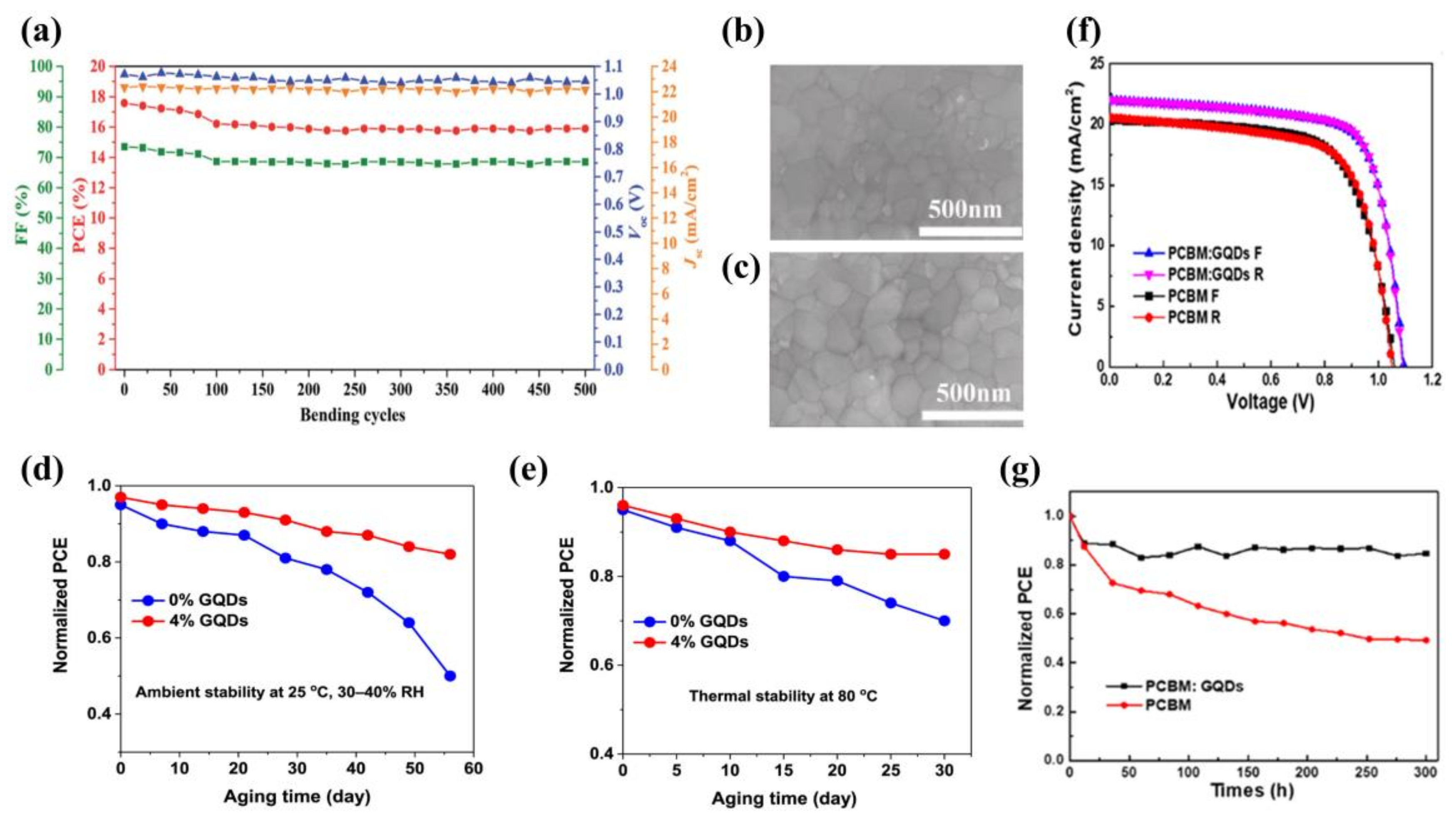
3.1.2. Graphene Quantum Dots as Additives in Electron Transport Layers
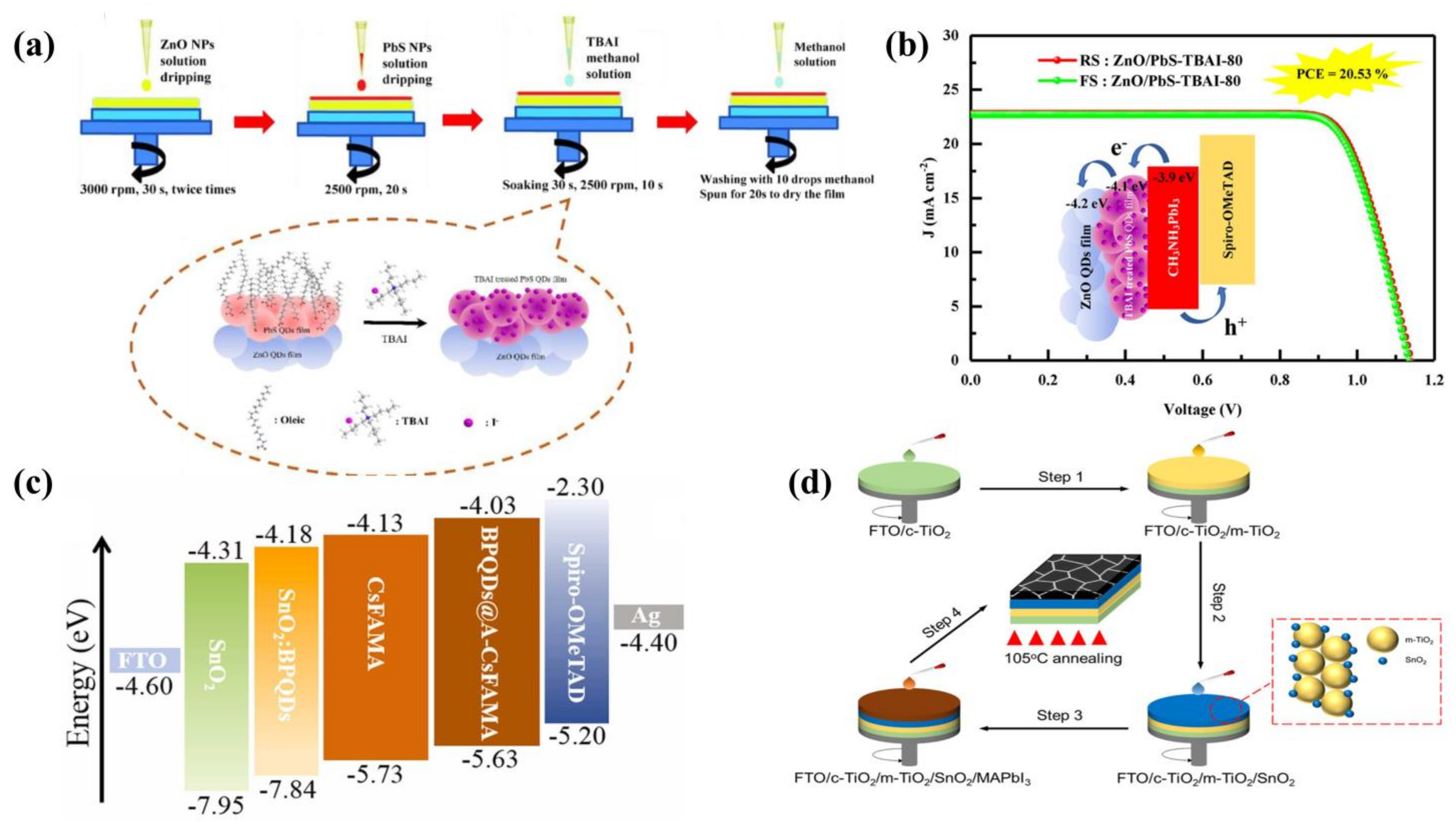
3.1.3. Other Quantum Dots as Additives in Electron Transport Layers
3.2. Quantum Dots as Additives in Perovskite Films
3.2.1. Carbon Quantum Dots as Additives in Perovskite Films
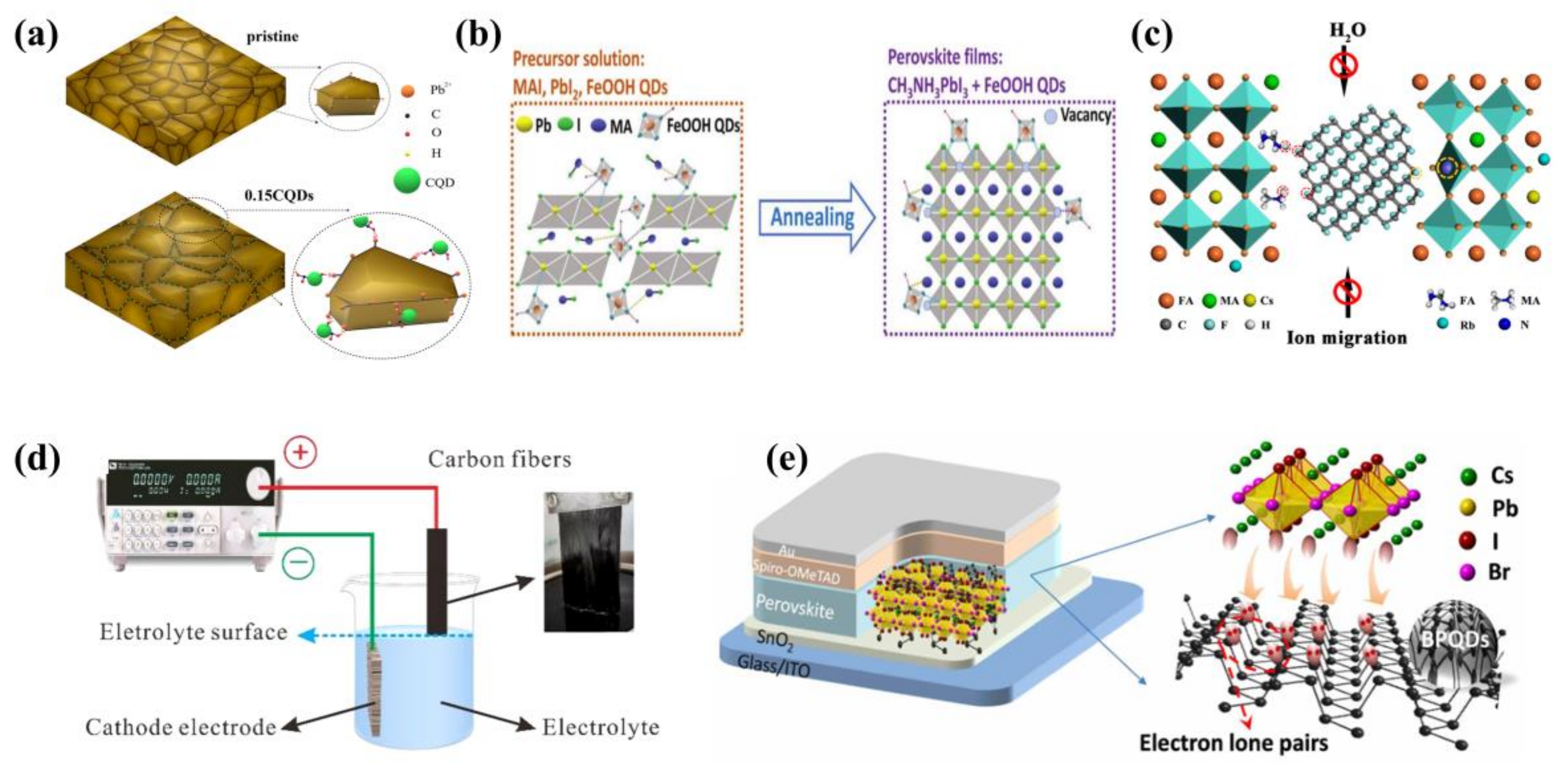
3.2.2. Graphene Quantum Dots as Additives in Perovskite Films
3.2.3. Other Quantum Dots as Additives in Perovskite Films
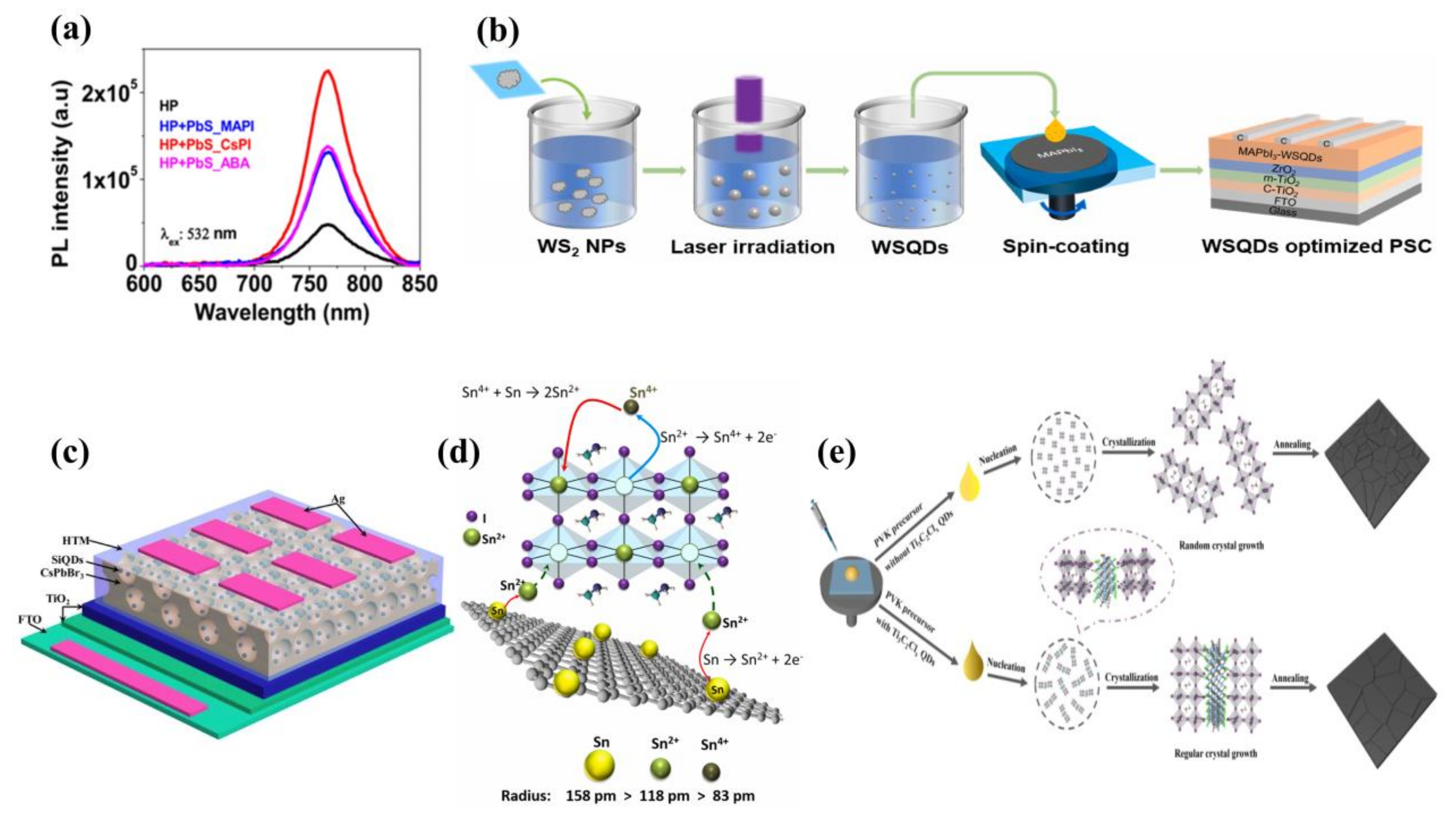
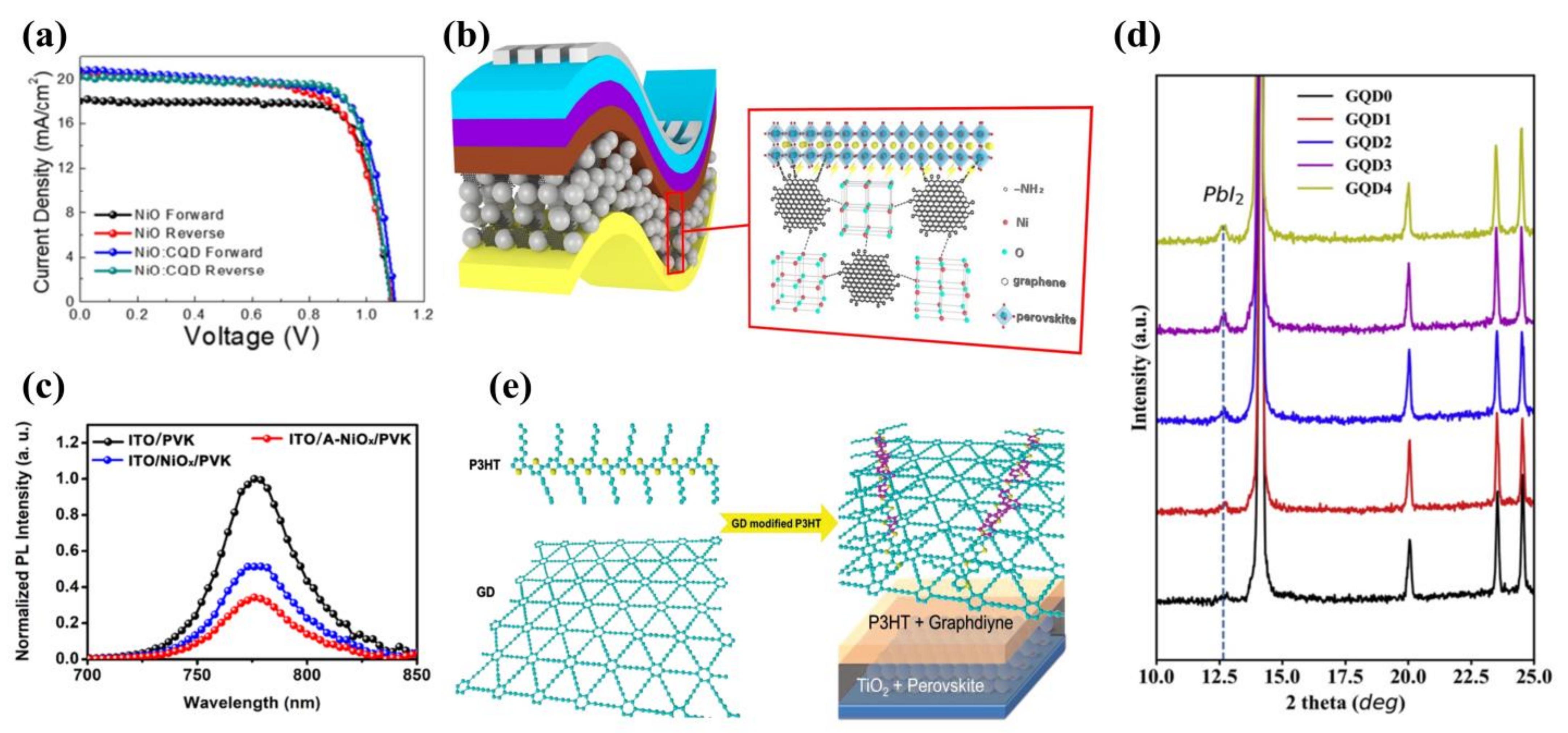
3.3. Quantum Dots as Additives in Hole Transport Layers
3.3.1. Carbon Quantum Dots as Additives in Hole Transport Layers
3.3.2. Graphene Quantum Dots as Additives in Hole Transport Layers
3.3.3. Other Quantum Dots as Additives in Hole Transport Layers
4. Quantum Dots as Electron Transport Materials
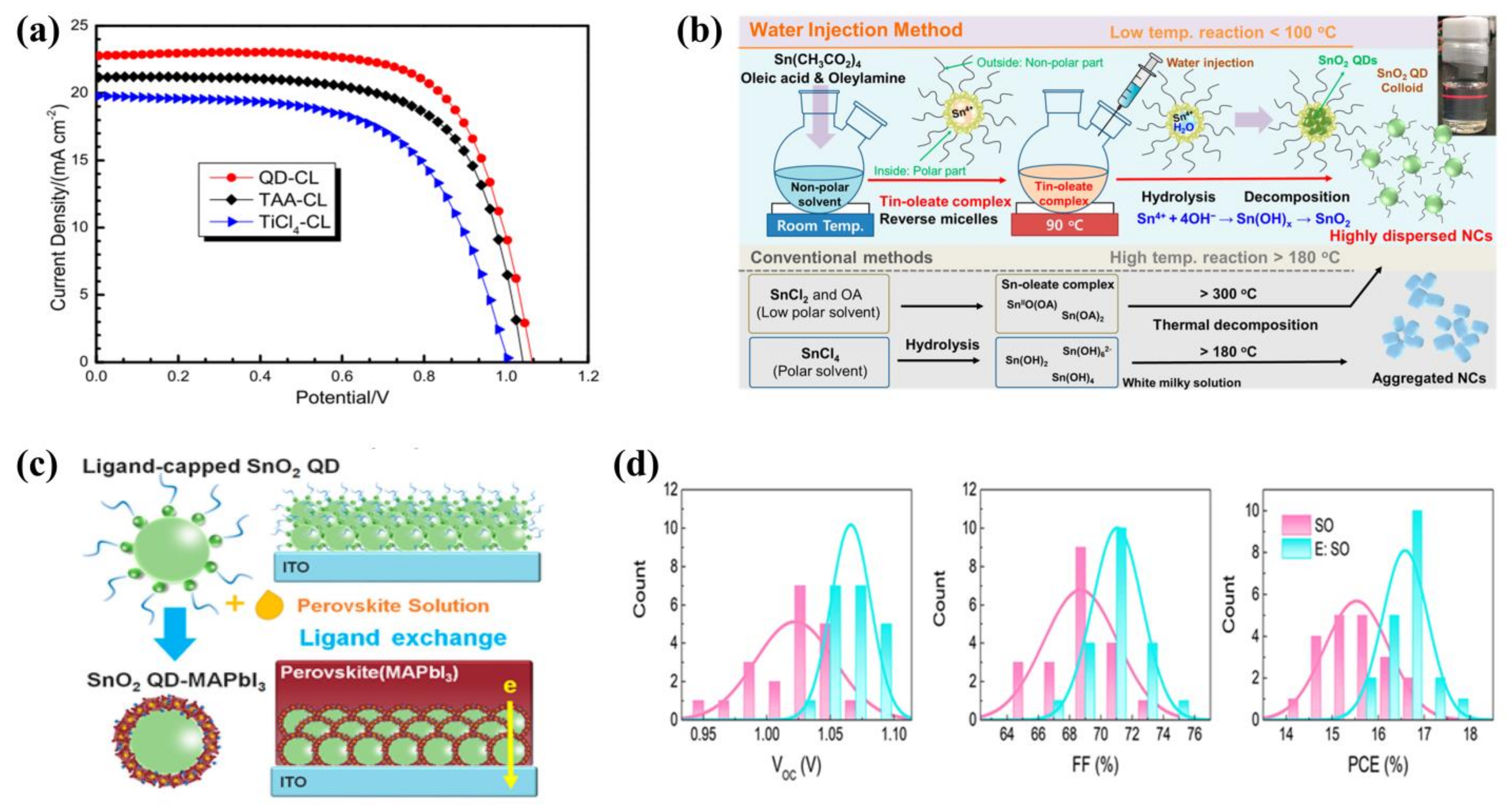
5. Quantum Dots as Hole Transport Materials
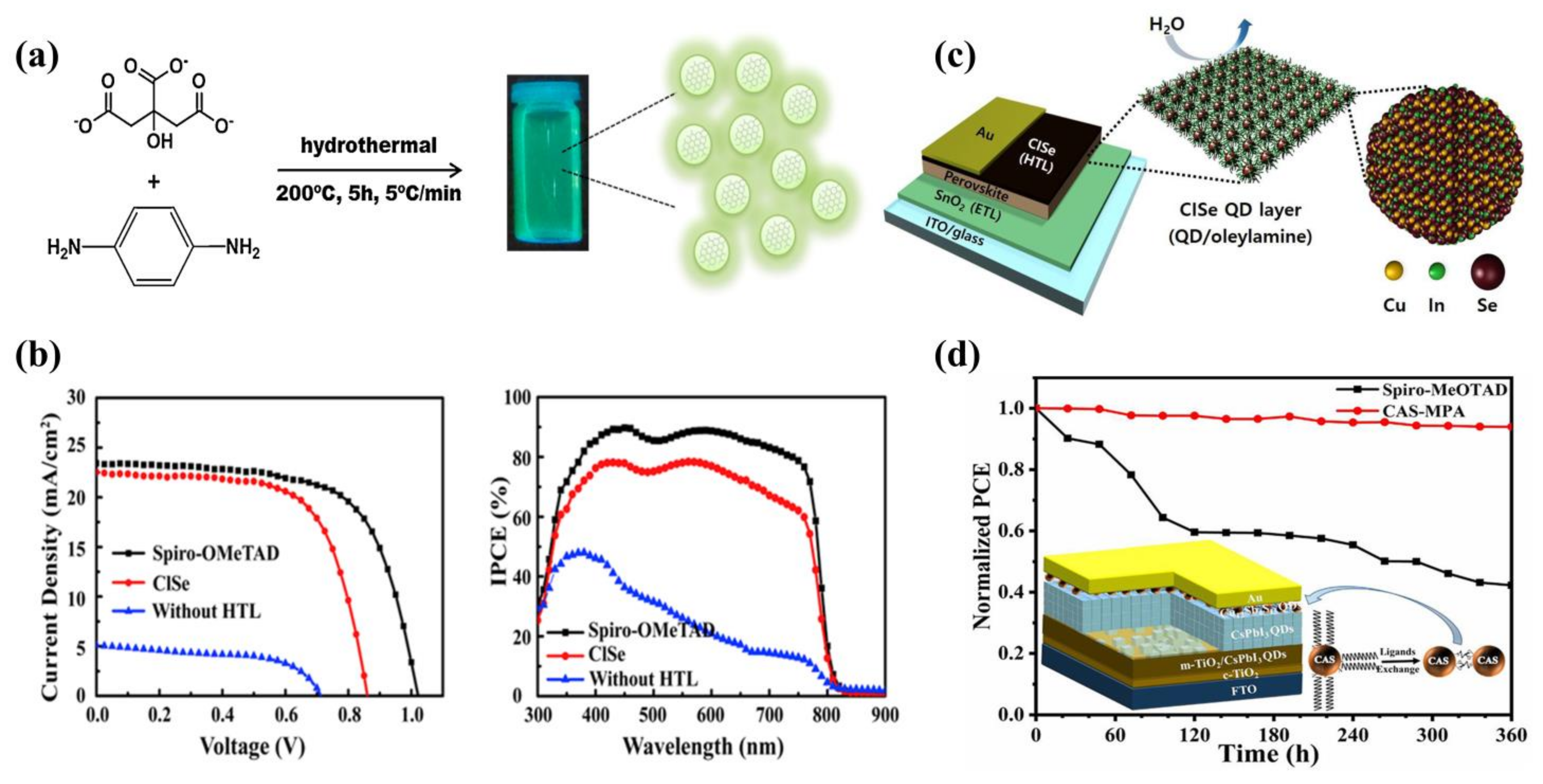
6. Perovskite Quantum Dots as Light-Absorbing Layers
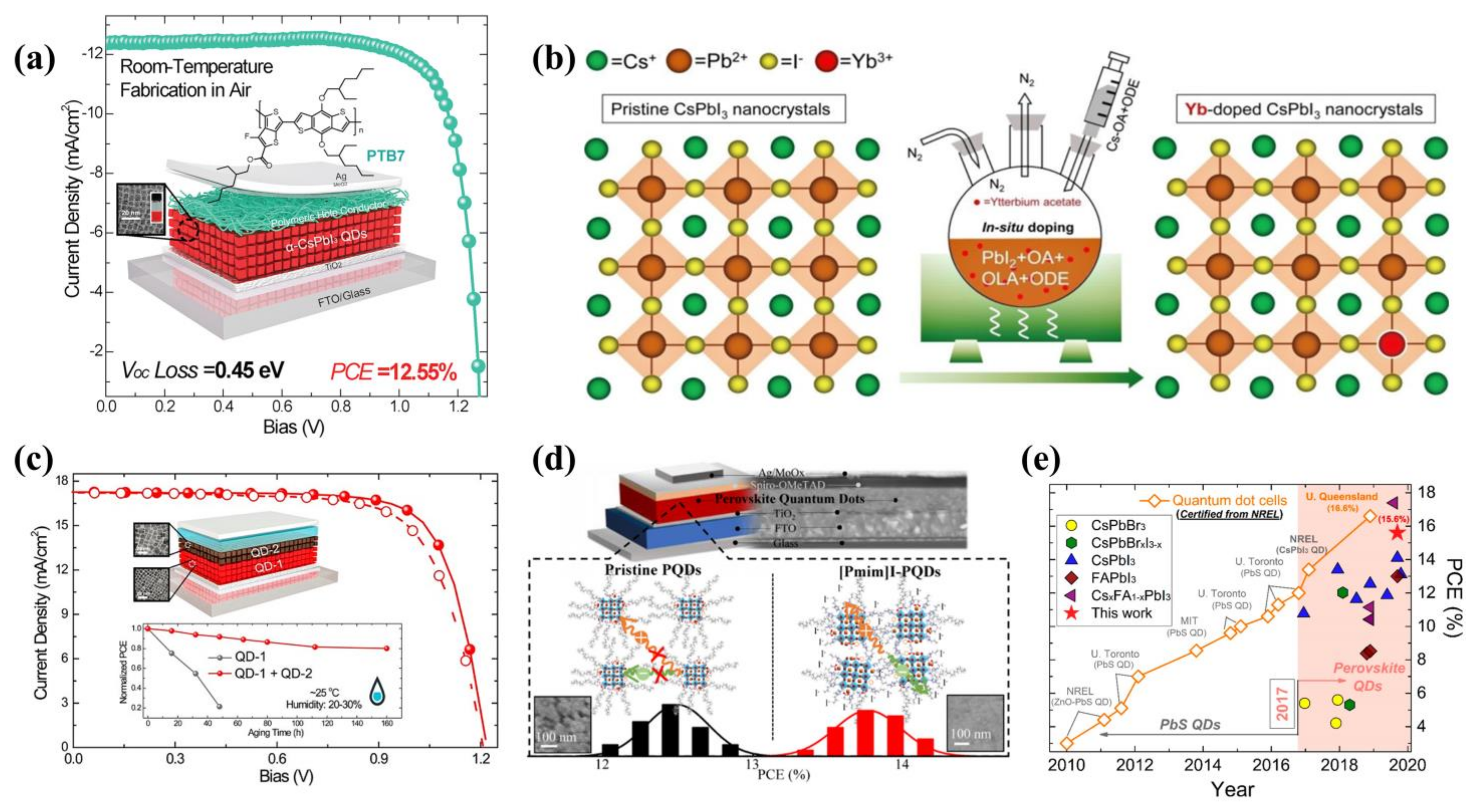
7. Quantum Dots as Luminescent Down-Shifting Materials
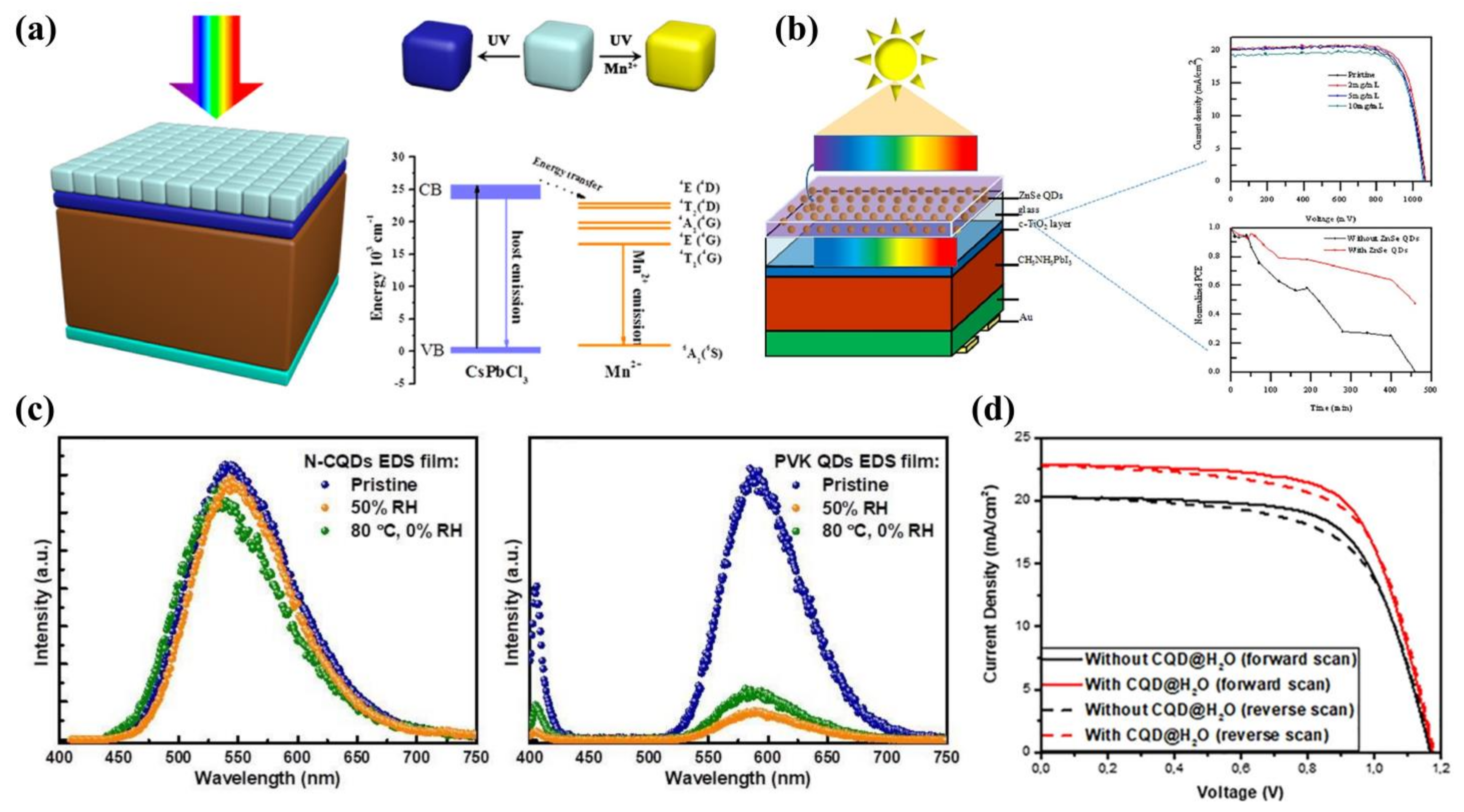
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| CQDs | carbon quantum dots |
| GQDs | graphene quantum dots |
| RCQDs | red carbon quantum dots |
| g-C3N4 QDs | graphitized carbon nitride quantum dots |
| EACQDs | carbon quantum dots prepared in ethyl acetate solvent |
| ASCQDs | anti-solvent carbon quantum dots |
| NCDs | nitrogen doped carbon dots |
| A-CQDs | carbon quantum dots prepared from acetone |
| CA-CQDs | carbon quantum dots prepared from anhydrous citric acid |
| GN-GQDs | graphite-nitrogen doped graphene quantum dots |
| F-GQDs | F doped in graphene quantum dots |
| AGQDs | graphene quantum dots with amino groups |
| N-GQDs | nitrogen-doped graphene quantum dots |
| CQDs@PMMA | polymethyl methacrylate with embedded carbon quantum dots |
| CQDs@H2O | H2O with embedded carbon quantum dots |
| CdSe QDs | CdSe quantum dots |
| CdS QDs | CdS quantum dots |
| PbS QDs | PbS quantum dots |
| BP QDs | black phosphorus quantum dots |
| SnO2 QDs | SnO2 quantum dots |
| rGO-Sn QDs | reduced graphene oxide sheets anchored with Sn quantum dots |
| AIGS QDs | Ag-In-Ga-S quantum dots |
| CIGSSe QDs | CuIn0.1Ga0.9(S0.9Se0.1)2 quantum dots |
| Yb-CsPbI3 QDs | Yb doped in CsPbI3 quantum dots |
| μGR-CsPbI3 QDs | µ-graphene crosslink CsPbI3 quantum dots |
| [Pmim]I-CsPbI3 QDs | 1-propyl-3-methylimidazolium iodide ionic liquid into CsPbI3 quantum dots |
| CsPbCl3:Mn QDs | Mn doped in CsPbCl3 quantum dots |
| Ce3+-CsPbI3 QDs | Ce3+ doped in CsPbI3 quantum dots |
References
- Heo, J.H.; Song, D.H.; Patil, B.R.; Im, S.H. Recent Progress of Innovative Perovskite Hybrid Solar Cells. Isr. J. Chem. 2015, 55, 966–977. [Google Scholar] [CrossRef]
- Moore, K.; Wei, W. Applications of carbon nanomaterials in perovskite solar cells for solar energy conversion. Nano Mater. Sci. 2021, 3, 276–290. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable energy and geopolitics: A review. Renew. Sustain. Energy Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive Engineering for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902579. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Causes and Solutions of Recombination in Perovskite Solar Cells. Adv. Mater. 2019, 31, 1803019. [Google Scholar] [CrossRef]
- Chao, L.; Niu, T.; Gao, W.; Ran, C.; Song, L.; Chen, Y.; Huang, W. Solvent Engineering of the Precursor Solution toward Large-Area Production of Perovskite Solar Cells. Adv. Mater. 2021, 33, 2005410. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.; Lee, S.-W.; Cho, K.J.; Park, S.; Lee, S.; Kang, Y.; Lee, H.-S.; Kim, D. Electric-Field-Induced Degradation of Methylammonium Lead Iodide Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3091–3096. [Google Scholar] [CrossRef]
- Kari, M.; Saghafi, K. Current-voltage hysteresis reduction of CH3NH3PbI3 planar perovskite solar cell by multi-layer absorber. Micro Nanostruct. 2022, 165, 207207. [Google Scholar] [CrossRef]
- Lin, Z. Relationship between ion vacancy mobility and hysteresis of perovskite solar cells. Chem. Phys. 2022, 554, 111422. [Google Scholar] [CrossRef]
- Wu, F.; Pathak, R.; Qiao, Q. Origin and alleviation of J-V hysteresis in perovskite solar cells: A short review. Catal. Today 2021, 374, 86–101. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, X.; Yang, J.; Qiu, Q.; Xie, T.; Liang, T. Application of Quantum Dot Interface Modification Layer in Perovskite Solar Cells: Progress and Perspectives. Nanomaterials 2022, 12, 2102. [Google Scholar] [CrossRef]
- Ye, M.; Biesold, G.M.; Zhang, M.; Wang, W.; Bai, T.; Lin, Z. Multifunctional quantum dot materials for perovskite solar cells: Charge transport, efficiency and stability. Nano Today 2021, 40, 101286. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, Y.; Ren, W.; Sunli, Z.; Xie, X.; Cui, Y.; Hao, Y. Application of quantum dots in perovskite solar cells. Nanotechnology 2021, 32, 482003. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, F.; Zhang, B.; Zhou, S.; Zhang, J.; Bai, Y.; Fan, L.; Hayat, T.; Alsaedi, A.; Tan, Z.a. Passivation of the grain boundaries of CH3NH3PbI3 using carbon quantum dots for highly efficient perovskite solar cells with excellent environmental stability. Nanoscale 2019, 11, 115–124. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, S.; Yuan, G.; Zhu, W.; Huang, Y.; Li, S.; Lin, M. Tailored graphene quantum dots to passivate defects and accelerate charge extraction for all-inorganic CsPbIBr2 perovskite solar cells. J. Alloys Compd. 2022, 895, 162529. [Google Scholar] [CrossRef]
- Ye, M.; Gong, J.; Lai, Y.; Lin, C.; Lin, Z. High-Efficiency Photoelectrocatalytic Hydrogen Generation Enabled by Palladium Quantum Dots-Sensitized TiO2 Nanotube Arrays. J. Am. Chem. Soc. 2012, 134, 15720–15723. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhu, P.; Liu, Y.; Qiu, Q.; Li, J.; Liang, T.; Xie, T. Enhanced photocatalytic degradation activity via a stable perovskite-type LaFeO3/In2S3 Z-scheme heterostructured photocatalyst: Unobstructed photoexcited charge behavior of Z-scheme photocatalytic system exploration. J. Alloys Compd. 2022, 901, 163628. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, P.; Xu, L.; Wang, D.; Lin, Y.; Xie, T. Photoelectrical properties of CdS/CdSe core/shell QDs modified anatase TiO2 nanowires and their application for solar cells. Phys. Chem. Chem. Phys. 2017, 19, 15724–15733. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhu, P.; Liu, Y.; Liang, T.; Xie, T.; Lin, Y. Highly efficient In2S3/WO3 photocatalysts: Z-scheme photocatalytic mechanism for enhanced photocatalytic water pollutant degradation under visible light irradiation. RSC Adv. 2021, 11, 3333–3341. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhuge, F.; Wang, Y.; Zhang, J.; Gan, L.; Zhou, X.; Li, H.; Zhai, T. Decorating Perovskite Quantum Dots in TiO2 Nanotubes Array for Broadband Response Photodetector. Adv. Funct. Mater. 2017, 27, 1703115. [Google Scholar] [CrossRef]
- Lu, J.; Sheng, X.; Tong, G.; Yu, Z.; Sun, X.; Yu, L.; Xu, X.; Wang, J.; Xu, J.; Shi, Y.; et al. Ultrafast Solar-Blind Ultraviolet Detection by Inorganic Perovskite CsPbX3 Quantum Dots Radial Junction Architecture. Adv. Mater. 2017, 29, 1700400. [Google Scholar] [CrossRef]
