Augmented Therapeutic Potential of EC-Synthetic Retinoids in Caco-2 Cancer Cells Using an In Vitro Approach
Abstract
1. Introduction
2. Results
2.1. Anti-Proliferative Activity of Different Synthetic Retinoids on Caco-2 Cell Line (Individually and in Combinations)
2.2. Apoptotic and Cell Cycle Analysis of Retinoids in Individual and Combinatorial Effect
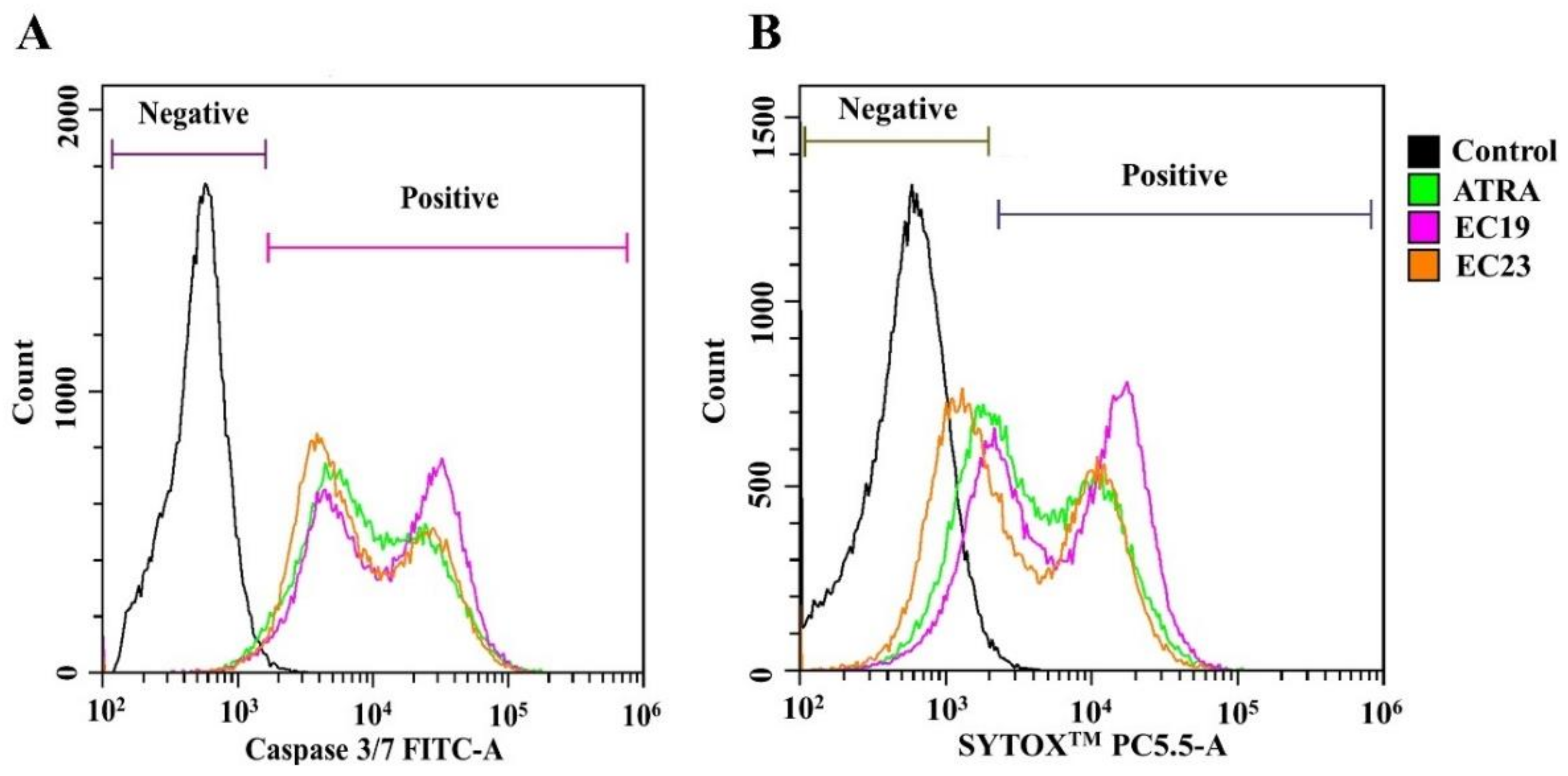
2.3. Retinoids Induce Caspase-3/7 Activity with Detectable Apoptosis and Necrosis in Caco-2
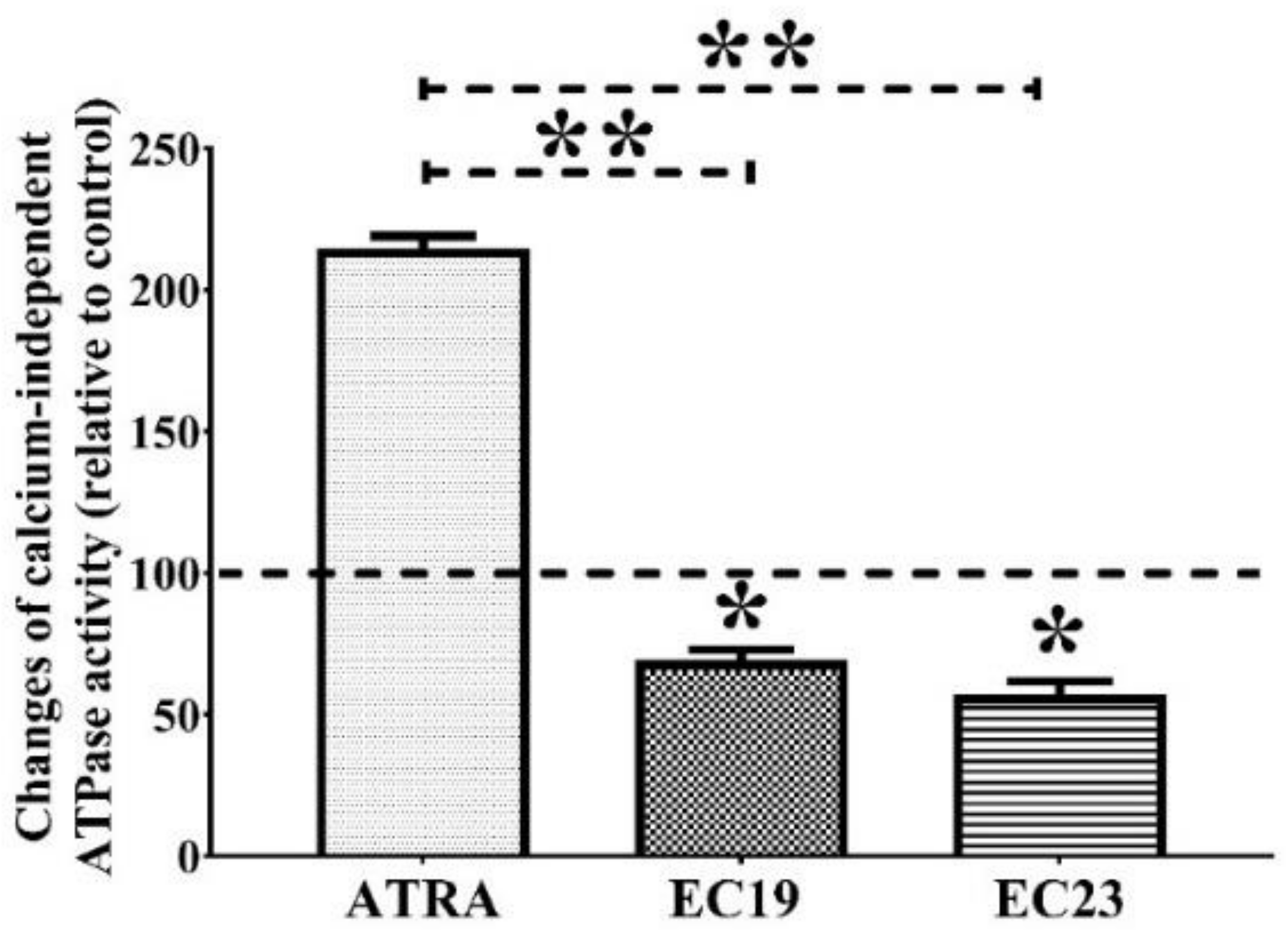
2.4. EC19 and EC23 Interfere with the ATP-Catabolizing Activity of Calcium-Independent ATPases in Caco-2 Cells
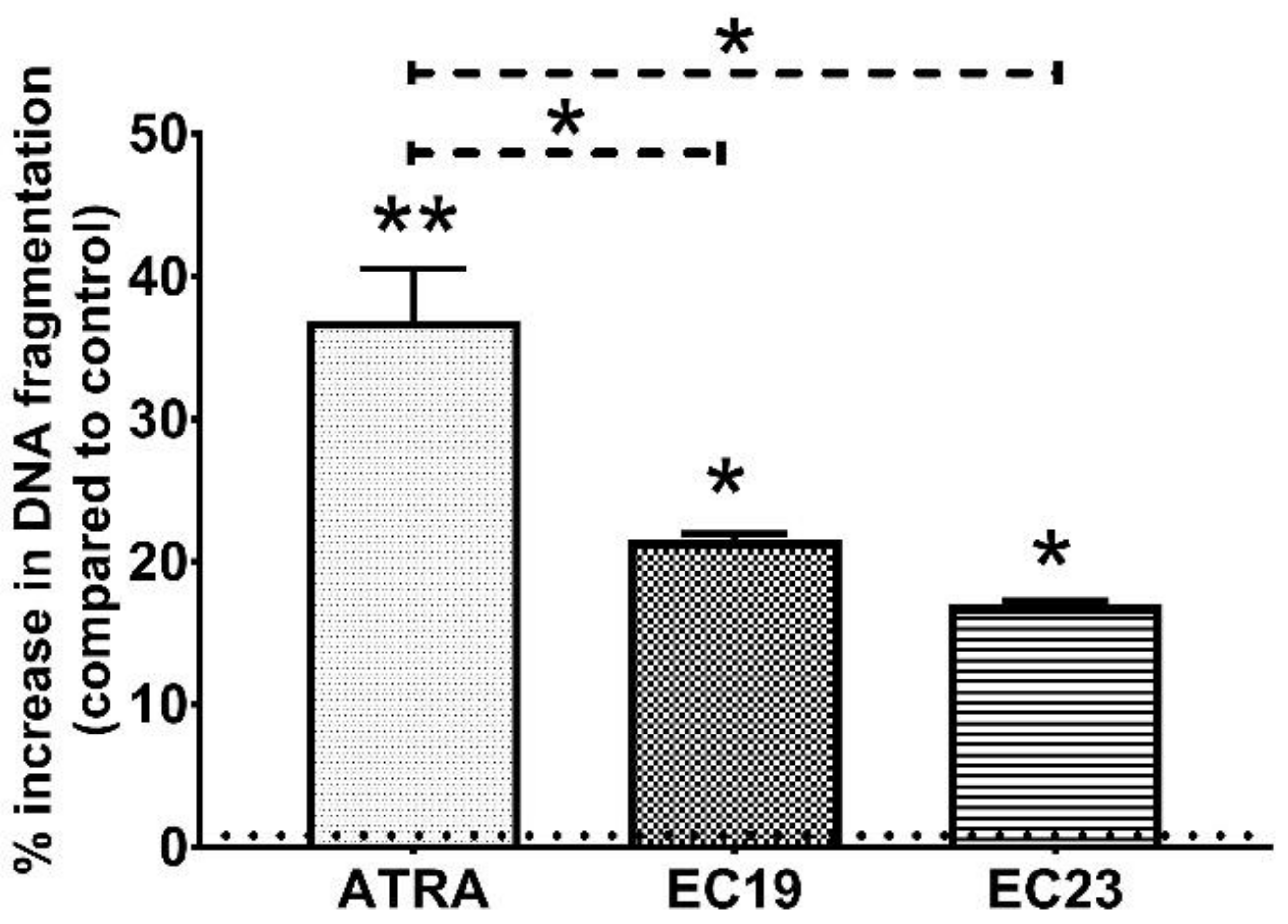
2.5. EC19 and EC23 Induce DNA Fragmentation and Genotoxicity in Caco-2 Cells
2.6. Confirmation of DNA Damage Effect of EC-Synthetic Retinoids Using Advanced Immunocytochemistry Analysis of Phosphorylated H2AX (HCS DNA Damage)
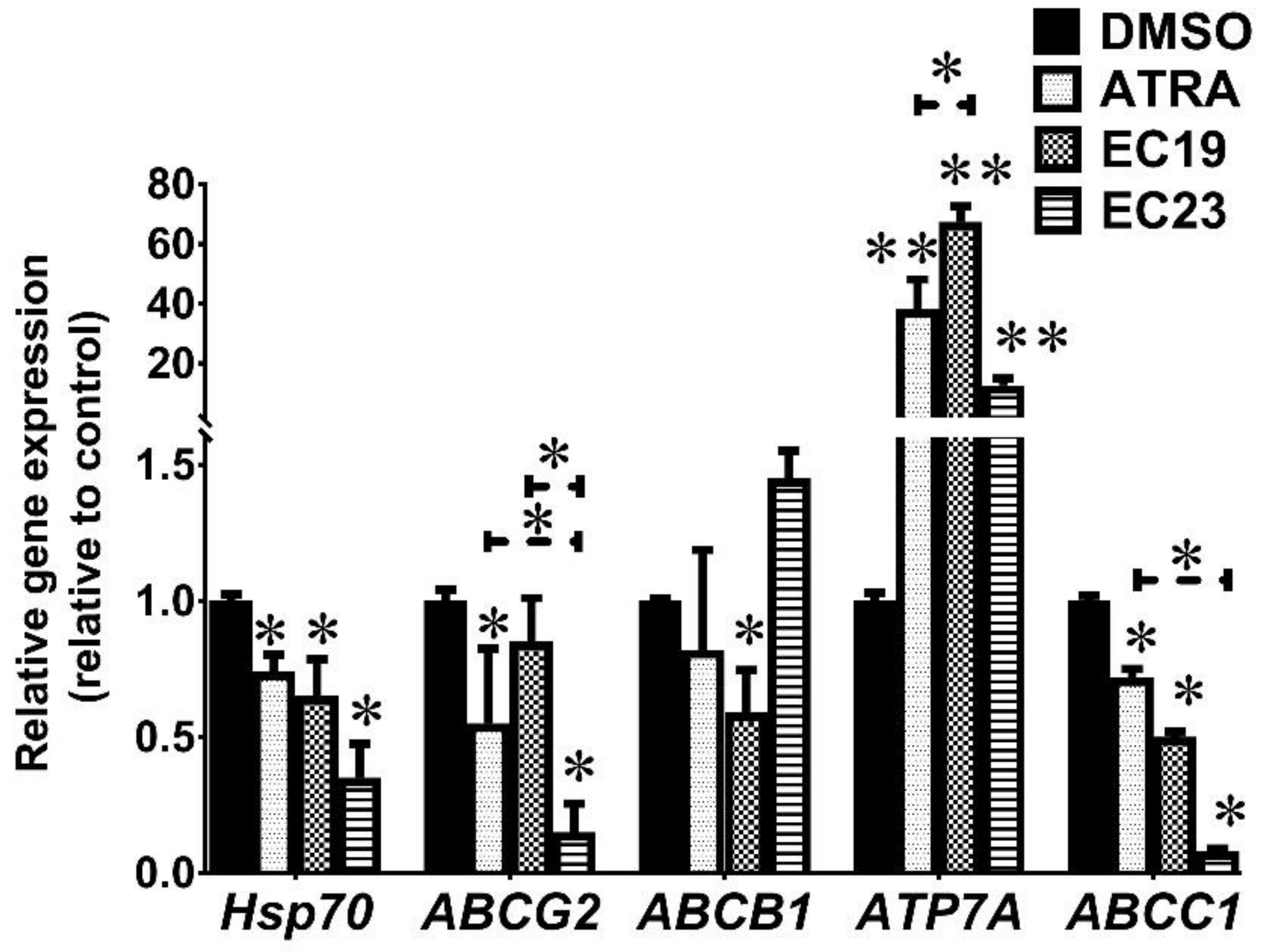
2.7. EC19 and EC23 Modulate the Chemoresistance-Related Genes and Proteins and Caco-2-Supporting ATPases Levels
3. Discussion
3.1. The Potential Application for Anti-Cancer Activity of EC-Synthetic Retinoids
3.2. RAR-Dependent Activity of EC-Synthetic Retinoids
3.3. EC-Synthetic Retinoids Induce Cellular Apoptosis, Necrosis, and Cell Cycle Arrest Individually and in Synergistic Combinations
3.4. EC-Synthetic Retinoids like Other Retinoids May Mediate Their Activities through Additional RAR-Independent Pathways
3.4.1. EC-Synthetic Retinoids Reduce ATPase Activity
3.4.2. EC-Synthetic Retinoids Induce DNA Fragmentation in Caco-2 Cells
3.4.3. EC-Synthetic Retinoids Modulate the Chemoresistance-Related Genes and Proteins
4. Materials and Methods
4.1. Retinoids and Chemical Reagents
4.2. Cell Culture
4.3. Antiproliferative Activities of Single Synthetic Retinoids and in Combination
4.4. Flow Cytometry Analysis of the Combinatorial Effect of RAR-Agonists and EC-Synthetic Retinoids
4.5. Assessment of the Pro-Apoptotic and Pro-Necrotic Activity
4.6. Measurement of Calcium-Independent ATPase Activity
4.7. DNA Fragmentation Assay
4.8. Immunocytochemistry Analysis of DNA Damage Using HCS DNA Damage
4.9. Gene Expression Analysis Using RT-qPCR
4.10. Western Blotting Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2020, e1291. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Rawal, R.M. Genetic and epigenetic modulation of drug resistance in cancer: Challenges and opportunities. Curr. Drug Metab. 2019, 20, 1114–1131. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, E.; Mora, A.; La Sala, D.; Roberti, A.; Zaki, N.; Badidi, E.; Taranta, M.; Cinti, C. Separate and combined effects of DNMT and HDAC inhibitors in treating human multi-drug resistant osteosarcoma HosDXR150 cell line. PLoS ONE 2014, 9, e95596. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559–84571. [Google Scholar] [CrossRef]
- Chène, P. The ATPases: A new family for a family-based drug design approach. Expert Opin. Ther. Targets 2003, 7, 453–461. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Nozaki, S.; Kitahara, H.; Ohara, T.; Kato, K.; Kawashiri, S.; Yamamoto, E. Copper efflux transporter (ATP7B) contributes to the acquisition of cisplatin-resistance in human oral squamous cell lines. Oncol. Rep. 2007, 18, 987–991. [Google Scholar] [CrossRef]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. The human ATP-binding cassette transporter genes: From the bench to the bedside. Curr. Mol. Med. 2001, 1, 45–65. [Google Scholar] [CrossRef]
- Lehne, G. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr. Drug Targets 2000, 1, 85–99. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Cheung, B.B.; Marshall, G.M. Targeting ATP7A to increase the sensitivity of neuroblastoma cells to retinoid therapy. Curr. Cancer Drug Targets 2011, 11, 826–836. [Google Scholar] [CrossRef]
- Theile, D.; Wizgall, P. Acquired ABC-transporter overexpression in cancer cells: Transcriptional induction or Darwinian selection? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1621–1632. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-relevant ABC transporter for anti-cancer drug resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef] [PubMed]
- Darby, R.A.; Callaghan, R.; McMahon, R.M. P-glycoprotein inhibition: The past, the present and the future. Curr. Drug Metab. 2011, 12, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Kordestani, L.; Basseville, A.; Kurdziel, K.; Fojo, A.T.; Bates, S.E. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updat. 2012, 15, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Kurlandsky, S.B.; Gamble, M.V.; Ramakrishnan, R.; Blaner, W.S. Plasma delivery of retinoic acid to tissues in the rat. J. Biol. Chem. 1995, 270, 17850–17857. [Google Scholar] [CrossRef]
