Uromodulin Regulates Murine Aquaporin−2 Activity via Thick Ascending Limb–Collecting Duct Cross−Talk during Water Deprivation
Abstract
1. Introduction
2. Results
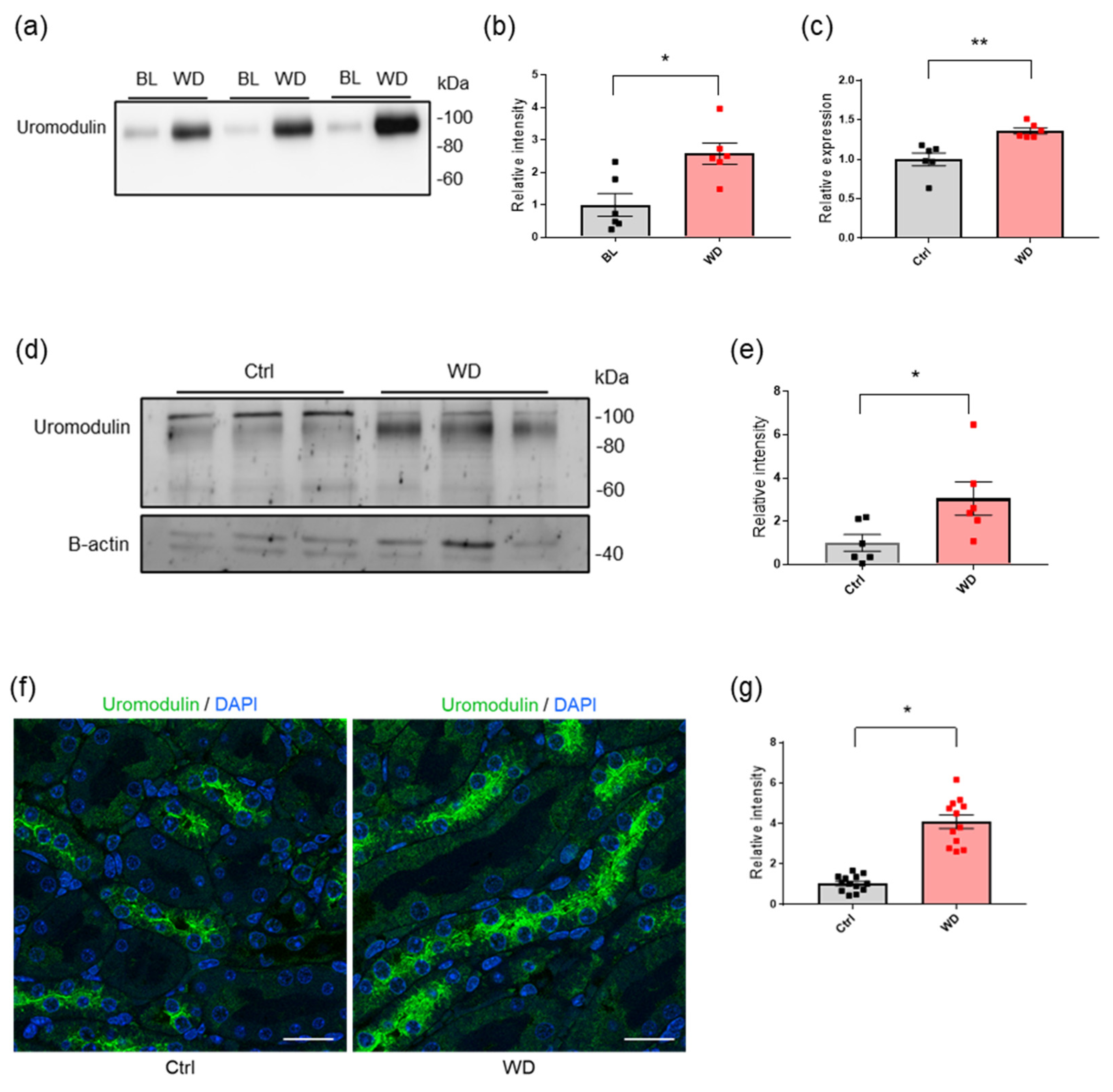
2.1. Water Deprivation Increases the Urinary Secretion of Uromodulin
2.2. Water Deprivation and Free Water Reabsorption
2.3. Uromodulin Adheres to the Apical Surface of the CD
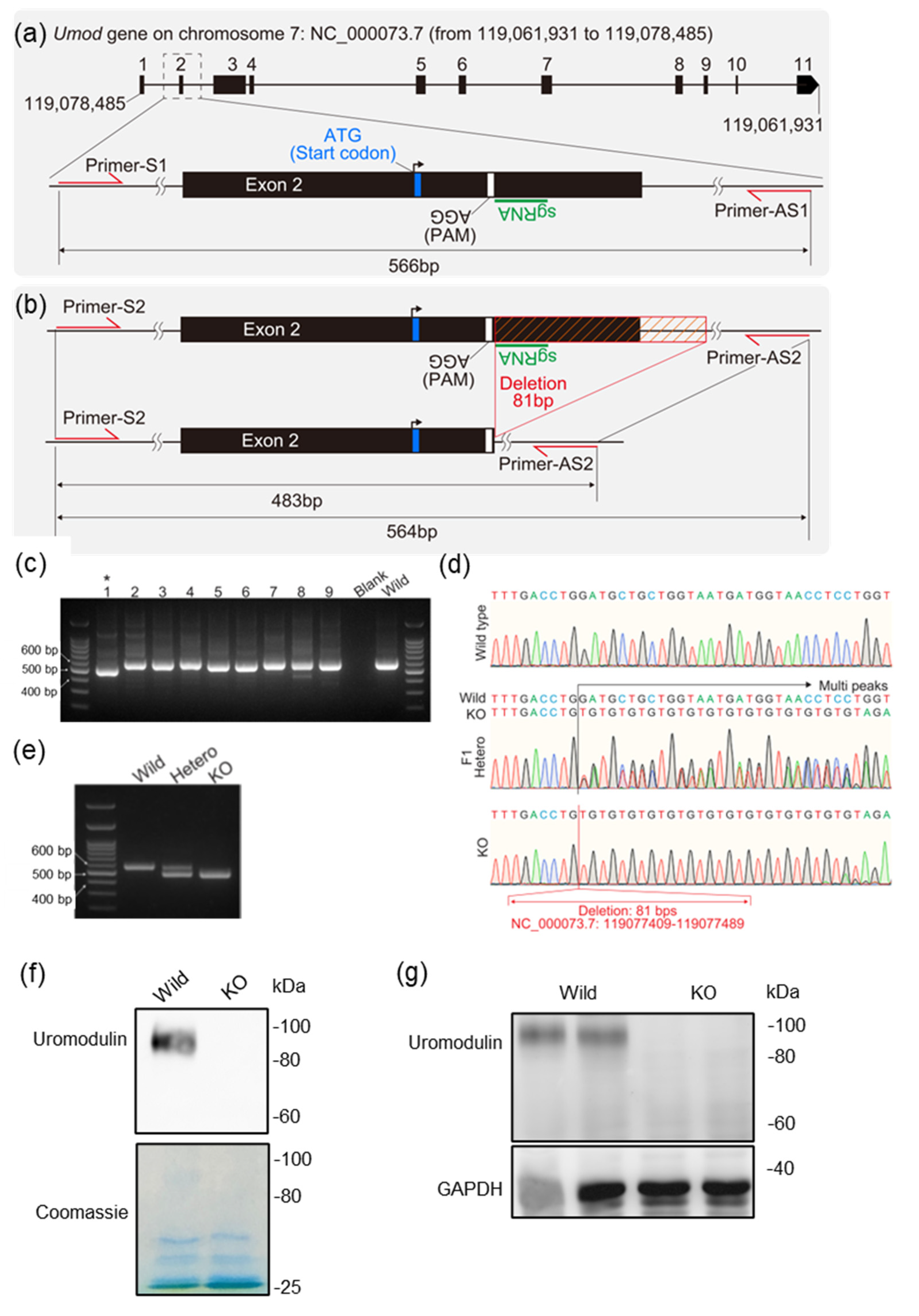
2.4. Generation of Uromodulin Knock-Out Mice
2.5. Uromodulin-Deficient Mice Exhibit Defective AQP2 Activation
2.6. Effect of Uromodulin on AQP2 Phosphorylation in Mouse CD Cells
2.7. Uromodulin May Activate AQP2 through the Suppression of Endocytosis
3. Discussion
4. Materials and Methods
4.1. Generation of Knock−Out Mice
4.2. Experimental Protocol
4.3. Cell Culture and Treatment
4.4. Reverse Transcriptase−Polymerase Chain Reaction (RT−PCR) Analysis
4.5. Western Blot Analysis
4.6. Immunostaining Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sands, J.M.; Layton, H.E.; Fenton, R.A. Urine concentration and dilution. In Brenner and Rector’s the Kidney, 9th ed.; Taal, M.W., Chertow, G.M., Marsden, P.A., Skorecki, K., Yu, A.S., Brenner, B.M., Eds.; Saunders: Philadelphia, PA, USA, 2012; Volume 1, pp. 326–352. [Google Scholar]
- Burg, M.B. Thick ascending limb of Henle’s loop. Kidney Int. 1982, 22, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Coffman, T.M.; Crowley, S.D. Kidney in hypertension: Guyton redux. Hypertension 2008, 51, 811–816. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lifton, R.P.; Gharavi, A.G.; Geller, D.S. Molecular mechanisms of human hypertension. Cell 2001, 104, 545–556. [Google Scholar] [CrossRef]
- Pennica, D.; Kohr, W.J.; Kuang, W.J.; Glaister, D.; Aggarwal, B.B.; Chen, E.Y.; Goeddel, D.V. Identification of Human Uromodulin as the Tamm-Horsfall Urinary Glycoprotein. Science 1987, 236, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.C.; Gorry, M.C.; Hart, P.S.; Woodard, A.S.; Shihabi, Z.; Sandhu, J.; Shirts, B.; Xu, L.; Zhu, H.; Barmada, M.M.; et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J. Med. Genet. 2002, 39, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.; Alper, S.L.; Antignac, C.; Bleyer, A.J.; Chauveau, D.; Dahan, K.; Deltas, C.; Hosking, A.; Kmoch, S.; Rampoldi, L.; et al. Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management—A KDIGO consensus report. Kidney Int. 2015, 88, 676–683. [Google Scholar] [CrossRef]