- Jin, J.; Chen, C.; Li, H.; Cheng, Y.; Xu, L.; Dong, B.; Song, H.; Dai, Q. Enhanced Performance and Photostability of Perovskite Solar Cells by Introduction of Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2017, 9, 14518–14524. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Huang, W.; Yao, E.-P.; Han, J.; Chen, Z.; Liu, S.; Shen, Y.; Wang, M.; Yang, Y. Carbon Quantum Dots/TiOx Electron Transport Layer Boosts Efficiency of Planar Heterojunction Perovskite Solar Cells to 19%. Nano Lett. 2017, 17, 2328–2335. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, J.; Yuan, S.; Li, N.; Qiu, Z.; Jia, J.; Liu, Y.; Dong, J.; Lv, P.; Cao, B. Efficient and stable planar perovskite solar cells with carbon quantum dots-doped PCBM electron transport layer. New J. Chem. 2019, 43, 7130–7135. [Google Scholar] [CrossRef]
- Chen, J.; Dong, H.; Zhang, L.; Li, J.; Jia, F.; Jiao, B.; Xu, J.; Hou, X.; Liu, J.; Wu, Z. Graphitic carbon nitride doped SnO2 enabling efficient perovskite solar cells with PCEs exceeding 22%. J. Mater. Chem. A 2020, 8, 2644–2653. [Google Scholar] [CrossRef]
- Hui, W.; Yang, Y.; Xu, Q.; Gu, H.; Feng, S.; Su, Z.; Zhang, M.; Wang, J.; Li, X.; Fang, J.; et al. Red-Carbon-Quantum-Dot-Doped SnO2 Composite with Enhanced Electron Mobility for Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2020, 32, 1906374. [Google Scholar] [CrossRef]
- Xie, J.; Huang, K.; Yu, X.; Yang, Z.; Xiao, K.; Qiang, Y.; Zhu, X.; Xu, L.; Wang, P.; Cui, C.; et al. Enhanced Electronic Properties of SnO2 via Electron Transfer from Graphene Quantum Dots for Efficient Perovskite Solar Cells. ACS Nano 2017, 11, 9176–9182. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Arivazhagan, V.; Xiao, K.; Qiang, Y.; Huang, K.; Hu, M.; Cui, C.; Yu, X.; Yang, D. Efficient and highly light stable planar perovskite solar cells with graphene quantum dots doped PCBM electron transport layer. Nano Energy 2017, 40, 345–351. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, W.; Xie, F.; Li, Y.; Abate, A.; Wei, M. Graphene quantum dots decorated TiO2 mesoporous film as an efficient electron transport layer for high-performance perovskite solar cells. J. Power Sources 2018, 402, 320–326. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kermanpur, A.; Atapour, M.; Adhami, S.; Heidari, R.H.; Khorshidi, E.; Irannejad, N.; Rezaie, B. Performance enhancement of mesoscopic perovskite solar cells with GQDs-doped TiO2 electron transport layer. Sol. Energy Mater. Sol. Cells 2020, 208, 110407. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, C.; Zhang, H.; Dong, H.; Chen, D.; Zhu, W.; Xi, H.; Chang, J.; Lin, Z.; Zhang, J.; et al. Boosting performance of perovskite solar cells with Graphene quantum dots decorated SnO2 electron transport layers. Appl. Surf. Sci. 2020, 507, 145099. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S.; Yin, X.; Han, J.; Tai, M.; Zhao, X.; Chen, H.; Gu, Y.; Wang, N.; Lin, H. Enhancing electron transport via graphene quantum dot/SnO2 composites for efficient and durable flexible perovskite photovoltaics. J. Mater. Chem. A 2019, 7, 1878–1888. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, W.; Jiang, Z.; Zhang, Y.; Ni, C. Graphene quantum dots doping SnO2 for improving carrier transport of perovskite solar cells. Ceram. Int. 2021, 47, 29712–29721. [Google Scholar] [CrossRef]
- Nagaraj, G.; Mohammed, M.K.A.; Shekargoftar, M.; Sasikumar, P.; Sakthivel, P.; Ravi, G.; Dehghanipour, M.; Akin, S.; Shalan, A.E. High-performance perovskite solar cells using the graphene quantum dot–modified SnO2/ZnO photoelectrode. Mater. Today Energy 2021, 22, 100853. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, T.; Leng, C.; Zang, Z.; Wang, M.; Hu, W.; Tang, X.; Lu, S.; Fang, L.; Zhou, M. Performance improvement of perovskite solar cells by employing a CdSe quantum dot/PCBM composite as an electron transport layer. J. Mater. Chem. A 2017, 5, 17499–17505. [Google Scholar] [CrossRef]
- Kumnorkaew, P.; Rattanawichai, N.; Ratanatawanate, C.; Yoriya, S.; Lohawet, K.; Zhao, Y.; Vas-Umnuay, P. Influence of PbS Quantum Dots-Doped TiO2 Nanotubes in TiO2 Film as an Electron Transport Layer for Enhanced Perovskite Solar Cell Performance. IEEE J. Photovolt. 2020, 10, 287–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Wu, Y.; Zhou, Q.; Shi, Z.; Zhuang, X.; Liu, B.; Dong, B.; Bai, X.; Xu, W.; et al. Double-layer synergistic optimization by functional black phosphorus quantum dots for high-efficiency and stable planar perovskite solar cells. Nano Energy 2021, 90, 106610. [Google Scholar] [CrossRef]
- Gu, B.; Du, Y.; Chen, B.; Zhao, R.; Lu, H.; Xu, Q.; Guo, C. Black Phosphorus Quantum Dot-Engineered Tin Oxide Electron Transport Layer for Highly Stable Perovskite Solar Cells with Negligible Hysteresis. ACS Appl. Mater. Interfaces 2022, 14, 11264–11272. [Google Scholar] [CrossRef]
- Pang, Z.; Yang, S.; Sun, Y.; He, L.; Wang, F.; Fan, L.; Chi, S.; Sun, X.; Yang, L.; Yang, J. Hydrophobic PbS QDs layer decorated ZnO electron transport layer to boost photovoltaic performance of perovskite solar cells. Chem. Eng. J. 2022, 439, 135701. [Google Scholar] [CrossRef]
- Zhou, J.; Lyu, M.; Zhu, J.; Li, G.; Li, Y.; Jin, S.; Song, J.; Niu, H.; Xu, J.; Zhou, R. SnO2 Quantum Dot-Modified Mesoporous TiO2 Electron Transport Layer for Efficient and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 5, 3052–3063. [Google Scholar] [CrossRef]
- Ali, S.M.; Ramay, S.M.; Aziz, M.H.; ur-Rehman, N.; AlGarawi, M.S.; AlGhamd, S.S.; Mahmood, A.; Alkhuraiji, T.S.; Atiq, S. Efficiency enhancement of perovskite solar cells by incorporation of CdS quantum dot through fast electron injection. Org. Electron. 2018, 62, 21–25. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Long, H. Recent advances in carbon nanomaterial-optimized perovskite solar cells. Mater. Today Energy 2021, 21, 100769. [Google Scholar] [CrossRef]
- Guo, R.; Zeng, D.; Xie, Y.; Ling, Y.; Zhou, D.; Jiang, L.; Jiao, W.; Zhao, J.; Li, S. Carbon nitride quantum dots (CNQDs)/TiO2 nanoparticle heterojunction photocatalysts for enhanced ultraviolet-visible-light-driven bisphenol a degradation and H2 production. Int. J. Hydrogen Energy 2020, 45, 22534–22544. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L.; et al. Efficiency Enhancement of Perovskite Solar Cells through Fast Electron Extraction: The Role of Graphene Quantum Dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef]
- Tsikritzis, D.; Rogdakis, K.; Chatzimanolis, K.; Petrović, M.; Tzoganakis, N.; Najafi, L.; Martín-García, B.; Oropesa-Nuñez, R.; Bellani, S.; Del Rio Castillo, A.E.; et al. A two-fold engineering approach based on Bi2Te3 flakes towards efficient and stable inverted perovskite solar cells. Mater. Adv. 2020, 1, 450–462. [Google Scholar] [CrossRef]
- Lv, Z.; He, L.; Jiang, H.; Ma, X.; Wang, F.; Fan, L.; Wei, M.; Yang, J.; Yang, L.; Yang, N. Diluted-CdS Quantum Dot-Assisted SnO2 Electron Transport Layer with Excellent Conductivity and Suitable Band Alignment for High-Performance Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 16326–16335. [Google Scholar] [CrossRef]
