The Eyelid Meibomian Gland Deficiency in Fucosyltransferase 1 Knockout Mice
Abstract
:1. Introduction
2. Results
2.1. Expression of FUT1 and Its Fucosylated Products in the Eyelid and MGs
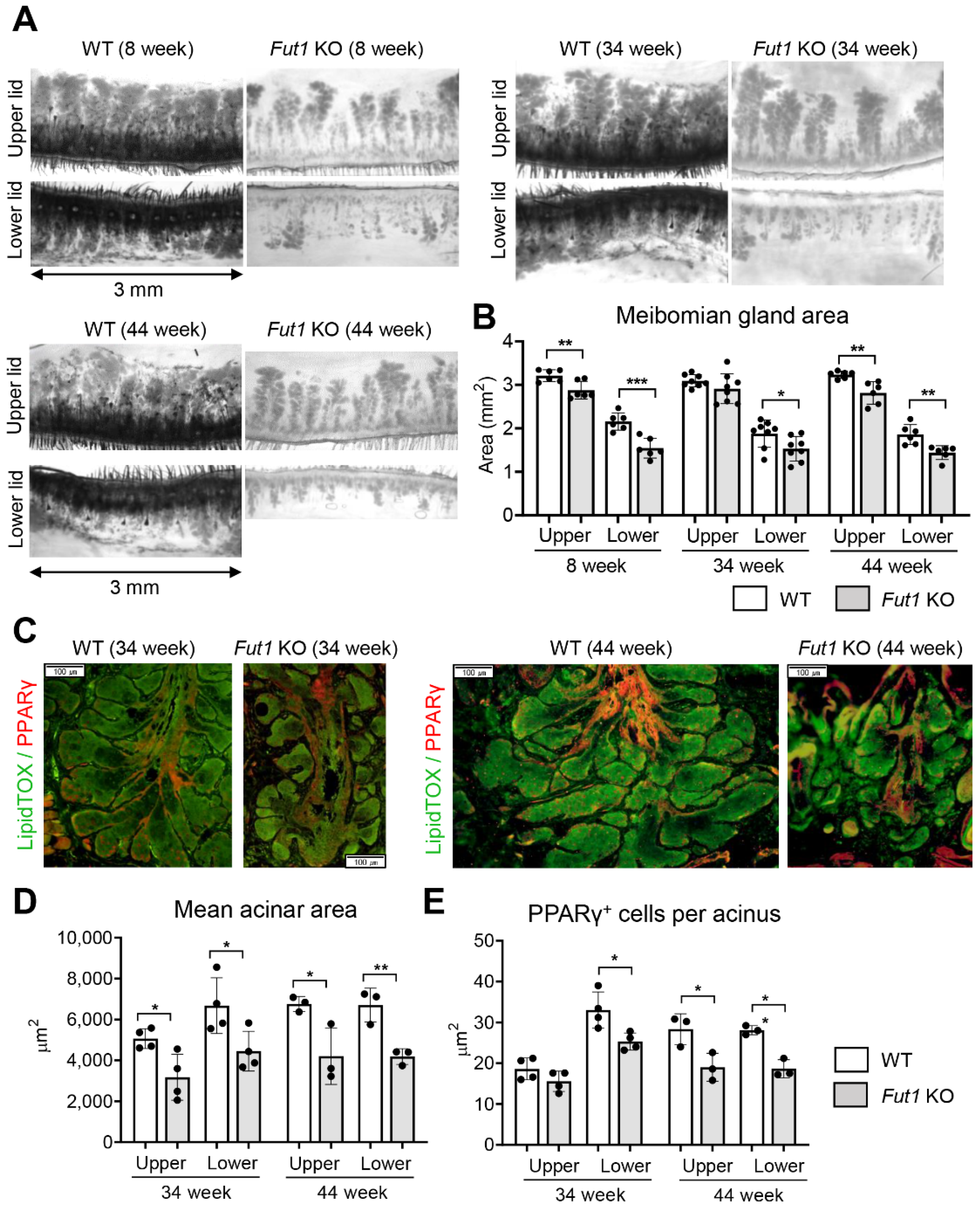
2.2. Effects of FUT1 Deficiency on MG Development and Function with Age
2.3. Effects of FUT1 Deficiency on Genes Related to Inflammation and Meibocyte Development
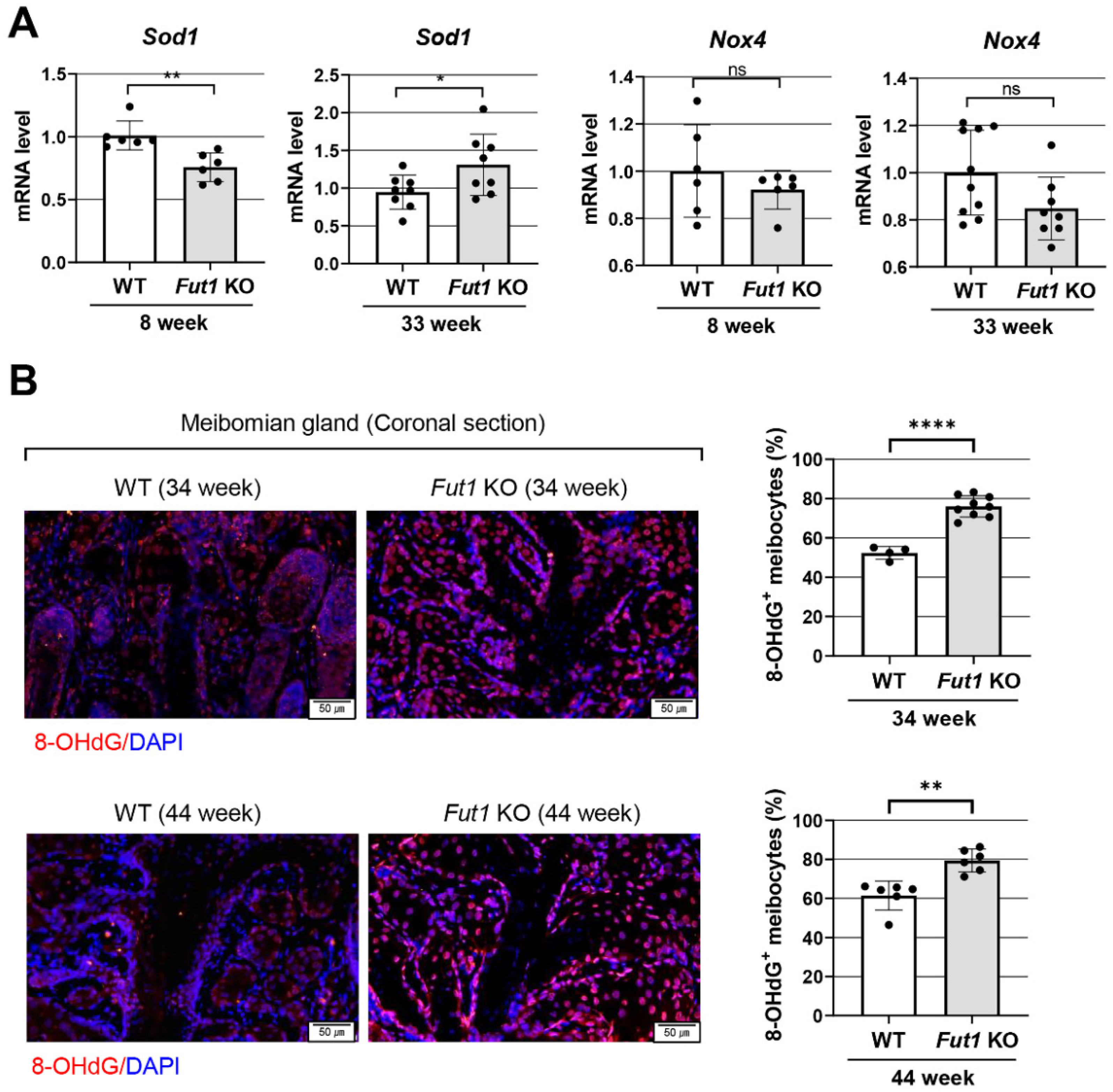
2.4. Effects of FUT1 Deficiency on Genes Related to Oxidative-Stress-Related Markers
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Transillumination Meibography
4.3. Immunohistochemistry
4.4. Western Blot
4.5. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shan, M.; Yang, D.; Dou, H.; Zhang, L. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 162, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41r–53r. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; McKinney, A.; Yang, Y.-W.; Tran, V.M.; Phillips, J.J. Glycosylation Alterations in Lung and Brain Cancer. Adv. Cancer Res. 2015, 126, 305–344. [Google Scholar] [CrossRef]
- Loong, J.H.; Wong, T.-L.; Tong, M.; Sharma, R.; Zhou, L.; Ng, K.-Y.; Yu, H.-J.; Li, C.-H.; Man, K.; Lo, C.-M.; et al. Glucose deprivation—Induced aberrant FUT1-mediated fucosylation drives cancer stemness in hepatocellular carcinoma. J. Clin. Investig. 2021, 131, e143377. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.T.; Brown, S.J.; Winterhalter, A.C.; Lust, M.; Salvaris, E.J.; Selan, C.; Nandurkar, H.H.; Desmond, P.V.; Cowan, P.J.; D’Apice, A.J. Glycosylation changes in hFUT1 transgenic mice increase TCR signaling and apoptosis resulting in thymocyte maturation arrest. Mol. Immunol. 2008, 45, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Garcher, C.; Bara, J.; Bron, A.; Oriol, R. Expression of mucin peptide and blood group ABH—And Lewis-related carbohydrate antigens in normal human conjunctiva. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1184–1191. [Google Scholar]
- Guzman-Aranguez, A.; Argüeso, P. Structure and Biological Roles of Mucin-type O-glycans at the Ocular Surface. Ocul. Surf. 2010, 8, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Panjwani, N.; Ahmad, S.; Raizman, M.B. Cell surface glycoproteins of corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1995, 36, 355–363. [Google Scholar]