- Brunati, M.; Perucca, S.; Han, L.; Cattaneo, A.; Consolato, F.; Andolfo, A.; Schaeffer, C.; Olinger, E.; Peng, J.; Santambrogio, S.; et al. The serine protease hepsin mediates urinary secretion and polymerization of Zona Pellucida domain protein uromodulin. Elife 2015, 4, e08887. [Google Scholar] [CrossRef]
- Santambrogio, S.; Cattaneo, A.; Bernascone, I.; Schwend, T.; Jovine, L.; Bachi, A.; Rampoldi, L. Urinary uromodulin carries an intact ZP domain generated by a conserved C-terminal proteolytic cleavage. Biochem. Biophys. Res. Commun. 2008, 370, 410–413. [Google Scholar] [CrossRef]
- Schaeffer, C.; Santambrogio, S.; Perucca, S.; Casari, G.; Rampoldi, L. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol. Biol. Cell 2009, 20, 589–599. [Google Scholar] [CrossRef]
- Dussol, B.; Berland, Y. Urinary kidney stone inhibitors. Where are we? Nephrol. Dial. Transplant. 1996, 11, 1222–1224. [Google Scholar] [CrossRef][Green Version]
- Mo, L.; Huang, H.Y.; Zhu, X.H.; Shapiro, E.; Hasty, D.L.; Wu, X.R. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004, 66, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, W. A glycoprotein in urine binds bacteria and blocks infections. Science 2020, 369, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.L.; Stanisich, J.J.; Sauer, M.M.; Lin, C.W.; Eras, J.; Zyla, D.S.; Trück, J.; Devuyst, O.; Aebi, M.; Pilhofer, M.; et al. Architecture and function of human uromodulin filaments in urinary tract infections. Science 2020, 369, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Raffi, H.S.; Bates, J.M., Jr.; Laszik, Z.; Kumar, S. Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J. Urol. 2009, 181, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Bartz, T.M.; Ix, J.H.; Chonchol, M.; Shlipak, M.G.; Devarajan, P.; Bennett, M.R.; Sarnak, M.J. Urinary Uromodulin and Risk of Urinary Tract Infections: The Cardiovascular Health Study. Am. J. Kidney Dis. 2017, 69, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Maeoka, Y.; McCormick, J.A. NaCl cotransporter activity and Mg2+ handling by the distal convoluted tubule. Am. J. Physiol. Ren. Physiol. 2020, 319, F1043–F1053. [Google Scholar] [CrossRef]