- Zou, H.; Guo, D.; He, B.; Yu, J.; Fan, K. Enhanced photocurrent density of HTM-free perovskite solar cells by carbon quantum dots. Appl. Surf. Sci. 2018, 430, 625–631. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, S.; Zhang, H.; Li, L.; Fu, Z. Surface passivation with nitrogen-doped carbon dots for improved perovskite solar cell performance. J. Mater. Sci. 2018, 53, 9180–9190. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Zhang, Y.; Hu, R.; Jiang, M.; Zhang, R.; Lv, H.; Tian, J.; Chu, L.; Zhang, J.; et al. Enhancing the Performance of Inverted Perovskite Solar Cells via Grain Boundary Passivation with Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhu, G.; Shao, Y. Improving the power conversion efficiency of perovskite solar cells by adding carbon quantum dots. J. Mater. Sci. 2020, 55, 2937–2946. [Google Scholar] [CrossRef]
- Xu, T.; Wan, Z.; Tang, H.; Zhao, C.; Lv, S.; Chen, Y.; Chen, L.; Qiao, Q.; Huang, W. Carbon quantum dot additive engineering for efficient and stable carbon-based perovskite solar cells. J. Alloys Compd. 2021, 859, 157784. [Google Scholar] [CrossRef]
- Li, S.; He, Z.; Li, Y.; Liu, K.; Chen, M.; Yang, Y.; Li, X. Laser induced anti-solvent carbon quantum dots in defect passivation for effective perovskite solar cells. J. Alloys Compd. 2021, 889, 161561. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Yang, Y.; Li, X. Laser fabricated carbon quantum dots in anti-solvent for highly efficient carbon-based perovskite solar cells. J. Colloid Interface Sci. 2021, 600, 691–700. [Google Scholar] [CrossRef]
- Fang, X.; Ding, J.; Yuan, N.; Sun, P.; Lv, M.; Ding, G.; Zhu, C. Graphene quantum dot incorporated perovskite films: Passivating grain boundaries and facilitating electron extraction. Phys. Chem. Chem. Phys. 2017, 19, 6057–6063. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, T.; Zhang, L.; Li, X.; Zou, H.; Yu, J. Enhanced Performance of Planar Perovskite Solar Cell by Graphene Quantum Dot Modification. ACS Sustain. Chem. Eng. 2018, 6, 8631–8640. [Google Scholar] [CrossRef]
- Gan, X.; Yang, S.; Zhang, J.; Wang, G.; He, P.; Sun, H.; Yuan, H.; Yu, L.; Ding, G.; Zhu, Y. Graphite-N Doped Graphene Quantum Dots as Semiconductor Additive in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 37796–37803. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Wang, L.; Pei, Y.; Wang, Z.; Zhang, Y.; Lin, H.; Li, X. Exfoliated Fluorographene Quantum Dots as Outstanding Passivants for Improved Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 22992–23001. [Google Scholar] [CrossRef]
- Han, J.; Yin, X.; Nan, H.; Zhou, Y.; Yao, Z.; Li, J.; Oron, D.; Lin, H. Enhancing the Performance of Perovskite Solar Cells by Hybridizing SnS Quantum Dots with CH3NH3PbI3. Small 2017, 13, 1700953. [Google Scholar] [CrossRef]
- Han, J.; Luo, S.; Yin, X.; Zhou, Y.; Nan, H.; Li, J.; Li, X.; Oron, D.; Shen, H.; Lin, H. Hybrid PbS Quantum-Dot-in-Perovskite for High-Efficiency Perovskite Solar Cell. Small 2018, 14, 1801016. [Google Scholar] [CrossRef]
- Fan, L.; Liu, S.; Lei, Y.; Qi, R.; Guo, L.; Yang, X.; Meng, Q.; Dai, Q.; Zheng, Z. Unusually Dispersed AgI Quantum Dots For Efficient HTL-Free CH3NH3PbI3 Photovoltaics. ACS Appl. Mater. Interfaces 2019, 11, 45568–45577. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Q.; Liu, T.; Ren, J.; Li, S.; Tai, M.; Lin, H.; He, H.; Wang, J.; Wang, N. Goethite Quantum Dots as Multifunctional Additives for Highly Efficient and Stable Perovskite Solar Cells. Small 2019, 15, 1904372. [Google Scholar] [CrossRef]
- Ngo, T.T.; Masi, S.; Mendez, P.F.; Kazes, M.; Oron, D.; Seró, I.M. PbS quantum dots as additives in methylammonium halide perovskite solar cells: The effect of quantum dot capping. Nanoscale Adv. 2019, 1, 4109–4118. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Tang, R.; Li, H.; Fu, L.; Li, B.; Yin, L. Fluorescence resonance energy transfer effect enhanced high performance of Si quantum Dots/CsPbBr3 inverse opal heterostructure perovskite solar cells. J. Power Sources 2019, 439, 227065. [Google Scholar] [CrossRef]
- Gong, X.; Guan, L.; Li, Q.; Li, Y.; Zhang, T.; Pan, H.; Sun, Q.; Shen, Y.; Grätzel, C.; Zakeeruddin, S.M.; et al. Black phosphorus quantum dots in inorganic perovskite thin films for efficient photovoltaic application. Sci. Adv. 2020, 6, eaay5661. [Google Scholar] [CrossRef] [Green Version]
- Masi, S.; Echeverría-Arrondo, C.; Salim, K.M.M.; Ngo, T.T.; Mendez, P.F.; López-Fraguas, E.; Macias-Pinilla, D.F.; Planelles, J.; Climente, J.I.; Mora-Seró, I. Chemi-Structural Stabilization of Formamidinium Lead Iodide Perovskite by Using Embedded Quantum Dots. ACS Energy Lett. 2020, 5, 418–427. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Zhang, C.; Yang, Y.; Li, X. Laser generated WS2 quantum dots for effective charge transport in high-performance carbon-based perovskite solar cells. J. Power Sources 2022, 518, 230766. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Jiang, J.; Tian, C.; Wang, X.; Wang, L.; Zhang, Z.; Wu, X.; Zheng, Y.; Liang, J.; et al. Chlorine-terminated MXene quantum dots for improving crystallinity and moisture stability in high-performance perovskite solar cells. Chem. Eng. J. 2022, 432, 134382. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Rho, W.-Y.; Kohan, M.; Im, Y.H.; Mathur, S.; Hahn, Y.-B. Suppression of Sn2+/Sn4+ oxidation in tin-based perovskite solar cells with graphene-tin quantum dots composites in active layer. Nano Energy 2021, 90, 106495. [Google Scholar] [CrossRef]
- Xu, S.; Kang, C.; Huang, Z.; Zhang, Z.; Rao, H.; Pan, Z.; Zhong, X. Dual-Functional Quantum Dot Seeding Growth of High-Quality Air-Processed CsPbI2Br Film for Carbon-Based Perovskite Solar Cells. Solar RRL 2022, 6, 2100989. [Google Scholar] [CrossRef]
- Zhang, T.; Ban, H.; Sun, Q.; Pan, H.; Yu, H.; Zhang, Z.; Zhang, X.; Shen, Y.; Wang, M. Preventing inhomogeneous elemental distribution and phase segregation in mixed Pb-Sn inorganic perovskites via incorporating PbS quantum dots. J. Energy Chem. 2022, 65, 179–185. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.; Yang, Y.; Liu, J.; Yin, X.; Que, W. CuInSe2 quantum dots doped MAPbI3 films with reduced trap density for perovskite solar cells. J. Alloys Compd. 2022, 906, 164292. [Google Scholar] [CrossRef]
- Ou, Y.; Lu, J.; Zhong, X.; Li, X.; Wu, S.; Chen, P.; Zhou, L. Efficiency improvement of Cs2AgBiBr6 perovskite solar cells with modification of SnS quantum dots. Mater. Lett. 2022, 312, 131672. [Google Scholar] [CrossRef]
- Subramanian, A.; Akram, J.; Hussain, S.; Chen, J.; Qasim, K.; Zhang, W.; Lei, W. High-Performance Photodetector Based on a Graphene Quantum Dot/CH3NH3PbI3 Perovskite Hybrid. ACS Appl. Electron. Mater. 2020, 2, 230–237. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Q.; Wang, M.; Ma, H.; Pei, M.; Bian, J.; Shi, Y. Efficient Planar Perovskite Solar Cells with Carbon Quantum Dot-Modified spiro-MeOTAD as a Composite Hole Transport Layer. ACS Appl. Mater. Interfaces 2021, 13, 56265–56272. [Google Scholar] [CrossRef]
- Benetti, D.; Jokar, E.; Yu, C.-H.; Fathi, A.; Zhao, H.; Vomiero, A.; Wei-Guang Diau, E.; Rosei, F. Hole-extraction and photostability enhancement in highly efficient inverted perovskite solar cells through carbon dot-based hybrid material. Nano Energy 2019, 62, 781–790. [Google Scholar] [CrossRef]
- Kim, J.K.; Nguyen, D.N.; Lee, J.-H.; Kang, S.; Kim, Y.; Kim, S.-S.; Kim, H.-K. Carbon quantum dot-incorporated nickel oxide for planar p-i-n type perovskite solar cells with enhanced efficiency and stability. J. Alloys Compd. 2020, 818, 152887. [Google Scholar] [CrossRef]