- Yu, V.C.; Delsert, C.; Andersen, B.; Holloway, J.M.; Devary, O.V.; Näär, A.M.; Kim, S.Y.; Boutin, J.M.; Glass, C.K.; Rosenfeld, M.G. RXR beta: A coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell 1991, 67, 1251–1266. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Umesono, K.; Noonan, D.J.; Heyman, R.A.; Evans, R.M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 1992, 358, 771–774. [Google Scholar] [CrossRef]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef]
- Marill, J.; Idres, N.; Capron, C.C.; Nguyen, E.; Chabot, G.G. Retinoic acid metabolism and mechanism of action: A review. Curr. Drug Metab. 2003, 4, 10. [Google Scholar] [CrossRef]
- Soprano, D.R.; Harnish, D.C.; Soprano, K.J.; Kochhar, D.M.; Jiang, H. Correlations of RAR isoforms and cellular retinoid-binding proteins mRNA levels with retinoid-induced teratogenesis. J. Nutr. 1993, 123, 367–371. [Google Scholar] [CrossRef]
- Nagpal, I.; Wei, L.-N. All-trans retinoic acid as a versatile cytosolic signal modulator mediated by CRABP1. Int. J. Mol. Sci. 2019, 20, 3610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wongsiriroj, N.; Blaner, W.S. The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surgery Nutr. 2014, 3, 126–139. [Google Scholar] [CrossRef]
- Butsri, S.; Kukongviriyapan, V.; Senggunprai, L.; Kongpetch, S.; Prawan, A. All-trans-retinoic acid induces RARB-dependent apoptosis via ROS induction and enhances cisplatin sensitivity by NRF2 downregulation in cholangiocarcinoma cells. Oncol. Lett. 2022, 23, 17. [Google Scholar] [CrossRef]
- De Vos, S.; Koeffler, H.P. Differentiation Induction in Leukemia and Lymphoma. Nutr. Oncol. 2006, 491–506. [Google Scholar] [CrossRef]
- Tarapcsák, S.; Szalóki, G.; Telbisz, Á.; Gyöngy, Z.; Matúz, K.; Csősz, É.; Nagy, P.; Holb, I.J.; Rühl, R.; Nagy, L.; et al. Interactions of retinoids with the ABC transporters P-glycoprotein and Breast Cancer Resistance Protein. Sci. Rep. 2017, 7, 41376. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, Y.; Zhu, D.; Yang, W.; Wang, W.; Hu, Y.; Yang, B.; He, Q. Nutlin-1 strengthened anti-proliferation and differentiation-inducing activity of ATRA in ATRA-treated p-glycoprotein deregulated human myelocytic leukemia cells. Investig. New Drugs 2012, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, G.; Collins, M.D.; Bürgin, H.; Hummler, H.; Nau, H. Embryotoxic doses of vitamin A to rabbits result in low plasma but high embryonic concentrations of all-trans-retinoic acid: Risk of vitamin A exposure in humans. J. Nutr. 1996, 126, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Audette, M.C.; Petropoulos, S.; Gibb, W.; Matthews, S.G. Placental drug transporters and their role in fetal protection. Placenta 2012, 33, 137–142. [Google Scholar] [CrossRef]
- Januchowski, R.; Wojtowicz, K.; Sterzyfska, K.; Sosifska, P.; Andrzejewska, M.; Zawierucha, P.; Nowicki, M.; Zabel, M. Inhibition of ALDH1A1 activity decreases expression of drug transporters and reduces chemotherapy resistance in ovarian cancer cell lines. Int. J. Biochem. Cell Biol. 2016, 78, 248–259. [Google Scholar] [CrossRef]
- Gerrard, G.; Payne, E.; Baker, R.J.; Jones, D.T.; Potter, M.; Prentice, H.G.; Ethell, M.; McCullough, H.; Burgess, M.; Mehta, A.B.; et al. Clinical effects and P-glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine. Haematologica 2004, 89, 782–790. [Google Scholar]
- Ito, F.; Miura, M.; Fujioka, Y.; Abumiya, M.; Kobayashi, T.; Takahashi, S.; Yoshioka, T.; Kameoka, Y.; Takahashi, N. The BCRP inhibitor febuxostat enhances the effect of nilotinib by regulation of intracellular concentration. Int. J. Hematol. 2021, 113, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.R.; Haffez, H. The potential roles of retinoids in combating drug resistance in cancer: Implications of ATP-binding cassette (ABC) transporters. Open Biol. 2022, 12, 220001. [Google Scholar] [CrossRef] [PubMed]
- Christie, V.B.; Barnard, J.H.; Batsanov, A.S.; Bridgens, C.E.; Cartmell, E.B.; Collings, J.C.; Maltman, D.J.; Redfern, C.P.F.; Marder, T.B.; Przyborski, S.; et al. Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation. Org. Biomol. Chem. 2008, 6, 3497–3507. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.R.; Soror, S.H.; Elnagar, M.R.; Haffez, H. Revealing the Potential Application of EC-Synthetic Retinoid Analogues in Anticancer Therapy. Molecules 2021, 26, 506. [Google Scholar] [CrossRef] [PubMed]
- Haffez, H.; Khatib, T.; McCaffery, P.; Przyborski, S.; Redfern, C.; Whiting, A. Neurogenesis in Response to Synthetic Retinoids at Different Temporal Scales. Mol. Neurobiol. 2018, 55, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Haffez, H.; Chisholm, D.R.; Valentine, R.; Pohl, E.; Redfern, C.; Whiting, A. The molecular basis of the interactions between synthetic retinoic acid analogues and the retinoic acid receptors. MedChemComm 2017, 8, 578–592. [Google Scholar] [CrossRef]
- Jumarie, C.; Malo, C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J. Cell Physiol. 1991, 149, 24–33. [Google Scholar] [CrossRef]