- Mutig, K.; Kahl, T.; Saritas, T.; Godes, M.; Persson, P.; Bates, J.; Raffi, H.; Rampoldi, L.; Uchida, S.; Hille, C.; et al. Activation of the bumetanide-sensitive Na+, K+, 2Cl-Cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J. Biol. Chem. 2011, 286, 30200–30210. [Google Scholar] [CrossRef]
- Trudu, M.; Janas, S.; Lanzani, C.; Debaix, H.; Schaeffer, C.; Ikehata, M.; Citterio, L.; Demaretz, S.; Trevisani, F.; Ristagno, G.; et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat. Med. 2013, 19, 1655–1660. [Google Scholar] [CrossRef]
- Wolf, M.T.F.; Wu, X.R.; Huang, C.L. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int. 2013, 84, 130–137. [Google Scholar] [CrossRef]
- Kanbay, M.; Yilmaz, S.; Dincer, N.; Ortiz, A.; Sag, A.A.; Covic, A.; Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cherney, D.Z.I.; Johnson, R.J.; et al. Antidiuretic Hormone and Serum Osmolarity Physiology and Related Outcomes: What Is Old, What Is New, and What Is Unknown? J. Clin. Endocrinol. Metab. 2019, 104, 5406–5420. [Google Scholar] [CrossRef]
- Nielsen, S.; Chow, C.L.; Marples, D.; Christensen, E.I.; Kishore, B.K.; Knepper, M.A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin–CD water channels to plasma membrane. Proc. Natl. Acad. Sci. USA 1995, 92, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sasaki, S.; Fushimi, K.; Ishibashi, K.; Yaoita, E.; Kawasaki, K.; Marumo, F.; Kihara, I. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am. J. Physiol. 1995, 268, C1546–C1551. [Google Scholar] [CrossRef] [PubMed]
- Arnspang, E.C.; Login, F.G.; Koffman, J.S.; Sengupta, P.; Nejsum, L.N. AQP2 plasma membrane diffusion is altered by the degree of AQP2-S256 phosphorylation. Int. J. Mol. Sci. 2016, 17, 1804. [Google Scholar] [CrossRef]
- Fushimi, K.; Sasaki, S.; Marumo, F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 1997, 272, 14800–14804. [Google Scholar] [CrossRef] [PubMed]