- Li, W.; Cheng, N.; Cao, Y.; Zhao, Z.; Xiao, Z.; Zi, W.; Sun, Z. Boost the performance of inverted perovskite solar cells with PEDOT:PSS/Graphene quantum dots composite hole transporting layer. Org. Electron. 2020, 78, 105575. [Google Scholar] [CrossRef]
- Wang, Z.; Rong, X.; Wang, L.; Wang, W.; Lin, H.; Li, X. Dual Role of Amino-Functionalized Graphene Quantum Dots in NiOx Films for Efficient Inverted Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 8342–8350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Jin, Z.; Chen, Y.; Liu, H.; Wang, J.; Li, Y.; Liu, S. Graphdiyne Quantum Dots for Much Improved Stability and Efficiency of Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1701117. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, J.; Liu, H.; Xu, Y.; Lv, S.; Luo, Y.; Li, D.; Meng, Q.; Li, Y. Efficient CH3NH3PbI3 Perovskite Solar Cells Based on Graphdiyne (GD)-Modified P3HT Hole-Transporting Material. Adv. Energy Mater. 2015, 5, 1401943. [Google Scholar] [CrossRef]
- Zheng, J.; Li, F.; Chen, C.; Du, Q.; Jin, M.; Li, H.; Ji, M.; Shen, Z. Perovskite Solar Cells Employing a PbSO4(PbO)4 Quantum Dot-Doped Spiro-OMeTAD Hole Transport Layer with an Efficiency over 22%. ACS Appl. Mater. Interfaces 2022, 14, 2989–2999. [Google Scholar] [CrossRef]
- Petridis, K.; Kakavelakis, G.; Stylianakis, M.M.; Kymakis, E. Graphene-Based Inverted Planar Perovskite Solar Cells: Advancements, Fundamental Challenges, and Prospects. Chem. Asian J. 2018, 13, 240–249. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Cai, M.; Xie, F.; Wu, Y.; Lan, Z.; Yang, X.; Qiang, Y.; Islam, A.; Han, L. Stable Inverted Planar Perovskite Solar Cells with Low-Temperature-Processed Hole-Transport Bilayer. Adv. Energy Mater. 2017, 7, 1700763. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yang, B.; Stylianakis, M.M.; Kymakis, E.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A. Reduced Graphene Oxide Improves Moisture and Thermal Stability of Perovskite Solar Cells. Cell Rep. Phys. Sci. 2020, 1, 100053. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Seo, H.-K.; Nazeeruddin, M.K.; Shin, H.-S. An Insight into Atmospheric Plasma Jet Modified ZnO Quantum Dots Thin Film for Flexible Perovskite Solar Cell: Optoelectronic Transient and Charge Trapping Studies. J. Phys. Chem. C 2015, 119, 10379–10390. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; Zheng, M.; Huo, J.; Zhou, P.; Lan, Z.; Lin, J.; Huang, M. TiO2 quantum dots as superb compact block layers for high-performance CH3NH3PbI3 perovskite solar cells with an efficiency of 16.97%. Nanoscale 2015, 7, 20539–20546. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tavakoli, R.; Nourbakhsh, Z.; Waleed, A.; Virk, U.S.; Fan, Z. High Efficiency and Stable Perovskite Solar Cell Using ZnO/rGO QDs as an Electron Transfer Layer. Adv. Mater. Interfaces 2016, 3, 1500790. [Google Scholar] [CrossRef]
- Tan, F.; Xu, W.; Hu, X.; Yu, P.; Zhang, W. Highly Efficient Inverted Perovskite Solar Cells with CdSe QDs/LiF Electron Transporting Layer. Nanoscale Res. Lett. 2017, 12, 614. [Google Scholar] [CrossRef]
- Fu, N.; Huang, C.; Lin, P.; Zhu, M.; Li, T.; Ye, M.; Lin, S.; Zhang, G.; Du, J.; Liu, C.; et al. Black phosphorus quantum dots as dual-functional electron-selective materials for efficient plastic perovskite solar cells. J. Mater. Chem. A 2018, 6, 8886–8894. [Google Scholar] [CrossRef]
- Park, S.Y.; Baek, M.Y.; Ju, Y.; Kim, D.H.; Moon, C.S.; Noh, J.H.; Jung, H.S. Simultaneous Ligand Exchange Fabrication of Flexible Perovskite Solar Cells using Newly Synthesized Uniform Tin Oxide Quantum Dots. J. Phys. Chem. Lett. 2018, 9, 5460–5467. [Google Scholar] [CrossRef]
- Yang, G.; Chen, C.; Yao, F.; Chen, Z.; Zhang, Q.; Zheng, X.; Ma, J.; Lei, H.; Qin, P.; Xiong, L.; et al. Effective Carrier-Concentration Tuning of SnO2 Quantum Dot Electron-Selective Layers for High-Performance Planar Perovskite Solar Cells. Adv. Mater. 2018, 30, 1706023. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Z.; Wang, H.; Ye, F.; Ma, J.; Zheng, X.; Gui, P.; Xiong, L.; Wen, J.; Fang, G. A facile room temperature solution synthesis of SnO2 quantum dots for perovskite solar cells. J. Mater. Chem. A 2019, 7, 10636–10643. [Google Scholar] [CrossRef]
- Wang, E.; Chen, P.; Yin, X.; Wu, Y.; Que, W. Tailoring Electronic Properties of SnO2 Quantum Dots via Aluminum Addition for High-Efficiency Perovskite Solar Cells. Solar RRL 2019, 3, 1900041. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.N.; Wall, J.; Li, L.; Xing, G.; Zhang, Q.; Yan, F. Low-temperature processed highly efficient hole transport layer free carbon-based planar perovskite solar cells with SnO2 quantum dot electron transport layer. Mater. Today Phys. 2020, 13, 100204. [Google Scholar] [CrossRef]
- Wang, E.; Chen, P.; Yin, X.; Wu, Y.; Que, W. Novel ethanol vapor annealing treatment of SnO2 quantum dots film for highly efficient planar heterojunction perovskite solar cells. Org. Electron. 2020, 84, 105751. [Google Scholar] [CrossRef]
- Hossain, I.M.; Hudry, D.; Mathies, F.; Abzieher, T.; Moghadamzadeh, S.; Rueda-Delgado, D.; Schackmar, F.; Bruns, M.; Andriessen, R.; Aernouts, T.; et al. Scalable Processing of Low-Temperature TiO2 Nanoparticles for High-Efficiency Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 47–58. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, W.; Ke, W.; Clark, S.; Secor, E.B.; Song, T.B.; Kanatzidis, M.G.; Li, X.; Hersam, M.C. Millisecond-pulsed photonically-annealed tin oxide electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 24110–24115. [Google Scholar] [CrossRef]
- Huang, L.; Sun, X.; Li, C.; Xu, J.; Xu, R.; Du, Y.; Ni, J.; Cai, H.; Li, J.; Hu, Z.; et al. UV-Sintered Low-Temperature Solution-Processed SnO2 as Robust Electron Transport Layer for Efficient Planar Heterojunction Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 21909–21920. [Google Scholar] [CrossRef] [PubMed]
- Eliwi, A.A.; Malekshahi Byranvand, M.; Fassl, P.; Khan, M.R.; Hossain, I.M.; Frericks, M.; Ternes, S.; Abzieher, T.; Schwenzer, J.A.; Mayer, T.; et al. Optimization of SnO2 electron transport layer for efficient planar perovskite solar cells with very low hysteresis. Mater. Adv. 2022, 3, 456–466. [Google Scholar] [CrossRef]
- Ding, J.; Duan, J.; Guo, C.; Tang, Q. Toward charge extraction in all-inorganic perovskite solar cells by interfacial engineering. J. Mater. Chem. A 2018, 6, 21999–22004. [Google Scholar] [CrossRef]
- Li, Q.; Bai, J.; Zhang, T.; Nie, C.; Duan, J.; Tang, Q. CdZnSe@ZnSe colloidal alloy quantum dots for high-efficiency all-inorganic perovskite solar cells. Chem. Commun. 2018, 54, 9575–9578. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, W.; Liu, H.; Peng, J.; Cao, H.; Shao, G.; Xia, Z.; Ma, W.; Tang, J. PbS colloidal quantum dots as an effective hole transporter for planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 515–518. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Huang, Y.; Wei, J.; Liu, F.; Shao, Z.; Hu, L.; Chen, S.; Yang, S.; Tang, J.; et al. Efficient inorganic solid solar cells composed of perovskite and PbS quantum dots. Nanoscale 2015, 7, 9902–9907. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, J.; Huang, Y.; Li, Y.; Shao, Z.; Xu, Y.; Dai, S. Colloidal CuInS2 Quantum Dots as Inorganic Hole-Transporting Material in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 17482–17488. [Google Scholar] [CrossRef]