- Suruga, K.; Mochizuki, K.; Suzuki, R.; Goda, T.; Takase, S. Regulation of cellular retinol-binding protein type II gene expression by arachidonic acid analogue and 9-cis retinoic acid in caco-2 cells. Eur. J. Biochem. 1999, 262, 70–78. [Google Scholar] [CrossRef]
- Levin, M.S. Cellular retinol-binding proteins are determinants of retinol uptake and metabolism in stably transfected Caco-2 cells. J. Biol. Chem. 1993, 268, 8267–8276. [Google Scholar] [CrossRef]
- Lee, M.O.; Han, S.Y.; Jiang, S.; Park, J.H.; Kim, S.J. Differential effects of retinoic acid on growth and apoptosis in human colon cancer cell lines associated with the induction of retinoic acid receptor beta. Biochem. Pharmacol. 2000, 59, 485–496. [Google Scholar] [CrossRef]
- Lopez, E.; Figueroa, S.; Oset-Gasque, M.; Gonzalez, M. Apoptosis and necrosis: Two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol. 2003, 138, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Preethy, C.P.; Padmapriya, R.; Periasamy, V.S.; Riyasdeen, A.; Srinag, S.; Krishnamurthy, H.; Alshatwi, A.A.; Akbarsha, M.A. Antiproliferative property of n-hexane and chloroform extracts of Anisomeles malabarica (L). R. Br. in HPV16-positive human cervical cancer cells. J. Pharmacol. Pharmacother. 2012, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Burton, K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956, 62, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.K.; Gercel-Taylor, C. Use of diphenylamine in the detection of apoptosis. Methods Mol. Med. 2001, 39, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.K.; Taylor, D.D.; Wan, T.; O’Connor, D.M.; Doering, D.L.; Gerçel-Taylor, C. Apoptosis as a measure of chemosensitivity to cisplatin and taxol therapy in ovarian cancer cell lines. Gynecol. Oncol. 1997, 65, 13–22. [Google Scholar] [CrossRef]
- Lund, B.W.; Piu, F.; Gauthier, N.K.; Eeg, A.; Currier, E.; Sherbukhin, V.; Brann, M.R.; Hacksell, U.; Olsson, R. Discovery of a potent, orally available, and isoform-selective retinoic acid beta2 receptor agonist. J. Med. Chem. 2005, 48, 7517–7519. [Google Scholar] [CrossRef]
- Zhuang, Y.; Faria, T.N.; Chambon, P.; Gudas, L.J. Identification and characterization of retinoic acid receptor beta2 target genes in F9 teratocarcinoma cells. Mol. Cancer Res. 2003, 1, 619–630. [Google Scholar]
- Rogers, R.F.; Walton, M.I.; Cherry, D.L.; Collins, I.; Clarke, P.A.; Garrett, M.D.; Workman, P. CHK1 Inhibition Is Synthetically Lethal with Loss of B-Family DNA Polymerase Function in Human Lung and Colorectal Cancer Cells. Cancer Res. 2020, 80, 1735–1747. [Google Scholar] [CrossRef]
- Bengtsson, A.M.; Jönsson, G.; Magnusson, C.; Salim, T.; Axelsson, C.; Sjölander, A. The cysteinyl leukotriene 2 receptor contributes to all-transretinoic acid-induced differentiation of colon cancer cells. BMC Cancer 2013, 13, 336. [Google Scholar] [CrossRef]
- McCormack, S.A.; Viar, M.J.; Tague, L.; Johnson, L.R. Altered distribution of the nuclear receptor RARβ accompanies proliferation and differentiation changes caused by retinoic acid in Caco-2 cells. In Vitr. Cell. Dev. Biol. Anim. 1996, 32, 53–61. [Google Scholar] [CrossRef]
- Szondy, Z.; Reichert, U.; Bernardon, J.M.; Michel, S.; Tóth, R.; Ancian, P.; Ajzner, E.; Fesus, L. Induction of apoptosis by retinoids and retinoic acid receptor gamma-selective compounds in mouse thymocytes through a novel apoptosis pathway. Mol. Pharmacol. 1997, 51, 972–982. [Google Scholar] [CrossRef] [PubMed]
- le Maire, A.; Teyssier, C.; Balaguer, P.; Bourguet, W.; Germain, P. Regulation of RXR-RAR Heterodimers by RXR- and RAR-Specific Ligands and Their Combinations. Cells 2019, 8, 1392. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gu, X.; Zhang, Z.; Li, W.; Wang, X. Retinoic acid receptor gamma is targeted by microRNA-124 and inhibits neurite outgrowth. Neuropharmacology 2020, 163, 107657. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Goralski, M.; Capota, E.; Padrick, S.B.; Kim, J.; Xie, Y.; Nijhawan, D. The antitumor toxin CD437 is a direct inhibitor of DNA polymerase α. Nat. Chem. Biol. 2016, 12, 511–515. [Google Scholar] [CrossRef]
- Abdel-Samad, R.; Aouad, P.; Gali-Muhtasib, H.; Sweidan, Z.; Hmadi, R.; Kadara, H.; D’Andrea, E.L.; Fucci, A.; Pisano, C.; Darwiche, N. Mechanism of action of the atypical retinoid ST1926 in colorectal cancer: DNA damage and DNA polymerase α. Am. J. Cancer Res. 2018, 8, 39. [Google Scholar]
- Wan, X.; Duncan, M.D.; Nass, P.; Harmon, J.W. Synthetic retinoid CD437 induces apoptosis of esophageal squamous HET-1A cells through the caspase-3-dependent pathway. Anticancer Res. 2001, 21, 2657–2663. [Google Scholar]
- Czeczuga-Semeniuk, E.; Wołczyński, S.; Dabrowska, M.; Dziecioł, J.; Anchim, T. The effect of doxorubicin and retinoids on proliferation, necrosis and apoptosis in MCF-7 breast cancer cells. Folia Histochem. Cytobiol. 2004, 42, 221–227. [Google Scholar]