- Bokhove, M.; Nishimura, K.; Brunati, M.; Han, L.; de Sanctis, D.; Rampoldi, L.; Jovine, L. A structured interdomain linker directs self-polymerization of human uromodulin. Proc. Natl. Acad. Sci. USA 2016, 113, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.E.; Peek, H.; Baudet, M.L.; Harvey, S. Extrapituitary GH in the chicken: Underestimation of immunohistochemical staining Carnoy’s fixation. J. Endocrinol. 2003, 177, 223–234. [Google Scholar] [CrossRef][Green Version]
- Tokonami, N.; Takata, T.; Beyeler, J.; Ehrbar, I.; Yoshifuji, A.; Christensen, E.I.; Loffing, J.; Devuyst, O.; Olinger, E.G. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int. 2018, 94, 701–715. [Google Scholar] [CrossRef]
- Lu, H.; Sun, T.X.; Bouley, R.; Blackburn, K.; McLaughlin, M.; Brown, D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am. J. Physiol. Ren. Physiol. 2004, 286, F233–F243. [Google Scholar] [CrossRef]
- Sandoval, P.C.; Claxton, J.N.S.; Lee, J.W.; Saeed, F.; Hoffert, J.D.; Knepper, M.A. Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci. Rep. 2016, 6, 34863. [Google Scholar] [CrossRef]
- Robben, J.H.; Knoers, N.V.A.M.; Deen, P.M.T. Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol. Biol. Cell 2004, 12, 5693–5699. [Google Scholar] [CrossRef]
- Kuwahara, M.; Fushimi, K.; Terada, Y.; Bai, L.; Marumo, F.; Sasaki, S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J. Biol. Chem. 1995, 270, 10384–10387. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Tang, T.; Murray, F.; Schroth, J.; Insel, P.A.; Fenton, R.A.; Hammond, H.K.; Vallon, V. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J. Am. Soc. Nephrol. 2010, 21, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Bouley, R.; Chen, Y.; Matsuzaki, T.; Nunes, P.; Hasler, U.; Brown, D.; Lu, H.A.J. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient rattleboro rats through modulation of Rho GTPase. Am. J. Physiol. Ren. Physiol. 2011, 301, 309–318. [Google Scholar] [CrossRef]
- Scolari, F.; Izzi, C.; Ghiggeri, G.M. Uromodulin: From monogenic to multifactorial diseases. Nephrol. Dial. Transplant. 2015, 30, 1250–1256. [Google Scholar] [CrossRef]
- Nanamatsu, A.; Mori, T.; Ando, F.; Furusho, T.; Mandai, S.; Susa, K.; Sohara, E.; Rai, T.; Uchida, S. Vasopressin Induces Urinary Uromodulin Secretion by Activating PKA (Protein Kinase A). Hypertension 2021, 77, 1953–1963. [Google Scholar] [CrossRef]