- Wu, Q.; Xue, C.; Li, Y.; Zhou, P.; Liu, W.; Zhu, J.; Dai, S.; Zhu, C.; Yang, S. Kesterite Cu2ZnSnS4 as a Low-Cost Inorganic Hole-Transporting Material for High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 28466–28473. [Google Scholar] [CrossRef]
- Khanzada, L.S.; Levchuk, I.; Hou, Y.; Azimi, H.; Osvet, A.; Ahmad, R.; Brandl, M.; Herre, P.; Distaso, M.; Hock, R.; et al. Effective Ligand Engineering of the Cu2ZnSnS4 Nanocrystal Surface for Increasing Hole Transport Efficiency in Perovskite Solar Cells. Adv. Funct. Mater. 2016, 26, 8300–8306. [Google Scholar] [CrossRef]
- Paulo, S.; Stoica, G.; Cambarau, W.; Martinez-Ferrero, E.; Palomares, E. Carbon quantum dots as new hole transport material for perovskite solar cells. Synth. Met. 2016, 222, 17–22. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, X.; Kong, J.; Zhou, W.; Zhou, Z.; Tian, Q.; Meng, Y.; Wu, S.; Kou, D. Controlling the Band Gap to Improve Open-Circuit Voltage in Metal Chalcogenide based Perovskite Solar Cells. Electrochim. Acta 2016, 215, 374–379. [Google Scholar] [CrossRef]
- Xu, L.; Deng, L.-L.; Cao, J.; Wang, X.; Chen, W.-Y.; Jiang, Z. Solution-Processed Cu(In, Ga)(S, Se)2 Nanocrystal as Inorganic Hole-Transporting Material for Efficient and Stable Perovskite Solar Cells. Nanoscale Res. Lett. 2017, 12, 159. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Baek, W.; Kim, S.; Kang, G.; Han, I.K.; Hyeon, T.; Park, M. Moisture proof hole transport layers based on CISe quantum dots for highly stable and large active area perovskite solar cells. Appl. Surf. Sci. 2019, 496, 143610. [Google Scholar] [CrossRef]
- Tamilselvan, M.; Bhattacharyya, A.J. Tetrahedrite (Cu12Sb4S13) Ternary Inorganic Hole Conductor for Ambient Processed Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 4227–4234. [Google Scholar] [CrossRef]
- Xu, H.; Duan, J.; Zhao, Y.; Jiao, Z.; He, B.; Tang, Q. 9.13%—Efficiency and stable inorganic CsPbBr3 solar cells. Lead-free CsSnBr3-xIx quantum dots promote charge extraction. J. Power Sources 2018, 399, 76–82. [Google Scholar] [CrossRef]
- Duan, J.; Dou, D.; Zhao, Y.; Wang, Y.; Yang, X.; Yuan, H.; He, B.; Tang, Q. Spray-assisted deposition of CsPbBr3 films in ambient air for large-area inorganic perovskite solar cells. Mater. Today Energy 2018, 10, 146–152. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, X.; Chen, S.; Zhao, X.; Dai, S.; Xu, B. Hydrophobic Cu2O Quantum Dots Enabled by Surfactant Modification as Top Hole-Transport Materials for Efficient Perovskite Solar Cells. Adv. Sci. 2019, 6, 1801169. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Z.; Liu, Y.; Liu, Y.; Gao, H.; Mao, Y. An inorganic hole-transport material of CuInSe2 for stable and efficient perovskite solar cells. Org. Electron. 2019, 67, 168–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, J.; Yuan, H.; Wang, Y.; Yang, X.; He, B.; Tang, Q. Using SnO2 QDs and CsMBr3 (M=Sn, Bi, Cu) QDs as Charge-Transporting Materials for 10.6%—Efficiency All-Inorganic CsPbBr3 Perovskite Solar Cells with an Ultrahigh Open-Circuit Voltage of 1.610 V. Solar RRL 2019, 3, 1800284. [Google Scholar] [CrossRef]
- Li, F.; Wei, J.; Liao, G.; Guo, C.; Huang, Y.; Zhang, Q.; Jin, X.; Jiang, S.; Tang, Q.; Li, Q. Quaternary quantum dots with gradient valence band for all-inorganic perovskite solar cells. J. Colloid Interface Sci. 2019, 549, 33–41. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ren, D.; Liu, Y.; Zheng, A.; Zakeeruddin, S.M.; Dong, X.; Hagfeldt, A.; Grätzel, M.; Wang, P. SnS Quantum Dots as Hole Transporter of Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 3822–3829. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Mei, A.; Hu, B.; Yang, Z.; Chen, W. Bandgap aligned Cu12Sb4S13 quantum dots as efficient inorganic hole transport materials in planar perovskite solar cells with enhanced stability. Sustain. Energy Fuels 2019, 3, 831–840. [Google Scholar] [CrossRef]
- Zhou, Z.-J.; Deng, Y.-Q.; Zhang, P.-P.; Kou, D.-X.; Zhou, W.-H.; Meng, Y.-N.; Yuan, S.-J.; Wu, S.-X. Cu2ZnSnS4 Quantum Dots as Hole Transport Material for Enhanced Charge Extraction and Stability in All-Inorganic CsPbBr3 Perovskite Solar Cells. Solar RRL 2019, 3, 1800354. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Yang, Z.; Li, Q.; Wei, W.; Hu, B.; Chen, W. Cu12Sb4S13 Quantum Dots with Ligand Exchange as Hole Transport Materials in All-Inorganic Perovskite CsPbI3 Quantum Dot Solar Cells. ACS Appl. Energy Mater. 2020, 3, 3521–3529. [Google Scholar] [CrossRef]
- Majdi, M.; Eskandari, M.; Fathi, D. Textured HTM-free perovskite/PbS quantum dot solar cell: Optical and electrical efficiency improvement by light trapping control. Sol. Energy 2021, 230, 618–627. [Google Scholar] [CrossRef]
- Zhao, G.; Cai, Q.; Liu, X.; Li, P.; Zhang, Y.; Shao, G.; Liang, C. PbS QDs as Electron Blocking Layer Toward Efficient and Stable Perovskite Solar Cells. IEEE J. Photovolt. 2019, 9, 194–199. [Google Scholar] [CrossRef]
- Kasi Matta, S.; Zhang, C.; O’Mullane, A.P.; Du, A. Density Functional Theory Investigation of Carbon Dots as Hole-transport Material in Perovskite Solar Cells. ChemPhysChem 2018, 19, 3018–3023. [Google Scholar] [CrossRef]
- Panigrahi, S.; Jana, S.; Calmeiro, T.; Nunes, D.; Martins, R.; Fortunato, E. Imaging the Anomalous Charge Distribution Inside CsPbBr3 Perovskite Quantum Dots Sensitized Solar Cells. ACS Nano 2017, 11, 10214–10221. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Z.; Zhang, J.; Bai, D.; Bian, H.; Wang, K.; Sun, J.; Wang, Q.; Liu, S.F. All-Ambient Processed Binary CsPbBr3–CsPb2Br5 Perovskites with Synergistic Enhancement for High-Efficiency Cs–Pb–Br-Based Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 7145–7154. [Google Scholar] [CrossRef]
- Chen, K.; Zhong, Q.; Chen, W.; Sang, B.; Wang, Y.; Yang, T.; Liu, Y.; Zhang, Y.; Zhang, H. Short-Chain Ligand-Passivated Stable α-CsPbI3 Quantum Dot for All-Inorganic Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900991. [Google Scholar] [CrossRef]
- Ling, X.; Zhou, S.; Yuan, J.; Shi, J.; Qian, Y.; Larson, B.W.; Zhao, Q.; Qin, C.; Li, F.; Shi, G.; et al. 14.1% CsPbI3 Perovskite Quantum Dot Solar Cells via Cesium Cation Passivation. Adv. Energy Mater. 2019, 9, 1900721. [Google Scholar] [CrossRef]
- Liu, F.; Ding, C.; Zhang, Y.; Kamisaka, T.; Zhao, Q.; Luther, J.M.; Toyoda, T.; Hayase, S.; Minemoto, T.; Yoshino, K.; et al. GeI2 Additive for High Optoelectronic Quality CsPbI3 Quantum Dots and Their Application in Photovoltaic Devices. Chem. Mater. 2019, 31, 798–807. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Yuan, J.; Ling, X.; Zhou, S.; Qian, Y.; Ma, W. Efficient and stable CsPbI3 perovskite quantum dots enabled by in situ ytterbium doping for photovoltaic applications. J. Mater. Chem. A 2019, 7, 20936–20944. [Google Scholar] [CrossRef]
- Chen, K.; Jin, W.; Zhang, Y.; Yang, T.; Reiss, P.; Zhong, Q.; Bach, U.; Li, Q.; Wang, Y.; Zhang, H.; et al. High Efficiency Mesoscopic Solar Cells Using CsPbI3 Perovskite Quantum Dots Enabled by Chemical Interface Engineering. J. Am. Chem. Soc. 2020, 142, 3775–3783. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yang, Y.; Liu, X.; Ding, Y.; Arain, Z.; Li, X.; Li, Y.; Zhou, Z.; Dai, S.; Nazeeruddin, M.K. Quasi-quantum dot-induced stabilization of α-CsPbI3 perovskite for high-efficiency solar cells. J. Mater. Chem. A 2020, 8, 10226–10232. [Google Scholar] [CrossRef]