- Motomura, K.; Sakai, H.; Isobe, H.; Nawata, H. Effects of retinoids on the production of tumour necrosis factor-alpha and nitric oxide by lipopolysaccharide-stimulated rat Kupffer cells in vitro: Evidence for participation of retinoid X receptor signalling pathway. Cell Biochem. Funct. 1997, 15, 95–101. [Google Scholar] [CrossRef]
- Landecker, A.; Katayama, M.L.S.; Mammana, A.K.; Leitao, R.M.C.; Sachetta, T.; Gemperli, R.; Neves, R.I. Effects of retinoic and glycolic acids on neoangiogenesis and necrosis of axial dorsal skin flaps in rats. Aesthetic Plast. Surg. 2001, 25, 134–139. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Hur, G.M.; Kim, Y.-S.; Won, M.; Choksi, S.; Liu, Z.-G. The death domain kinase RIP has an important role in DNA damage-induced, p53-independent cell death. J. Biol. Chem. 2006, 281, 25011–25017. [Google Scholar] [CrossRef] [PubMed]
- Biton, S.; Ashkenazi, A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 2011, 145, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Y. Regulation of death receptors by synthetic retinoids. In Death Receptors in Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2005; pp. 189–200. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Cho, Y.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K.-M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- Kadigamuwa, C.; Choksi, S.; Xu, Q.; Cataisson, C.; Greenbaum, S.S.; Yuspa, S.H.; Liu, Z.-G. Role of retinoic acid Receptor-γ in DNA damage-induced necroptosis. Iscience 2019, 17, 74–86. [Google Scholar] [CrossRef]
- Zhang, M.L.; Tao, Y.; Zhou, W.Q.; Ma, P.C.; Cao, Y.P.; He, C.D.; Wei, J.; Li, L.J. All-trans retinoic acid induces cell-cycle arrest in human cutaneous squamous carcinoma cells by inhibiting the mitogen-activated protein kinase–activated protein 1 pathway. Clin. Exp. Dermatol. 2014, 39, 354–360. [Google Scholar] [CrossRef]
- Hsu, C.A.; Rishi, A.K.; Su-Li, X.; Gerald, T.M.; Dawson, M.I.; Schiffer, C.; Reichert, U.; Shroot, B.; Poirer, G.C.; Fontana, J.A. Retinoid induced apoptosis in leukemia cells through a retinoic acid nuclear receptor-independent pathway. Blood 1997, 89, 4470–4479. [Google Scholar] [CrossRef]
- Lin, E.; Chen, M.-C.; Huang, C.-Y.; Hsu, S.-L.; Huang, W.J.; Lin, M.-S.; Wu, J.C.-H.; Lin, H. All-trans retinoic acid induces DU145 cell cycle arrest through Cdk5 activation. Cell. Physiol. Biochem. 2014, 33, 1620–1630. [Google Scholar] [CrossRef]
- Hsu, S.-L.; Hsu, J.-W.; Liu, M.-C.; Chen, L.-Y.; Chang, C.-D. Retinoic acid-mediated G1 arrest is associated with induction of p27kip1 and inhibition of cyclin-dependent kinase 3 in human lung squamous carcinoma CH27 cells. Exp. Cell Res. 2000, 258, 322–331. [Google Scholar] [CrossRef]
- Petrie, K.; Urban-Wójciuk, Z.; Sbirkov, Y.; Graham, A.; Hamann, A.; Brown, G. Retinoic acid receptor γ is a therapeutically targetable driver of growth and survival in prostate cancer. Cancer Rep. 2020, 3, e1284. [Google Scholar] [CrossRef] [PubMed]
- El-Metwally, T.H.; Hussein, M.R.; Pour, P.M.; Kuszynksi, C.A.; Adrian, T.E. Natural retinoids inhibit proliferation and induce apoptosis in pancreatic cancer cells previously reported to be retinoid resistant. Cancer Biol. Ther. 2005, 4, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.; Brodie, A.; Njar, V. Inhibitory effects of retinoic acid metabolism blocking agents (RAMBAs) on the growth of human prostate cancer cells and LNCaP prostate tumour xenografts in SCID mice. Br. J. Cancer 2006, 94, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Koay, D.C.; Zerillo, C.; Narayan, M.; Harris, L.N.; DiGiovanna, M.P. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: Induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Res. 2010, 12, 19. [Google Scholar] [CrossRef]
- Emionite, L.; Galmozzi, F.; Grattarola, M.; Boccardo, F.; Vergani, L.; Toma, S. Histone deacetylase inhibitors enhance retinoid response in human breast cancer cell lines. Anticancer Res. 2004, 24, 4019–4024. [Google Scholar] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Dickinson, J.L.; Holloway, A.F.; Woods, G.M. Fucoidan enhances the therapeutic potential of arsenic trioxide and all-trans retinoic acid in acute promyelocytic leukemia, in vitro and in vivo. Oncotarget 2016, 7, 46028. [Google Scholar] [CrossRef]
- Telford, W.G.; Komoriya, A.; Packard, B.Z. Detection of localized caspase activity in early apoptotic cells by laser scanning cytometry. Cytometry 2002, 47, 81–88. [Google Scholar] [CrossRef]
- Krysko, D.V.; Kaczmarek, A.; Krysko, O.; Heyndrickx, L.; Woznicki, J.; Bogaert, P.; Cauwels, A.; Takahashi, N.; Magez, S.; Bachert, C. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell. Death Differ. 2011, 18, 1316–1325. [Google Scholar] [CrossRef]
- Cai, B.; Liao, C.; He, D.; Chen, J.; Han, J.; Lu, J.; Qin, K.; Liang, W.; Wu, X.; Liu, Z. Gasdermin E mediates photoreceptor damage by all-trans-retinal in the mouse retina. J. Biol. Chem. 2022, 298–314, 101553. [Google Scholar] [CrossRef]