- Olinger, E.; Lake, J.; Sheehan, S.; Schiano, G.; Takata, T.; Tokonami, N.; Debaix, H.; Consolato, F.; Rampoldi, L.; Korstanje, R.; et al. Hepsin-mediated Processing of Uromodulin is Crucial for Salt-sensitivity and Thick Ascending Limb Homeostasis. Sci. Rep. 2019, 9, 12287. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.Z.; Sanders, P.W. Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein. Am. J. Pathol. 2001, 158, 1859–1866. [Google Scholar] [CrossRef]
- Micanovic, R.; LaFavers, K.; Garimella, P.S.; Wu, X.R.; El-Achkar, T.M. Uromodulin (Tamm-Horsfall protein): Guardian of urinary and systemic homeostasis. Nephrol. Dial. Transplant. 2020, 35, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Knepper, M.A.; Kwon, T.H.; Nielsen, S. Molecular Physiology of Water Balance. N. Engl. J. Med. 2015, 372, 1349–1358. [Google Scholar] [CrossRef]
- Wilson, J.L.L.; Miranda, C.A.; Knepper, M.A. Vasopressin and the regulation of aquaporin-2. Clin. Exp. Nephrol. 2013, 17, 751–764. [Google Scholar] [CrossRef]
- Fairweather, S.J.; Shah, N.; Bröer, S. Heteromeric Solute Carriers: Function, Structure, Pathology and Pharmacology. Adv. Exp. Med. Biol. 2021, 21, 13–127. [Google Scholar] [PubMed]
- Errasti-Murugarren, E.; Palacín, M. Heteromeric Amino Acid Transporters in Brain: From Physiology to Pathology. Neurochem. Res. 2022, 47, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.A.; Sun, T.X.; Matsuzaki, T.; Yi, X.H.; Eswara, J.; Bouley, R.; Mckee, M.; Brown, D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J. Biol. Chem. 2007, 282, 28721–28732. [Google Scholar] [CrossRef]
- Li, W.; Jin, W.W.; Tsuji, K.; Chen, Y.; Nomura, N.; Su, L.; Yui, N.; Arthur, J.; Cotecchia, S.; Paunescu, T.G.; et al. Ezrin directly interacts with AQP2 and promotes its endocytosis. J. Cell Sci. 2017, 130, 2914–2925. [Google Scholar]
- van Balkom, S.B.M.; Boone, M.; Hendriks, G.; Kamsteeg, E.J.; Robben, J.H.; Stronks, H.C.; van der Voorde, A.; van Herp, F.; van der Sluijs, P.; Deen, P.M.T. LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J. Am. Soc. Nephrol. 2009, 20, 990–1001. [Google Scholar] [CrossRef]
- Park, E.J.; Lim, J.S.; Jung, H.J.; Kim, E.; Han, K.H.; Kwon, T.H. The role of 70-kDa heat shock protein in dDAVP-induced AQP2 trafficking in kidney collecting duct cells. Am. J. Physiol. Ren. Physiol. 2013, 304, 958–971. [Google Scholar] [CrossRef]