- Shivarudraiah, S.B.; Ng, M.; Li, C.H.A.; Halpert, J.E. All-Inorganic, Solution-Processed, Inverted CsPbI3 Quantum Dot Solar Cells with a PCE of 13.1% Achieved via a Layer-by-Layer FAI Treatment. ACS Appl. Energy Mater. 2020, 3, 5620–5627. [Google Scholar] [CrossRef]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [Green Version]
- Mali, S.S.; Shim, C.S.; Hong, C.K. Highly stable and efficient solid-state solar cells based on methylammonium lead bromide (CH3NH3PbBr3) perovskite quantum dots. NPG Asia Mater. 2015, 7, e208. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Liu, F.; Zhang, Y.; Hirotani, D.; Rin, X.; Hayase, S.; Minemoto, T.; Masuda, T.; Wang, R.; Shen, Q. Photoexcited hot and cold electron and hole dynamics at FAPbI3 perovskite quantum dots/metal oxide heterojunctions used for stable perovskite quantum dot solar cells. Nano Energy 2020, 67, 104267. [Google Scholar] [CrossRef]
- Liu, F.; Ding, C.; Zhang, Y.; Ripolles, T.S.; Kamisaka, T.; Toyoda, T.; Hayase, S.; Minemoto, T.; Yoshino, K.; Dai, S.; et al. Colloidal Synthesis of Air-Stable Alloyed CsSn1–xPbxI3 Perovskite Nanocrystals for Use in Solar Cells. J. Am. Chem. Soc. 2017, 139, 16708–16719. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, Z.; Chen, D.; Bai, D.; Bian, H.; Sun, J.; Zhu, G.; Wang, G.; Liu, S. µ-Graphene Crosslinked CsPbI3 Quantum Dots for High Efficiency Solar Cells with Much Improved Stability. Adv. Energy Mater. 2018, 8, 1800007. [Google Scholar] [CrossRef]
- Li, F.; Zhou, S.; Yuan, J.; Qin, C.; Yang, Y.; Shi, J.; Ling, X.; Li, Y.; Ma, W. Perovskite Quantum Dot Solar Cells with 15.6% Efficiency and Improved Stability Enabled by an α-CsPbI3/FAPbI3 Bilayer Structure. ACS Energy Lett. 2019, 4, 2571–2578. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, J.; Li, T.; Tao, C.; Deng, X.; Li, Z. Convenient preparation of CsSnI3 quantum dots, excellent stability, and the highest performance of lead-free inorganic perovskite solar cells so far. J. Mater. Chem. A 2019, 7, 7683–7690. [Google Scholar] [CrossRef]
- Han, R.; Zhao, Q.; Hazarika, A.; Li, J.; Cai, H.; Ni, J.; Zhang, J. Ionic Liquids Modulating CsPbI3 Colloidal Quantum Dots Enable Improved Mobility for High-Performance Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 4061–4070. [Google Scholar] [CrossRef]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1-xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Sanehira Erin, M.; Marshall Ashley, R.; Christians Jeffrey, A.; Harvey Steven, P.; Ciesielski Peter, N.; Wheeler Lance, M.; Schulz, P.; Lin Lih, Y.; Beard Matthew, C.; Luther Joseph, M. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 2017, 3, eaao4204. [Google Scholar] [CrossRef] [Green Version]
- Swarnkar, A.; Marshall Ashley, R.; Sanehira Erin, M.; Chernomordik Boris, D.; Moore David, T.; Christians Jeffrey, A.; Chakrabarti, T.; Luther Joseph, M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Lee, J.-W.; Dai, Z.; Wang, R.; Nuryyeva, S.; Liao, M.E.; Chang, S.-Y.; Meng, L.; Meng, D.; Sun, P.; et al. Surface Ligand Management for Stable FAPbI3 Perovskite Quantum Dot Solar Cells. Joule 2018, 2, 1866–1878. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Ling, X.; Yang, D.; Li, F.; Zhou, S.; Shi, J.; Qian, Y.; Hu, J.; Sun, Y.; Yang, Y.; et al. Band-Aligned Polymeric Hole Transport Materials for Extremely Low Energy Loss α-CsPbI3 Perovskite Nanocrystal Solar Cells. Joule 2018, 2, 2450–2463. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, X.; Jin, Z.; Zhang, J.; Gao, Z.; Li, Y.; Liu, S.F. Energy-Down-Shift CsPbCl3:Mn Quantum Dots for Boosting the Efficiency and Stability of Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1479–1486. [Google Scholar] [CrossRef]
- Wang, B.; Li, B.; Shen, T.; Li, M.; Tian, J. ZnSe quantum dots downshifting layer for perovskite solar cells. J. Energy Chem. 2018, 27, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wu, Y.; Liu, L.; Gao, Y.; Chen, X.; Bi, W.; Chen, X.; Liu, D.; Dai, Q.; Song, H. Interfacial Engineering and Photon Downshifting of CsPbBr3 Nanocrystals for Efficient, Stable, and Colorful Vapor Phase Perovskite Solar Cells. Adv. Sci. 2019, 6, 1802046. [Google Scholar] [CrossRef] [Green Version]
- Bian, H.; Wang, Q.; Yang, S.; Yan, C.; Wang, H.; Liang, L.; Jin, Z.; Wang, G.; Liu, S. Nitrogen-doped graphene quantum dots for 80% photoluminescence quantum yield for inorganic γ-CsPbI3 perovskite solar cells with efficiency beyond 16%. J. Mater. Chem. A 2019, 7, 5740–5747. [Google Scholar] [CrossRef]
- Chen, C.; Zhuang, X.; Bi, W.; Wu, Y.; Gao, Y.; Pan, G.; Liu, D.; Dai, Q.; Song, H. Chemical inhibition of reversible decomposition for efficient and super-stable perovskite solar cells. Nano Energy 2020, 68, 104315. [Google Scholar] [CrossRef]
- Maxim, A.A.; Sadyk, S.N.; Aidarkhanov, D.; Surya, C.; Ng, A.; Hwang, Y.-H.; Atabaev, T.S.; Jumabekov, A.N. PMMA Thin Film with Embedded Carbon Quantum Dots for Post-Fabrication Improvement of Light Harvesting in Perovskite Solar Cells. Nanomaterials 2020, 10, 291. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.M.; Dastjerdi, H.T.; Prochowicz, D.; Yadav, P.; Tavakoli, R.; Saliba, M.; Fan, Z. Highly efficient and stable inverted perovskite solar cells using down-shifting quantum dots as a light management layer and moisture-assisted film growth. J. Mater. Chem. A 2019, 7, 14753–14760. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Li, H.; Bi, W.; Chen, C.; Zhang, B.; Xu, L.; Dong, B.; Song, H.; Dai, Q. Efficient and stable perovskite solar cells through e-beam preparation of cerium doped TiO2 electron transport layer, ultraviolet conversion layer CsPbBr3 and the encapsulation layer Al2O3. Sol. Energy 2020, 198, 187–193. [Google Scholar] [CrossRef]
- Maxim, A.A.; Aidarkhanov, D.; Atabaev, T.S.; Jumabekov, A.N.; Ng, A. Light management in perovskite solar cell by incorporation of carbon quantum dots. Mater. Today Proc. 2022, 49, 2487–2490. [Google Scholar] [CrossRef]
- Anizelli, H.S.; Stoichkov, V.; Fernandes, R.V.; Duarte, J.L.; Laureto, E.; Kettle, J.; Visoly-Fisher, I.; Katz, E.A. Application of luminescence downshifting materials for enhanced stability of CH3NH3PbI3(1-x)Cl3x perovskite photovoltaic devices. Org. Electron. 2017, 49, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Khenkin, M.V.; Arnaoutakis, G.E.; Samoylova, N.A.; Barbé, J.; Lee, H.K.H.; Tsoi, W.C.; Katz, E.A. Initial Stages of Photodegradation of MAPbI3 Perovskite: Accelerated Aging with Concentrated Sunlight. Solar RRL 2020, 4, 1900270. [Google Scholar] [CrossRef]
- Mahon, N.S.; Korolik, O.V.; Khenkin, M.V.; Arnaoutakis, G.E.; Galagan, Y.; Soriūtė, V.; Litvinas, D.; Ščajev, P.; Katz, E.A.; Mazanik, A.V. Photoluminescence kinetics for monitoring photoinduced processes in perovskite solar cells. Sol. Energy 2020, 195, 114–120. [Google Scholar] [CrossRef]
- Binek, A.; Petrus, M.L.; Huber, N.; Bristow, H.; Hu, Y.; Bein, T.; Docampo, P. Recycling Perovskite Solar Cells to Avoid Lead Waste. ACS Appl. Mater. Interfaces 2016, 8, 12881–12886. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Hu, Z.; Huang, L.; Huang, X.; Jia, X.; Zhang, J.; Zhang, J.; Zhu, Y. In situ recycle of PbI2 as a step towards sustainable perovskite solar cells. Prog. Photovolt. 2017, 25, 1022–1033. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, H.J.; Sohn, C.H.; Gong, O.Y.; Choi, J.H.; Cho, K.H.; Han, G.S.; Nam, K.T.; Jung, H.S. High-Throughput Pb Recycling for Perovskite Solar Cells Using Biomimetic Whitlockite. Energy Environ. Mater. 2022, 0, 1–7. [Google Scholar] [CrossRef]

| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| CQDs (10 nm) | ITO/TiO2:CQDs/MAPbI3Cl3−x/Spiro-OMeTAD/Au | 1.136 | 21.36 | 78 | 18.89 | [26] |
| CQDs (4 nm) | FTO/PEDOT:PSS/MAPbI3/PCBM:CQDs/BCP/Ag | 0.97 | 22.30 | 79.6 | 18.10 | [27] |
| CQDs (~4.8 nm) | FTO/c-TiO2/m-TiO2:CQDs/MAPbClxI3−x/Spiro-OMeTAD/Au | 1.019 | 22.64 | 71.6 | 16.40 | [25] |
| g-C3N4 QDs (5~10 nm) | ITO/SnO2:g-C3N4 QDs/CsFAMA/Spiro-OMeTAD/Au | 1.176 | 24.03 | 78.3 | 22.13 | [28] |
| Red CQDs (~) | ITO/SnO2:RCQs/Cs0.05FA0.81MA0.14PbI2.25Br0.45/Spiro-OMeTAD /MoO3/Au | 1.14 | 24.1 | 82.9 | 22.77 | [29] |
| GQDs (~5 nm) | FTO/Au/SnO2:GQDs/ZnO/Perovskite/Spiro-OMeTAD/Au | 1.172 | 22.85 | 74 | 19.81 | [37] |
| GQDs (5~10 nm) | ITO/SnO2:GQDs/MAPbI3/Spiro-OMeTAD/Au | 1.13 | 23.05 | 78 | 20.31 | [30] |
| GQDs (~) | FTO/SnO2:GQDs/CsFAMA/Spiro-OMeTAD/Ag | 1.10 | 21.62 | 78 | 18.55 | [36] |
| GQDs (6 nm) | ITO/c-TiO2/m-TiO2:GQDs/Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3/Spiro-OMeTAD/Au | 0.97 | 21.92 | 67 | 14.36 | [33] |
| GQDs (~5 nm) | ITO/PCBM:GQDs/MAPbI3/Spiro-OMeTAD/Au | 1.09 | 22.03 | 73 | 17.56 | [31] |
| GQDs (~) | ITO/SnO2:GQDs/MAFAPbIxCl3−x /Spiro-OMeTAD/Ag | 1.11 | 24.40 | 78 | 21.10 | [34] |
| GQDs (3~5 nm) | FTO/c-TiO2/m-TiO2:GQDs/Perovskite/Spiro-OMeTAD/Ag | 1.08 | 24.92 | 76 | 20.45 | [32] |
| GQDs (5.4~10.9 nm) | FTO/SnO2:GQDs/CsFAMA/Spiro-OMeTAD/Au | 1.08 | 23.5 | 77 | 19.6 | [35] |
| CdSe QDs (10 nm) | ITO/PEDOT:PSS/CH3NH3PbI3−xClx/PCBM:CdSe QDs/LiF/Ag | 0.90 | 20.96 | 73.16 | 13.73 | [38] |
| CdS QDs (4~5 nm) | FTO/TiO2:CdS QDs/CH3NH3PbI3/Spiro-OMeTAD/Au | 0.94 | 16.7 | 64 | 10.52 | [44] |
| PbS QDs (~5 nm) | FTO/TNTs:PbS QDs/Cs0.05(FA0.85MA0.15)0.95Pb(I0.85Br0.15)3/Spiro-OMeTAD/Au | 1.14 | 23.38 | 56.03 | 14.95 | [39] |
| PbS QDs (1~3 nm) | ITO/ZnO:PbS QDs-TBAI-80/MAPbI3/PCBM/Ag | 1.14 | 22.80 | 78.99 | 20.53 | [42] |
| BP QDs (2~8 nm) | Glass/FTO/SnO2:BP QDs/BP QDs@A-CsFAMA/Spiro-OMeTAD/Ag | 1.22 | 23.53 | 79.6 | 22.85 | [40] |
| BP QDs (~) | FTO/SnO2:BP QDs/(FAPbI3)0.97(MAPbBr3)0.03/Spiro-OMeTAD/Ag | 1.13 | 24.4 | 76.1 | 21.0 | [41] |
| SnO2 QDs (4~5 nm) | FTO/c-TiO2/m-TiO2:SnO2 QDs/MAPbI3/Spiro-OMeTAD/Ag | 1.13 | 22.36 | 75.67 | 19.09 | [43] |
| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| CQDs (3~5 nm) | FTO/TiO2/ZrO2/CH3NH3PbI3:CQDs/Carbon | 0.79 | 16.40 | 61.2 | 7.62 | [50] |
| CQDs (~) | ITO/PTAA/CH3NH3PbI3:CQDs/Ti/Cu | 1.101 | 23.13 | 75.28 | 19.17 | [53] |
| CQDs (1.62~7.36 nm) | ITO/NiO/CH3NH3PbI3:CQDs/PCB61M/BCP/Ag | 1.07 | 21.68 | 78.78 | 18.24 | [52] |
| CQDs (3~5 nm) | ITO/c-TiO2/m-TiO2/MAPbI3:CQDs/Spiro-OMeTAD/Ag | 1.13 | 22.19 | 75 | 18.81 | [16] |
| EACQDs (2.65~5.37 nm) | FTO/c-TiO2/m-TiO2/ZrO2/MAPbI3:EACQDs/Carbon | 1.021 | 22.72 | 65.29 | 15.14 | [56] |
| ASCQDs (3.43 nm) | FTO/c-TiO2/m-TiO2/ZrO2/CH3NH3PbI3:ASCQDs/Carbon | 1.03 | 23.84 | 61.13 | 14.95 | [55] |
| NCDs (12 nm) | FTO/TiO2/CH3NH3PbI3:NCDs/Spiro-OMeTAD/Ag | 1.08 | 20.66 | 73.91 | 16.49 | [51] |
| A-CQDs (4 nm) | FTO/c-TiO2/m-TiO2/ZrO2/MAPbI3:A-CQDs/Carbon | 0.94 | 22.35 | 51.27 | 13.28 | [54] |
| GQDs (~3 nm) | FTO/c-TiO2/MAPbI3:GQDs/Spiro-OMeTAD/Au | 1.14 | 24.17 | 71.6 | 19.70 | [58] |
| GQDs (~20 nm) | FTO/c-TiO2/MAPbI3:GQDs/Spiro-OMeTAD/Au | 1.04 | 22.58 | 75 | 18.34 | [57] |
| GN-GQDs (1.5~5.5 nm) | FTO/NiO/MAPbI3:GN-GQDs/PCBM/BCP/Ag | 1.06 | 23.40 | 80 | 19.8 | [59] |
| F-GQDs (2~8 nm) | PEN/ITO/SnO2/Rb0.05Cs0.05(FA0.83MA0.17)0.90 Pb(I0.95Br0.05)3:F-GQDs/Spiro-OMeTAD/Ag | 1.106 | 22.87 | 80.67 | 20.40 | [60] |
| BP QDs (3~5 nm) | ITO/SnO2/CsPbI2Br:BP QDs/Spiro-OMeTAD/Au | 1.25 | 15.86 | 78 | 15.47 | [67] |
| Si QDs (~) | FTO/c-TiO2/m-TiO2/CsPbBr3:Si QDs/Spiro-OMeTAD/Ag | 1.42 | 7.80 | 75 | 8.31 | [66] |
| AgI QDs (~) | ITO/MAPbI3:AgI QDs/PCBM/Ag | 1.01 | 22.94 | 70.83 | 16.41 | [63] |
| FeOOH QDs (~3 nm) | FTO/c-TiO2/m-TiO2/Cs0.05FA0.81MA0.14Pb Br0.45I2.55:FeOOH QDs/Spiro-OMeTAD/Au | 1.17 | 23.12 | 77.6 | 21.0 | [64] |
| PbS QDs (~3.5 nm) | ITO/c-TiO2/m-TiO2/MAPbI3:PbS QDs/Spiro-OMeTAD/Au | 1.08 | 22.50 | 75 | 18.22 | [65] |
| PbS QDs (~4 nm) | ITO/c-TiO2/m-TiO2/MAPbI3:PbS QDs/Spiro-OMeTAD/Au | 1.02 | 23.50 | 77.2 | 18.60 | [62] |
| PbS QDs (4.7 nm) | FTO/SnO2/FAPbI3:PbS QDs/Spiro-OMeTAD/Au | 1.06 | 21.80 | 75 | 18.00 | [68] |
| PbS QDs (5~7 nm) | ITO/PEDOT: PSS/CsPb0.5Sn0.5BrI2: PbS QDs/PCBM/Ag | 0.66 | 20.08 | 61 | 8.03 | [73] |
| rGO-Sn QDs (6 nm) | FTO/SnO2/Al2O3/Graphene/FA0.8MA0.2SnI3:Sn QDs/Spiro-OMeTAD/Au | 0.68 | 16.63 | 68.1 | 7.70 | [71] |
| SnS QDs (3~4 nm) | FTO/c-TiO2/CH3NH3PbI3:SnS QDs/Spiro-OMeTAD/Au | 1.036 | 22.70 | 71.6 | 16.80 | [61] |
| SnS QDs (~10 nm) | FTO/m-TiO2/Cs2AgBiBr6:SnS QDs/Carbon | 1.02 | 3.74 | 51 | 1.95 | [75] |
| WS2 QDs (3 nm) | FTO/c-TiO2/m-TiO2/ZrO2/MAPbI3:WS2 QDs/Carbon | 1.057 | 23.08 | 69.09 | 16.85 | [69] |
| CdSe QDs (3.8 nm) | FTO/c-TiO2/m-TiO2/CsPbBrI2:MPA-CdSe QDs/Carbon | 1.25 | 14.47 | 80.1 | 14.49 | [72] |
| Ti3C2Clx QDs (8 nm) | FTO/SnO2/Rb0.05Cs0.05(FA0.83MA0.17)0.90 Pb(I0.83Br0.17)3:T3C2Clx QDs/PCBM/Ag | 1.19 | 22.27 | 80.42 | 21.31 | [70] |
| CuInSe2 QDs (12~25 nm) | ITO/SnO2/MAPbI3:CuInSe2 QDs/Spiro-OMeTAD/Ag | 1.09 | 21.09 | 78 | 18.04 | [74] |
| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| CQDs (4 nm) | FTO/SnO2/(FAPbI3)0.95(MAPbBr3)0.05/Spiro-OMeTAD:GQDs/Ag | 1.06 | 24.17 | 79.41 | 20.41 | [77] |
| CQDs (4~6 nm) | ITO/GO:CQDs/CH3NH3PbI3/PCBM/BCP/Ag | 0.953 | 21.0 | 80.1 | 16.2 | [78] |
| CQDs (3.2 nm) | ITO/NiO:CQDs/CH3NH3PbI3/PCBM/BCP/Ag | 1.08 | 20.22 | 77.15 | 16.91 | [79] |
| GQDs (15 nm) | FTO/PEDOT:PSS/GQDs/CH3NH3PbI3/PCBM/BCP/Ag | 1.002 | 21.41 | 75.31 | 16.16 | [80] |
| AGQDs (2 nm) | ITO/NiO:AGQDs/(FA0.83MA0.17)0.95Cs0.05Pb(I0.9Br0.1)3/PCBM/BCP/Ag | 1.067 | 22.5 | 81.5 | 19.55 | [81] |
| Graphdiyne QDs (3~5 nm) | FTO/TiO2/GD QDs/CH3NH3PbI3:GD QDs/Spiro-OMeTAD:GD QDs/Au | 1.124 | 22.48 | 78.7 | 19.89 | [82] |
| Graphdiyne QDs (50 nm) | FTO/TiO2/CH3NH3PbI3/P3HT:GD QDs/Au | 0.941 | 21.7 | 71.3 | 14.58 | [83] |
| PbSO4(PbO)4 QDs (5 nm) | ITO/SnO2/CsFAMA/Spiro-OMeTAD:PbSO4(PbO)4 QDs/Au | 1.142 | 24.80 | 80 | 22.66 | [84] |
| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| TiO2 QDs (3.6 nm) | FTO/TiO2 QDs/m-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 1.063 | 22.48 | 71 | 16.97 | [89] |
| ZnO QDs (~) | ITO-PET/Graphene/ZnO QDs (Apjet)/CH3NH3PbI3/Spiro-OMeTAD/Ag | 0.935 | 16.80 | 62 | 9.73 | [88] |
| ZnO/rGO QDs (5 nm) | FTO/ZnO/rGO QDs/CH3NH3PbI3/Spiro-OMeTAD/Au | 1.03 | 21.7 | 68 | 15.2 | [90] |
| SnO2 QDs (1.7~3.3 nm) | ITO/SnO2 QDs/MAPbI3/Spiro-OMeTAD/Ag | 1.08 | 21.85 | 74.28 | 17.66 | [98] |
| SnO2 QDs (2~4 nm) | FTO/SnO2 QDs/MA0.7FA0.3PbI3/Spiro-OMeTAD/Au | 1.08 | 23.40 | 74 | 18.80 | [95] |
| SnO2 QDs (1.7~3.3 nm) | FTO/Al:SnO2 QDs/MAPbI3/Spiro-OMeTAD/Ag | 1.06 | 22.78 | 75.41 | 18.20 | [96] |
| SnO2 QDs (3~5 nm) | FTO/SnO2 QDs/Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3/Spiro-OMeTAD/Ag | 1.13 | 23.05 | 79.8 | 20.79 | [94] |
| SnO2 QDs (2.9 nm) | ITO/SnO2 QDs/MAPbI3/Li-doped Spiro-OMeTAD/Au | 1.12 | 21.61 | 77 | 18.71 | [93] |
| SnO2 QDs (3 nm) | ITO/SnO2 QDs/Cs0.05FA0.81MA0.14PbI2.25Br0.45/Spiro-OMeTAD/Carbon | 1.08 | 22.19 | 56.64 | 13.64 | [97] |