- Estfanous, S.; Krause, K.; Anne, M.N.; Eltobgy, M.; Caution, K.; Abu Khweek, A.; Hamilton, K.; Badr, A.; Daily, K.; Carafice, C. Gasdermin D restricts Burkholderia cenocepacia infection in vitro and in vivo. Sci. Rep. 2021, 11, 20. [Google Scholar] [CrossRef]
- Sun, S.Y.; Yue, P.; Mao, L.; Dawson, M.I.; Shroot, B.; Lamph, W.W.; Heyman, R.A.; Chandraratna, R.A.; Shudo, K.; Hong, W.K.; et al. Identification of receptor-selective retinoids that are potent inhibitors of the growth of human head and neck squamous cell carcinoma cells. Clin. Cancer. Res. 2000, 6, 1563–1573. [Google Scholar] [PubMed]
- Sun, S.Y.; Yue, P.; Chandraratna, R.A.; Tesfaigzi, Y.; Hong, W.K.; Lotan, R. Dual mechanisms of action of the retinoid CD437: Nuclear retinoic acid receptor-mediated suppression of squamous differentiation and receptor-independent induction of apoptosis in UMSCC22B human head and neck squamous cell carcinoma cells. Mol. Pharmacol. 2000, 58, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Dallavalle, S.; Merlini, L.; Penco, S.; Pisano, C.; Carminati, P.; Giannini, G.; Vesci, L.; Gaetano, C.; Illy, B.; et al. A novel atypical retinoid endowed with proapoptotic and antitumor activity. J. Med. Chem. 2003, 46, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.M.; Yanase, T.; Nishi, Y.; Hirase, N.; Goto, K.; Takayanagi, R.; Nawata, H. A nuclear receptor system constituted by RAR and RXR induces aromatase activity in MCF-7 human breast cancer cells. Mol. Cell Endocrinol. 2000, 166, 137–145. [Google Scholar] [CrossRef]
- Xie, L.; Collins, J.F. Transcription factors Sp1 and Hif2α mediate induction of the copper-transporting ATPase (Atp7a) gene in intestinal epithelial cells during hypoxia. J. Biol. Chem. 2013, 288, 23943–23952. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Tanaka, T.; Kurokawa, H.; Matsuno, K.; Hayashida, Y.; Takahashi, T. Effect of copper and role of the copper transporters ATP7A and CTR1 in intracellular accumulation of cisplatin. Anticancer Res. 2007, 27, 2209–2216. [Google Scholar] [PubMed]
- Nyasae, L.; Bustos, R.; Braiterman, L.; Eipper, B.; Hubbard, A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: Copper-dependent redistribution between two intracellular sites. Am. J. Physiol. Gastroint. Liver Physiol. 2007, 292, G1181–G1194. [Google Scholar] [CrossRef]
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB J. 2021, 35, e21810. [Google Scholar] [CrossRef]
- Nagaraja, G.M.; Othman, M.; Fox, B.P.; Alsaber, R.; Pellegrino, C.M.; Zeng, Y.; Khanna, R.; Tamburini, P.; Swaroop, A.; Kandpal, R.P. Gene expression signatures and biomarkers of noninvasive and invasive breast cancer cells: Comprehensive profiles by representational difference analysis, microarrays and proteomics. Oncogene 2006, 25, 2328–2338. [Google Scholar] [CrossRef]
- Crnogorac-Jurcevic, T.; Gangeswaran, R.; Bhakta, V.; Capurso, G.; Lattimore, S.; Akada, M.; Sunamura, M.; Prime, W.; Campbell, F.; Brentnall, T.A.; et al. Proteomic analysis of chronic pancreatitis and pancreatic adenocarcinoma. Gastroenterology 2005, 129, 1454–1463. [Google Scholar] [CrossRef]
- Owatari, S.; Akune, S.; Komatsu, M.; Ikeda, R.; Firth, S.D.; Che, X.F.; Yamamoto, M.; Tsujikawa, K.; Kitazono, M.; Ishizawa, T.; et al. Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res. 2007, 67, 4860–4868. [Google Scholar] [CrossRef] [PubMed]
- Bohlken, A.; Cheung, B.B.; Bell, J.L.; Koach, J.; Smith, S.; Sekyere, E.; Thomas, W.; Norris, M.; Haber, M.; Lovejoy, D.B.; et al. ATP7A is a novel target of retinoic acid receptor beta2 in neuroblastoma cells. Br. J. Cancer 2009, 100, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Pratt, R.M. Influence of retinoids on sister chromatid exchanges and chromosomes in cultured human embryonic palatal mesenchymal cells. Teratog. Carcinog. Mutagen. 1991, 11, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hudhud, L.; Chisholm, D.R.; Whiting, A.; Steib, A.; Pohóczky, K.; Kecskés, A.; Szőke, É.; Helyes, Z. Synthetic Diphenylacetylene-Based Retinoids Induce DNA Damage in Chinese Hamster Ovary Cells without Altering Viability. Molecules 2022, 27, 977. [Google Scholar] [CrossRef] [PubMed]
- Gercel-Taylor, C. Diphenylamine assay of DNA fragmentation for chemosensitivity testing. In Methods in Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, pp. 79–82. [Google Scholar] [CrossRef]
- Zuco, V.; Zanchi, C.; Lanzi, C.; Beretta, G.L.; Supino, R.; Pisano, C.; Barbarino, M.; Zanier, R.; Bucci, F.; Aulicino, C. Development of resistance to the atypical retinoid, ST1926, in the lung carcinoma cell line H460 is associated with reduced formation of DNA strand breaks and a defective DNA damage response. Neoplasia 2005, 7, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Goda, K.; Bacsó, Z.; Szabó, G. Multidrug resistance through the spectacle of P-glycoprotein. Curr. Cancer Drug Targets 2009, 9, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Mark, M.E.; Cai, X.; Mao, Q. Fluorescence resonance energy transfer (FRET) analysis demonstrates dimer/oligomer formation of the human breast cancer resistance protein (BCRP/ABCG2) in intact cells. Int. J. Biochem. Mol. Biol. 2010, 1, 11. [Google Scholar]