- Benavente, M.C.R.; Argüeso, P. Glycosylation pathways at the ocular surface. Biochem. Soc. Trans. 2018, 46, 343–350. [Google Scholar] [CrossRef]
- Kim, K.W.; Ryu, J.S.; Ko, J.H.; Kim, J.Y.; Kim, H.J.; Lee, H.J.; Oh, J.-H.; Chung, J.H.; Oh, J.Y. FUT1 deficiency elicits immune dysregulation and corneal opacity in steady state and under stress. Cell Death Dis. 2020, 11, 285. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Sullivan, D.A.; Chen, D.; Hatton, M.P.; Kam, W.R.; Liu, Y. Biomarkers for Progenitor and Differentiated Epithelial Cells in the Human Meibomian Gland. Stem Cells Transl. Med. 2018, 7, 887–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The International Workshop on Meibomian Gland Dysfunction: Executive Summary. Investig. Opthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hsu, H.-C.; Mountz, J.D.; Allen, J.G. Unmasking Fucosylation: From Cell Adhesion to Immune System Regulation and Diseases. Cell Chem. Biol. 2018, 25, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Jester, J.V.; Potma, E.; Brown, D.J. PPARγ Regulates Mouse Meibocyte Differentiation and Lipid Synthesis. Ocul. Surf. 2016, 14, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, S.; Iwanishi, H.; Arita, R.; Shirai, K.; Sumioka, T.; Kokado, M.; Jester, J.; Saika, S. Ocular surface inflammation impairs structure and function of meibomian gland. Exp. Eye Res. 2017, 163, 78–84. [Google Scholar] [CrossRef]
- Sabeti, S.; Kheirkhah, A.; Yin, J.; Dana, R. Management of meibomian gland dysfunction: A review. Surv. Ophthalmol. 2020, 65, 205–217. [Google Scholar] [CrossRef]
- Dahlhoff, M.; Camera, E.; Schafer, M.; Emrich, D.; Riethmacher, D.; Foster, A.; Paus, R.; Schneider, M.R. Sebaceous lipids are essential for water repulsion, protection against UVB-induced apoptosis and ocular integrity in mice. Development 2016, 143, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.T.; Ntambi, J.M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. 2008, 19, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, M.; Man, W.C.; Ntambi, J.M. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J. Nutr. 2001, 131, 2260–2268. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Parfitt, G.; Brown, D.J.; Jester, J.V. Meibocyte differentiation and renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp. Eye Res. 2017, 163, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tellefsen Nøland, S.; Badian, R.A.; Utheim, T.P.; Utheim Ø, A.; Stojanovic, A.; Tashbayev, B.; Raeder, S.; Dartt, D.A.; Chen, X. Sex and age differences in symptoms and signs of dry eye disease in a Norwegian cohort of patients. Ocul. Surf. 2021, 19, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Muntz, A.; Lim, J.; Kim, J.S.; Lacerda, L.; Arora, A.; Craig, J.P. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. Ocul. Surf. 2020, 18, 736–741. [Google Scholar] [CrossRef]
- McCulley, J.P.; Shine, W.E. Meibomian Gland Function and the Tear Lipid Layer. Ocul. Surf. 2003, 1, 97–106. [Google Scholar] [CrossRef]
- Sun, M.; Puri, S.; Parfitt, G.J.; Mutoji, N.; Coulson-Thomas, V.J. Hyaluronan Regulates Eyelid and Meibomian Gland Morphogenesis. Investig. Opthalmol. Vis. Sci. 2018, 59, 3713–3727. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Carbe, C.; Powers, A.; Zhang, E.E.; Esko, J.D.; Grobe, K.; Feng, G.-S.; Zhang, X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 2008, 135, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantelli, F.; Schaffer, L.; Dana, R.; Head, S.R.; Argüeso, P. Glycogene Expression in Conjunctiva of Patients with Dry Eye: Downregulation of Notch Signaling. Investig. Opthalmol. Vis. Sci. 2009, 50, 2666–2672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sun, P.; Liu, J.; Fu, L.; Yan, J.; Liu, Y.; Yu, L.; Wang, X.; Yan, Q. Suppression of FUT1/FUT4 expression by siRNA inhibits tumor growth. Biochim. Biophys Acta 2008, 1783, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-Y.; Oh, J.-H.; Lee, D.H.; Lee, S.; Chung, J.H. Blood Type B antigen modulates cell migration through regulating cdc42 expression and activity in HaCaT cells. J. Cell. Physiol. 2013, 228, 2243–2251. [Google Scholar] [CrossRef]
- Isozaki, T.; Ruth, J.H.; Amin, M.A.; Campbell, P.L.; Tsou, P.-S.; Ha, C.M.; Haines, G.K.; Edhayan, G.; Koch, A.E. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Res. Ther. 2014, 16, R28. [Google Scholar] [CrossRef] [Green Version]
- Bu, J.; Zhang, M.; Wu, Y.; Jiang, N.; Guo, Y.; He, X.; He, H.; Jeyalatha, M.V.; Reinach, P.S.; Liu, Z.; et al. High-Fat Diet Induces Inflammation of Meibomian Gland. Investig. Opthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M.; von Lindenfels, V.; Garbe, A.; Kampik, A. Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay. Ophthalmology 2016, 123, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.J.; Yu, C.; Mathew, R.; Kunnen, C.M.; Kalnitsky, J.; Redfern, R.L.; Leonardi, A.; Perez, V.L.; MacLeod, A.S.; Gupta, P.K.; et al. Neutrophils cause obstruction of eyelid sebaceous glands in inflammatory eye disease in mice. Sci. Transl. Med. 2018, 10, eaas9164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, J.J.; Okeley, N.M.; Zeng, W.; Law, C.-L.; Senter, P.D.; Gardai, S.J. Understanding the mechanism of 2FF-induced immune modulation (Abstract). Cancer Res. 2016, 76, 4005. [Google Scholar] [CrossRef]
- Vaneev, A.; Kost, O.; Eremeev, N.; Beznos, O.; Alova, A.; Gorelkin, P.; Erofeev, A.; Chesnokova, N.; Kabanov, A.; Klyachko, N. Superoxide Dismutase 1 Nanoparticles (Nano-SOD1) as a Potential Drug for the Treatment of Inflammatory Eye Diseases. Biomedicines 2021, 9, 396. [Google Scholar] [CrossRef]
- Domino, S.E.; Zhang, L.; Gillespie, P.J.; Saunders, T.L.; Lowe, J.B. Deficiency of reproductive tract alpha (1,2) fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha (1,2) fucosyltransferase locus. Mol. Cell Biol. 2001, 21, 8336–8345. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.H.; Ryu, J.S.; Hwang, H.S.; Kim, M.K. Comparative Analysis of Age-Related Changes in Lacrimal Glands and Meibomian Glands of a C57BL/6 Male Mouse Model. Int. J. Mol. Sci. 2020, 21, 4169. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, C.H.; Ryu, J.S.; Ko, J.H.; Kim, Y.K.; Oh, J.-H.; Chung, J.H.; Oh, J.Y. The Eyelid Meibomian Gland Deficiency in Fucosyltransferase 1 Knockout Mice. Int. J. Mol. Sci. 2022, 23, 9464. https://doi.org/10.3390/ijms23169464
Yoon CH, Ryu JS, Ko JH, Kim YK, Oh J-H, Chung JH, Oh JY. The Eyelid Meibomian Gland Deficiency in Fucosyltransferase 1 Knockout Mice. International Journal of Molecular Sciences. 2022; 23(16):9464. https://doi.org/10.3390/ijms23169464
Chicago/Turabian StyleYoon, Chang Ho, Jin Suk Ryu, Jung Hwa Ko, Yeon Kyung Kim, Jang-Hee Oh, Jin Ho Chung, and Joo Youn Oh. 2022. "The Eyelid Meibomian Gland Deficiency in Fucosyltransferase 1 Knockout Mice" International Journal of Molecular Sciences 23, no. 16: 9464. https://doi.org/10.3390/ijms23169464
APA StyleYoon, C. H., Ryu, J. S., Ko, J. H., Kim, Y. K., Oh, J.-H., Chung, J. H., & Oh, J. Y. (2022). The Eyelid Meibomian Gland Deficiency in Fucosyltransferase 1 Knockout Mice. International Journal of Molecular Sciences, 23(16), 9464. https://doi.org/10.3390/ijms23169464