| BP QDs (4.7 nm) | ITO-PEN/BP QDs/FA0.85MA0.15PbI0.25Br0.5/Spiro-OMeTAD/Au | 1.03 | 16.77 | 65.2 | 11.26 | [92] |
| CdSe QDs (5.5 nm) | ITO/PEDOT:PSS/MAPbI3/CdSe QDs/LiF/Ag | 0.99 | 20.5 | 69.9 | 14.2 | [91] |
| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| CQDs (~) | FTO/c-TiO2/m-TiO2/MAPbI3/CQDs/Au | 0.515 | 7.83 | 74 | 3 | [110] |
| PbS QDs (3 nm) | ITO/PbS QDs/MAPbI3/PCBM/Al | 0.86 | 12.1 | 72 | 7.5 | [105] |
| PbS QDs (3.6 nm) | FTO/c-TiO2/m-TiO2/CH3NH3PbI3/PbS QDs/Au | 0.87 | 18.69 | 49 | 7.88 | [106] |
| PbS QDs (3.5 nm) | FTO/c-TiO2/m-TiO2/CH3NH3PbI3/PbS QDs/Au | 0.97 | 19.03 | 61.34 | 11.32 | [126] |
| PbS QDs (~) | FTO/TiO2/m-TiO2/CH3NH3PbI3/PbS QDs/Au | 0.8 | 29.3 | 83 | 19.52 | [125] |
| CuInS2 QDs (4 nm) | FTO/c-TiO2/m-TiO2/MAPbI3/CuInS2/ZnS QDs/Au | 0.924 | 18.6 | 48.7 | 8.38 | [107] |
| CuIn1.5Se3 QDs (4 nm) | ITO/SnO2/MAPbBr3/CuIn1.5Se3 QDs/Au | 0.979 | 20.46 | 68.5 | 13.72 | [113] |
| CuInSe2 QDs (7 nm) | ITO/SnO2/FAMAPbI3BrCl/CuInSe2 QDs/Au | 0.86 | 22.5 | 66 | 12.8 | [118] |
| SnS QDs (4~7 nm) | FTO/c-TiO2/(CsPbI3)0.05(FAPbI3)0.79(MAPbI3)0.16/SnS QDs/Au | 0.944 | 22.96 | 63.3 | 13.72 | [121] |
| MoS2 QDs (4~7 nm) | FTO/c-TiO2/m-TiO2/CsPbBr3/MoS2 QDs/Carbon | 1.307 | 6.55 | 79.4 | 6.80 | [116] |
| Cu2O QDs (8~10 nm) | FTO/c-TiO2/m-TiO2/Cs0.05FA0.81MA0.14PbI2.55Br0.45/Cu2O QDs/Au | 1.15 | 22.2 | 74.2 | 18.90 | [117] |
| CsSnBr3 QDs (20 nm) | FTO/SnO2 QDs/CsPbBr3/CsSnBr3 QDs/Carbon | 1.61 | 7.8 | 84.4 | 10.60 | [119] |
| Ag-In-Ga-S QDs (~) | FTO/c-TiO2/m-TiO2/CsPbBr3/AIGS QDs/Carbon | 1.46 | 7.43 | 80.31 | 8.46 | [120] |
| Cu12Sb4S13 QDs (5.7 nm) | FTO/c-TiO2/CH3NH3PbI3/Cu12Sb4S13 QDs/Au | 1.05 | 21.85 | 61.6 | 14.13 | [122] |
| Cu12Sb4S13 QDS (5~6 nm) | FTO/c-TiO2/m-TiO2/CsPbI3 QDs/Cu12Sb4S13 QDs/Au | 1.04 | 18.28 | 52.9 | 10.02 | [124] |
| Cu12Sb4S13 QDs (9 nm) | FTO/c-TiO2/CH3NH3PbI3/Cu12Sb4S13 QDs/Au | 0.8 | 18.08 | 45 | 6.5 | [114] |
| Cu2ZnSnS4 QDs (20 nm) | FTO/c-TiO2/CH3NH3PbI3/Cu2ZnSnS4 QDs/Au | 1.06 | 20.54 | 58.7 | 12.75 | [108] |
| Cu2ZnSnS4 QDs (8 nm) | FTO/m-TiO2/c-TiO2/CsPbBr3/Cu2ZnSnS4 QDs/Ag | 0.94 | 7.36 | 70.01 | 4.84 | [123] |
| Cu2ZnSnS4 QDs (5.15 nm) | ITO/Cu2ZnSnS4-LF QDs/Perovskite/PCBM/Ag | 0.92 | 20.7 | 81 | 15.4 | [109] |
| Cu2ZnSnSe4 QDs (~) | FTO/TiO2/CH3NH3PbI3/Cu2ZnSnSe4 QDs/Au | 0.808 | 19.37 | 62.1 | 9.72 | [111] |
| CuIn0.1Ga0.9(S0.9Se0.1)2 QDs (10 nm) | FTO/c-TiO2/m-TiO2/CH3NH3PbI3/CIGSSe QDs/Au | 0.94 | 17.66 | 54.88 | 9.15 | [112] |
| CsSnBr2I QDs (1.7–3.8 nm) | FTO/c-TiO2/m-TiO2/CsPbBr3/CsSnBr2I QDs/Carbon | 1.39 | 8.70 | 76 | 9.13 | [115] |
| CuInS2@ZnS QDs (5.6~6.4 nm) | FTO/c-TiO2/m-TiO2/CsPbBr3/CuInS2@ZnS QDs/Carbon | 1.45 | 7.47 | 77.73 | 8.42 | [103] |
| CdZnSe@ZnSe QDs (6 nm) | FTO/c-TiO2/m-TiO2/CsPbBr3/CdZnSe@ZnSe QDs/Carbon | 1.498 | 7.25 | 79.6 | 8.65 | [104] |
| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| MAPbI3 QDs (2~3 nm) | FTO/c-TiO2/m-TiO2/MAPbI3 QDs/Iodine redox electrolyte/Pt | 0.71 | 15.82 | 59 | 6.54 | [137] |
| MAPbBr3 QDs (2 nm) | FTO/c-TiO2/m-TiO2/MAPbBr3 QDs/PTAA/Au | 1.11 | 14.07 | 73 | 11.40 | [138] |
| FAPbI3 QDs (17.7 nm) | ITO/SnO2/FAPbI3 QDs/Spiro-OMeTAD/Au | 1.10 | 11.83 | 64 | 8.38 | [148] |
| FAPbI3 QDs (7~12 nm) | ITO/c-TiO2/FAPbI3 QDs/Spiro-OMeTAD/Au | 1.05 | 13.3 | 67 | 9.40 | [139] |
| CsPbI3 QDs (3~12.5 nm) | ITO/c-TiO2/CsPbI3 QDs/Spiro-OMeTAD/MoOx/Al | 1.23 | 13.47 | 65 | 10.77 | [147] |
| CsPbI3 QDs (10 nm) | FTO/c-TiO2/CsPbI3 QDs/MoOx/Al | 1.20 | 14.37 | 78 | 13.40 | [146] |
| CsPbI3 QDs (8 nm) | FTO/c-TiO2/CsPbI3 QDs/PTB7/MoO3/Ag | 1.27 | 12.39 | 80 | 12.55 | [149] |
| CsPbI3 QDs (10 nm) | FTO/c-TiO2/CsPbI3 QDs/Spiro-OMeTAD/Au | 1.04 | 16.98 | 67 | 11.87 | [130] |
| Yb-CsPbI3 QDs (12 nm) | FTO/c-TiO2/Yb-CsPbI3 QDs/PTB7/MoO3/Ag | 1.25 | 14.18 | 74 | 13.12 | [133] |
| CsPbI3 QDs (12 nm) | FTO/c-TiO2/CsPbI3 QDs/Spiro-OMeTAD/Au | 1.11 | 14.80 | 74 | 12.15 | [132] |
| CsPbI3 QDs (~) | FTO/c-TiO2/CsPbI3 QDs/PTAA/MoO3/Ag | 1.25 | 14.96 | 76 | 14.10 | [131] |
| CsPbI3 QDs (9.1 nm) | FTO/c-TiO2/m-TiO2/CsPbI3 QDs/Spiro-OMeTAD/Au | 1.06 | 17.77 | 76 | 14.32 | [134] |
| CsPbI3 QDs (30 nm) | FTO/c-TiO2/CsPbI3 QDs/Spiro-OMeTAD/Au | 1.06 | 16.87 | 75 | 13.38 | [135] |
| CsPbI3 QDs (~) | FTO/NiOx/CsPbI3 QDs/C60/ZnO/Ag | 1.19 | 14.25 | 77.6 | 13.1 | [136] |
| CsPbBr3 QDs (7.2 nm) | FTO/c-TiO2/m-TiO2/CsPbBr3 QDs/Spiro-OMeTAD/Au | 0.86 | 8.55 | 57 | 4.21 | [128] |
| CsPbBr3 QDs (15~20 nm) | FTO/ZnO/CsPbBr3 QDs/Spiro-OMeTAD/Au | 1.43 | 6.17 | 77 | 6.81 | [129] |
| CsSn0.6Pb0.4I3 QDs (11~14 nm) | FTO/c-TiO2/m-TiO2/CsSn0.6Pb0.4I3 QDs/Spiro-OMeTAD/Au | 0.26 | 0.90 | 42 | 0.1 | [140] |
| CsSnI3 QDs (30 nm) | FTO/PEDOT:PSS/CsSnI3 QDs/PCBM/Ag | 0.42 | 23.79 | 41 | 4.13 | [143] |
| Cs0.5FA0.5PbI3 QDs (12 nm) | ITO/SnO2/Cs0.5FA0.5PbI3 QDs/Spiro-OMeTAD/Au | 1.17 | 18.30 | 78 | 16.60 | [145] |
| α-CsPbI3/FAPbI3 QDs (~) | FTO/TiO2/α-CsPbI3 QDs/FAPbI3 QDs/PTAA/MoO3/Ag | 1.22 | 17.26 | 0.74 | 15.6 | [142] |
| μGR-CsPbI3 QDs (10 nm) | FTO/TiO2/μGR-CsPbI3 QDs/PTAA/Au | 1.18 | 13.59 | 72.6 | 11.64 | [141] |
| [Pmim]I-CsPbI3 QDs (~) | FTO/TiO2/[Pmim]I-CsPbI3 QDs/Spiro-OMeTAD/MoOx/Ag | 1.20 | 15.65 | 75.1 | 14.14 | [144] |
| QDs | Device Structure | Voc | Jsc | FF % | PCE % | Ref. |
|---|---|---|---|---|---|---|
| CQDs@PMMA QDs (3~7 nm) | CQDs@PMMA/Glass/FTO/SnO2/Cs0.05(MA0.17FA0.83)0.95Pb(I0.84Br0.16)3Spiro-OMeTAD/Au | 1.13 | 23.21 | 68.19 | 17.86 | [155] |
| CQDs@H2O QDs (3~7 nm) | CQDs@H2O/Glass/FTO/SnO2/Cs0.05(MA0.17FA0.83)0.95Pb(I0.84Br0.16)3/Spiro-OMeTAD/Au | 1.18 | 22.8 | 65 | 17.60 | [158] |
| N-GQDs (3.8 nm) | N-GQDs/Glass/FTO/TiO2/γ-CsPbI3/PTAA/Au | 1.106 | 19.15 | 75.6 | 16.02 | [153] |
| ZnSe QDs (~) | ZnSe QDs/Glass/FTO/c-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 1.1 | 20.3 | 79.9 | 17.30 | [151] |
| CdSe/CdS QDs (~) | CdSe/CdS QDs/Glass/ITO/NiO/CsFAPbI3/C60/BCP/Ag | 1.143 | 23.6 | 76.8 | 20.70 | [156] |
| CsPbCl3:Mn QDs (8 nm) | CsPbCl3:Mn QDs/Glass/FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 1.105 | 22.03 | 76.3 | 18.57 | [150] |
| Ce3+-CsPbI3 QDs (~) | Ce3+-CsPbI3 QDs/Glass/FTO/c-SnO2/IBrFA0.83MA0.17Pb(I0.83Br0.17)3/PDPP4T/Au | 1.19 | 24.13 | 79.7 | 22.16 | [154] |
| CsPbBr3@SiO2 QDs (8 nm) | CsPbBr3@SiO2 QDs/Glass/FTO/c-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 1.06 | 24.6 | 79.6 | 20.80 | [152] |
| CsPbBr3 QDs (12 nm) | CsPbBr3 QDs/FTO/Ce-TiO2/CsFAMAPbI3/Spiro-OMeTAD/Au/Al2O3 | 1.112 | 22.26 | 78.13 | 19.34 | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, J.; Luo, X.; Li, Y.; Qiu, Q.; Xie, T. Selection, Preparation and Application of Quantum Dots in Perovskite Solar Cells. Int. J. Mol. Sci. 2022, 23, 9482. https://doi.org/10.3390/ijms23169482
Zhou Y, Yang J, Luo X, Li Y, Qiu Q, Xie T. Selection, Preparation and Application of Quantum Dots in Perovskite Solar Cells. International Journal of Molecular Sciences. 2022; 23(16):9482. https://doi.org/10.3390/ijms23169482
Chicago/Turabian StyleZhou, Yankai, Jiayan Yang, Xingrui Luo, Yingying Li, Qingqing Qiu, and Tengfeng Xie. 2022. "Selection, Preparation and Application of Quantum Dots in Perovskite Solar Cells" International Journal of Molecular Sciences 23, no. 16: 9482. https://doi.org/10.3390/ijms23169482
APA StyleZhou, Y., Yang, J., Luo, X., Li, Y., Qiu, Q., & Xie, T. (2022). Selection, Preparation and Application of Quantum Dots in Perovskite Solar Cells. International Journal of Molecular Sciences, 23(16), 9482. https://doi.org/10.3390/ijms23169482