- Hamada, H.; Tsuruo, T. Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1986, 83, 7785–7789. [Google Scholar] [CrossRef]
- König, J.; Müller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef]
- Zakeri-Milani, P.; Valizadeh, H. Intestinal transporters: Enhanced absorption through P-glycoprotein-related drug interactions. Expert Opin. Drug Metab. Toxicol. 2014, 10, 859–871. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.D.; Strong, J.M.; Reynolds, K.S.; Huang, S.M. A regulatory viewpoint on transporter-based drug interactions. Xenobiotica 2008, 38, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Jouan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay. Pharmaceutics 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.T.; Shi, X.J.; Yuan, Y.X.; Qiu, Y.Y.; Zou, Y.; Liu, C.; Yu, H.; He, X.; Xu, K.; Yin, P.H. Bufalin reverses ABCB1-mediated drug resistance in colorectal cancer. Oncotarget 2017, 8, 48012–48026. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.K.; Zhang, G.N.; Al Rihani, S.B.; Wei, M.N.; Gupta, P.; Zhang, X.Y.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study. Cancer Lett. 2017, 396, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.L.; Amory, J.K.; Walsh, T.J.; Isoherranen, N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J. Lipid Res. 2012, 53, 587–598. [Google Scholar] [CrossRef]
- Wise, E.M.; Graber, E.M. Clinical pearl: Comedone extraction for persistent macrocomedones while on isotretinoin therapy. J. Clin. Aesthet. Dermatol. 2011, 4, 20–21. [Google Scholar]
- Skeel, R.T.; Huang, J.; Manola, J.; Wilding, G.; Dreicer, R.; Walker, P.; Muggia, F.; Crawford, E.D.; Dutcher, J.P.; Loehrer, P.J. A phase II study of 13-cis retinoic acid plus interferon alpha-2a in advanced stage penile carcinoma: An Eastern Cooperative Oncology Group study (E3893). Cancer Investig. 2003, 21, 41–46. [Google Scholar] [CrossRef]
- Veal, G.J.; Errington, J.; Rowbotham, S.E.; Illingworth, N.A.; Malik, G.; Cole, M.; Daly, A.K.; Pearson, A.D.; Boddy, A.V. Adaptive dosing approaches to the individualization of 13-cis-retinoic acid (isotretinoin) treatment for children with high-risk neuroblastoma. Clin. Cancer Res. 2013, 19, 469–479. [Google Scholar] [CrossRef]
- Reynolds, C.P.; Matthay, K.K.; Villablanca, J.G.; Maurer, B.J. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003, 197, 185–192. [Google Scholar] [CrossRef]
- Cardin, G.B.; Mantha, M.; Jumarie, C. Resistance to cadmium as a function of Caco-2 cell differentiation: Role of reactive oxygen species in cadmium- but not zinc-induced adaptation mechanisms. BioMetals 2009, 22, 753–769. [Google Scholar] [CrossRef]
- Roufayel, R.; Kadry, S. Molecular Chaperone HSP70 and Key Regulators of Apoptosis—A Review. Curr. Mol. Med. 2019, 19, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stokes, J., 3rd; Singh, U.P.; Scissum Gunn, K.; Acharya, A.; Manne, U.; Mishra, M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Zahiri, E.; Ehtiati, S.; Norouzi, M.; Rahmani, F.; Fiuji, H.; Avan, A.; Ferns, G.A.; Khazaei, M.; Hashemy, S.I.; et al. Therapeutic potency of heat-shock protein-70 in the pathogenesis of colorectal cancer: Current status and perspectives. Biochem. Cell Biol. 2019, 97, 85–90. [Google Scholar] [CrossRef]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef]
- Li, J.; Sun, L.; Xu, C.; Yu, F.; Zhou, H.; Zhao, Y.; Zhang, J.; Cai, J.; Mao, C.; Tang, L.; et al. Structure insights into mechanisms of ATP hydrolysis and the activation of human heat-shock protein 90. Acta Biochim. Biophys. Sin. 2012, 44, 300–306. [Google Scholar] [CrossRef]
- Verkhivker, G.M. Dynamics-based community analysis and perturbation response scanning of allosteric interaction networks in the TRAP1 chaperone structures dissect molecular linkage between conformational asymmetry and sequential ATP hydrolysis. Biochim. Biophys. Acta. Proteins Proteom. 2018, 1866, 899–912. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Boudesco, C.; Cause, S.; Jego, G.; Garrido, C. Hsp70: A Cancer Target Inside and Outside the Cell. In Chaperones. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1709, pp. 371–396. [Google Scholar] [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Gilliams-Francis, K.L.; Quaye, A.A.; Naegele, J.R. PARP cleavage, DNA fragmentation, and pyknosis during excitotoxin-induced neuronal death. Exp. Neurol. 2003, 184, 359–372. [Google Scholar] [CrossRef]
- Renvoizé, C.; Biola, A.; Pallardy, M.; Bréard, J. Apoptosis: Identification of dying cells. Cell Biol. Toxicol. 1998, 14, 111–120. [Google Scholar] [CrossRef]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.I.; Harmon, B.V.; Gobé, G.C.; Kerr, J.F. Patterns of cell death. Methods Achiev. Exp. Pathol. 1988, 13, 18–54. [Google Scholar] [PubMed]
- Cohen, J.J. Apoptosis. Immunol. Today 1993, 14, 126–130. [Google Scholar] [CrossRef]
- Cohen, G.M.; Sun, X.M.; Snowden, R.T.; Ormerod, M.G.; Dinsdale, D. Identification of a transitional preapoptotic population of thymocytes. J. Immunol. 1993, 151, 566–574. [Google Scholar]
- Stewart, B.W. Mechanisms of apoptosis: Integration of genetic, biochemical, and cellular indicators. J. Natl. Cancer Inst. 1994, 86, 1286–1296. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Ou, X. Activity of fenretinide plus chemotherapeutic agents in small-cell lung cancer cell lines. Cancer Chemother. Pharmacol. 1999, 43, 145–150. [Google Scholar] [CrossRef]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Blasiak, J. All-Trans Retinoic Acid Modulates DNA Damage Response and the Expression of the VEGF-A and MKI67 Genes in ARPE-19 Cells Subjected to Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef]
- Barna, G.; Sebestyén, A.; Weischede, S.; Peták, I.; Mihalik, R.; Formelli, F.; Kopper, L. Different ways to induce apoptosis by fenretinide and all-trans-retinoic acid in human B lymphoma cells. Anticancer Res. 2005, 25, 4179–4185. [Google Scholar]
- Bailly, A.P.; Perrin, A.; Serrano-Macia, M.; Maghames, C.; Leidecker, O.; Trauchessec, H.; Martinez-Chantar, M.L.; Gartner, A.; Xirodimas, D.P. The Balance between Mono- and NEDD8-Chains Controlled by NEDP1 upon DNA Damage Is a Regulatory Module of the HSP70 ATPase Activity. Cell Rep. 2019, 29, 212–224.e8. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jiang, Y.; Chen, Y. Predicting drug combination index and simulating the network-regulation dynamics by mathematical modeling of drug-targeted EGFR-ERK signaling pathway. Sci. Rep. 2017, 7, 40752. [Google Scholar] [CrossRef] [PubMed]
- Wali, V.B.; Sylvester, P.W. Synergistic antiproliferative effects of gamma-tocotrienol and statin treatment on mammary tumor cells. Lipids 2007, 42, 1113–1123. [Google Scholar] [CrossRef]
- Erdem, S.S.; Obeidin, V.A.; Yigitbasi, T.; Tumer, S.S.; Yigit, P. Verteporfin mediated sequence dependent combination therapy against ovarian cancer cell line. J. Photochem. Photobiol. B 2018, 183, 266–274. [Google Scholar] [CrossRef]
- Chan, K.M.; Delfert, D.; Junger, K.D. A direct colorimetric assay for Ca2+ -stimulated ATPase activity. Anal. Biochem. 1986, 157, 375–380. [Google Scholar] [CrossRef]
- White, M.A.; Lin, W.; Cheng, X. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV-2 Nsp13 Helicase. J. Phys. Chem. Lett. 2020, 11, 9144–9151. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Willig, J.B.; Vianna, D.R.B.; Beckenkamp, A.; Beckenkamp, L.R.; Sévigny, J.; Wink, M.R.; Buffon, A.; Pilger, D.A. Imatinib mesylate affects extracellular ATP catabolism and expression of NTPDases in a chronic myeloid leukemia cell line. Purinergic Signal. 2020, 16, 29–40. [Google Scholar] [CrossRef]
- Leal, D.B.; Streher, C.A.; Neu, T.N.; Bittencourt, F.P.; Leal, C.A.; da Silva, J.E.; Morsch, V.M.; Schetinger, M.R. Characterization of NTPDase (NTPDase1; ecto-apyrase; ecto-diphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes. Biochim. Biophys. Acta 2005, 1721, 9–15. [Google Scholar] [CrossRef]
- Frankfurt, O.S.; Krishan, A. Enzyme-linked immunosorbent assay (ELISA) for the specific detection of apoptotic cells and its application to rapid drug screening. J. Immunol. Methods 2001, 253, 133–144. [Google Scholar] [CrossRef]
- Frankfurt, O.S.; Robb, J.A.; Sugarbaker, E.V.; Villa, L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp. Cell Res. 1996, 226, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Frankfurt, O.S.; Krishan, A. Identification of apoptotic cells by formamide-induced dna denaturation in condensed chromatin. J. Histochem. Cytochem. 2001, 49, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Drug(s) (Single/Combinations) | # IC50 (µM) ± SEM (Single) | # IC50 (µM) ± SEM (Combination) | ×IC50 (µM) (Combination) | Individual * IC50 (µM) (within the Combination) | DRI | CI | |
|---|---|---|---|---|---|---|---|
| Retinoid (A) | Retinoid (B) | ||||||
| Individual Retinoids | |||||||
| ATRA | 97.70 ± 9.0 | ----- | ----- | ----- | ----- | ----- | ----- |
| EC19 | 27.20 ± 1.8 | ----- | ----- | ----- | ----- | ----- | ----- |
| EC23 | 23.00 ± 1.2 | ----- | ----- | ----- | ----- | ----- | ----- |
| CD437 | 2.80 ± 0.7 | ----- | ----- | ----- | ----- | ----- | ----- |
| AC261066 | 26.90 ± 2.1 | ----- | ----- | ----- | ----- | ----- | ----- |
| CD2665 | 27.90 ± 3.1 | ----- | ----- | ----- | ----- | ----- | ----- |
| Agonists combinations | |||||||
| ATRA (A) and CD437 (B) | ----- | 5.8 ± 1.2 ns | 2.07 | 202.23 | 5.80 | 0.48 | 4.1 |
| EC19 (A) and CD437 (B) | ----- | <0.1 *** | 0.04 | 1.09 | 0.11 | 24.95 | 0.1 |
| EC23 (A) and CD437 (B) | ----- | 0.82 ± 0.1 ** | 0.29 | 6.67 | 0.81 | 3.45 | 0.6 |
| ATRA (A) and AC261066 (B) | ----- | 47.90 ± 7.0 ns | 1.78 | 173.9 | 47.89 | 0.56 | 3.6 |
| EC19 (A) and AC261066 (B) | ----- | 1.37 ± 0.3 *** | 0.05 | 4.89 | 1.34 | 5.56 | 0.2 |
| EC23 (A) and AC261066 (B) | ----- | 1.76 ± 0.4 *** | 0.07 | 6.84 | 1.88 | 3.36 | 0.4 |
| Antagonist combination | |||||||
| CD2665 (A) and ATRA (B) | ----- | 1.70 ± 0.3 *** | 0.06 | 1.67 | 5.86 | 16.67 | 0.12 |
| CD2665 (A) and EC19 (B) | ----- | 24.28 ± 2.5 ns | 0.87 | 24.27 | 23.66 | 1.14 | 1.74 |
| CD2665 (A) and EC23 (B) | ----- | 25.80 ± 4.2 ns | 0.92 | 25.67 | 21.16 | 1.1 | 1.84 |
| CD2665 (A) then ATRA (B) | ----- | 61.4 ± 6.6 ns | 2.201 | 61.41 | 215.04 | 0.45 | 8.3 |
| CD2665 (A) then EC19 (B) | ----- | 2.97 ± 0.8 *** | 0.106 | 2.96 | 2.88 | 9.44 | 0.2 |
| CD2665 (A) then EC23 (B) | ----- | 4.27 ± 0.3 *** | 0.153 | 4.27 | 3.52 | 6.53 | 0.3 |
| Retinoids (Individual and Combinations) | Apoptosis Analysis of Caco-2 Cell Line # | |||
|---|---|---|---|---|
| % Viable Cells (LL) | % Early Apoptotic Cells (UL) | % Late Apoptotic Cells (LR) | % Necrotic Cells (UR) | |
| Control | 98.11 ± 5.6 | 0.00 | 1.87 ± 0.6 | 0.02 |
| ATRA | 67.21 ± 4.5 * | 27.40 ± 1.6 *** | 1.27 ± 0.6 | 4.12 ± 0.7 *** |
| EC19 | 64.80 ± 3.5 * | 11.04 ± 2.1 *** | 5.06 ± 1.2 * | 19.10 ± 2.0 *** |
| EC23 | 76.00 ± 4.8 * | 15.76 ± 2.5 *** | 1.77 ± 0.9 | 6.47 ± 1.3 *** |
| CD437 | 62.23± 5.2 * | 17.42 ± 1.4 *** | 7.51 ± 0.46 ** | 12.84 ± 1.1 *** |
| AC261066 | 67.21 ± 4.7 * | 27.40 ± 2.8 *** | 1.27 ± 0.91 | 4.12 ± 1.9 *** |
| ATRA + CD437 | 39.86 ± 2.5 ** | 43.33 ± 3.6 *** | 3.44 ± 1.0 * | 13.37 ± 1.3 *** |
| EC19 + CD437 | 36.68 ± 1.9 ** | 48.15 ± 4.1 *** | 3.41 ± 1.1 * | 11.76 ± 4.1 *** |
| EC23 + CD437 | 41.17 ± 2.2 ** | 41.57 ± 2.9 *** | 4.33 ± 1.4 * | 12.93 ± 2.2 *** |
| ATRA+ AC261066 | 31.59 ± 1.5 ** | 55.39 ± 3.5 *** | 2.38 ± 1.1 * | 10.64 ± 1.6 *** |
| EC19 + AC261066 | 20.01 ± 2.1 *** | 63.43 ± 5.3 *** | 0.91 ± 0.3 | 15.65 ± 2.5 *** |
| EC23 + AC261066 | 33.91 ± 1.6 ** | 52.21 ± 5.2 *** | 3.29 ± 1.3 * | 10.59 ± 1.7 *** |
| Retinoids (Individual and Combinations) | Cell Cycle Analysis of Caco-2 Cell Line # | |||
|---|---|---|---|---|
| % SubG0-G1 | % G0-G1 | % S | % G2M | |
| Control | 0.09 | 53.46 ± 3.9 | 13.01 ± 1.3 | 33.07 ± 2.2 |
| ATRA | 0.00 | 0.67 *** | 4.25 ± 3.2 *** | 94.73 ± 5.4 *** |
| EC19 | 0.00 | 8.32 ± 1.4 *** | 20.15 ± 2.2 * | 70.86 ± 6.1 *** |
| EC23 | 0.00 | 3.28 ± 0.9 *** | 7.58 ± 3.9 * | 88.86 ± 5.4 *** |
| CD437 | 0.00 | 3.78 ± 0.9 *** | 8.76 ± 0.9 * | 86.99 ± 0.9 *** |
| AC261066 | 0.00 | 2.20 ± 0.9 *** | 7.49 ± 0.9 * | 90.16 ± 0.9 *** |
| ATRA + CD437 | 11.75 ± 4.8 *** | 42.08 ± 4.2 * | 20.17 ± 3.5 * | 25.54 ± 3.7 |
| EC19 + CD437 | 22.54 ± 5.2 *** | 36.06 ± 2.1 * | 25.70 ± 2.4 * | 15.51 ± 2.9 * |
| EC23 + CD437 | 23.76 ± 3.4 *** | 51.60 ± 4.3 | 10.23 ± 1.7 | 14.34 ± 2.2 * |
| ATRA+ AC261066 | 18.63 ± 2.1 *** | 38.47 ± 2.2 * | 23.53 ± 2.9 * | 19.01 ± 1.9 * |
| EC19 + AC261066 | 52.24 ± 3.6 *** | 30.83 ± 1.6 * | 12.34 ± 3.1 | 4.63 ± 2.8 *** |
| EC23 + AC261066 | 36.02 ± 2.5 *** | 41.07 ± 2.9 * | 10.93 ± 1.1 | 11.99 ± 7.1 * |
| Key Parameters | Control # | ATRA # | EC19 # | EC23 # |
|---|---|---|---|---|
| % Live cells (SYTOX™ −ve/Caspase-3/7 −ve) | 80.56 ± 6.39 | 4.66 ± 3.35 ** | 12.31 ± 1.09 ** | 4.69 ± 0.33 ** |
| % Apoptotic cells (SYTOX™ −ve/Caspase-3/7 +ve) | 1.74 ± 1.22 | 11.75 ± 2.92 * | 6.70 ± 1.36 * | 11.76 ± 0.88 * |
| % Necrotic cells (SYTOX™ +ve/Caspase-3/7 +ve) | 10.75 ± 1.69 | 82.69 ± 7.13 * | 79.05 ± 1.384 * | 81.75 ± 3.83 * |
| Gene | Primer Sequence |
|---|---|
| P-glycoprotein 1/ABCB1 | F: 5′-TCACCAAGCGGCTCCGATACAT-3′ R: 5′-CCCGGCTGTTGTCTCCATAGGC-3′ |
| BCRP/ABCG2 | F: 5′-TATAGCTCAGATCATTGTCACAGTC-3′ R: 5′-GTTGGTCGTCAGGAAGAAGAG-3′ |
| MRP1/ABCC1 | F: 5′-CGGAAACCATCCACGACCCTAATC -3′ R: 5′-ACCTCCTCATTCGCATCCACCTGG-3′ |
| Hsp70 | F: 5′-ACACGAATCCCTGCGGTAAAA-3′ R: 5′-GCAGGCGATAAGATGGCACA-3′ |
| ATP7A | F: 5′-TGAACAGTCATCACCTTCATCGTC-3′ R: 5′-GCGATCAAGCCACACAGTTCA-3′ |
| β-actin | F: 5′-GCACCACACCTTCTACAATGAGC-3′ R: 5′-GGATAGCACAGCCTGGATAGCAAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, M.R.; Ibrahim, E.; Elnagar, M.R.; Soror, S.H.; Haffez, H. Augmented Therapeutic Potential of EC-Synthetic Retinoids in Caco-2 Cancer Cells Using an In Vitro Approach. Int. J. Mol. Sci. 2022, 23, 9442. https://doi.org/10.3390/ijms23169442
Abdelaal MR, Ibrahim E, Elnagar MR, Soror SH, Haffez H. Augmented Therapeutic Potential of EC-Synthetic Retinoids in Caco-2 Cancer Cells Using an In Vitro Approach. International Journal of Molecular Sciences. 2022; 23(16):9442. https://doi.org/10.3390/ijms23169442
Chicago/Turabian StyleAbdelaal, Mohamed R., Esraa Ibrahim, Mohamed R. Elnagar, Sameh H. Soror, and Hesham Haffez. 2022. "Augmented Therapeutic Potential of EC-Synthetic Retinoids in Caco-2 Cancer Cells Using an In Vitro Approach" International Journal of Molecular Sciences 23, no. 16: 9442. https://doi.org/10.3390/ijms23169442
APA StyleAbdelaal, M. R., Ibrahim, E., Elnagar, M. R., Soror, S. H., & Haffez, H. (2022). Augmented Therapeutic Potential of EC-Synthetic Retinoids in Caco-2 Cancer Cells Using an In Vitro Approach. International Journal of Molecular Sciences, 23(16), 9442. https://doi.org/10.3390/ijms23169442