- Hosokawa, K.; Takata, T.; Sugihara, T.; Matono, T.; Koda, M.; Kanda, T.; Taniguchi, S.; Ida, A.; Mae, Y.; Yamamoto, M.; et al. Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipid deposition in renal tubules. Int. J. Mol. Sci. 2019, 21, 190. [Google Scholar] [CrossRef]
- Hamada, S.; Takata, T.; Yamada, K.; Yamamoto, M.; Mae, Y.; Iyama, T.; Ikeda, S.; Kanda, T.; Sugihara, T.; Isomoto, H. Steatosis is involved in the progression of kidney disease in a high-fat-diet-induced non-alcoholic steatohepatitis mouse model. PLoS ONE 2022, 17, e0265461. [Google Scholar] [CrossRef]
- Iyama, T.; Takata, T.; Yamada, K.; Mae, Y.; Taniguchi, S.; Ida, A.; Ogawa, M.; Yamamoto, M.; Hamada, S.; Fukuda, S.; et al. A novel method for assessing the renal biopsy specimens using an activatable fluorescent probe. Sci. Rep. 2020, 10, 12094. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, T.; Hamada, S.; Mae, Y.; Iyama, T.; Ogihara, R.; Seno, M.; Nakamura, K.; Takata, M.; Sugihara, T.; Isomoto, H. Uromodulin Regulates Murine Aquaporin−2 Activity via Thick Ascending Limb–Collecting Duct Cross−Talk during Water Deprivation. Int. J. Mol. Sci. 2022, 23, 9410. https://doi.org/10.3390/ijms23169410
Takata T, Hamada S, Mae Y, Iyama T, Ogihara R, Seno M, Nakamura K, Takata M, Sugihara T, Isomoto H. Uromodulin Regulates Murine Aquaporin−2 Activity via Thick Ascending Limb–Collecting Duct Cross−Talk during Water Deprivation. International Journal of Molecular Sciences. 2022; 23(16):9410. https://doi.org/10.3390/ijms23169410
Chicago/Turabian StyleTakata, Tomoaki, Shintaro Hamada, Yukari Mae, Takuji Iyama, Ryohei Ogihara, Misako Seno, Kazuomi Nakamura, Miki Takata, Takaaki Sugihara, and Hajime Isomoto. 2022. "Uromodulin Regulates Murine Aquaporin−2 Activity via Thick Ascending Limb–Collecting Duct Cross−Talk during Water Deprivation" International Journal of Molecular Sciences 23, no. 16: 9410. https://doi.org/10.3390/ijms23169410
APA StyleTakata, T., Hamada, S., Mae, Y., Iyama, T., Ogihara, R., Seno, M., Nakamura, K., Takata, M., Sugihara, T., & Isomoto, H. (2022). Uromodulin Regulates Murine Aquaporin−2 Activity via Thick Ascending Limb–Collecting Duct Cross−Talk during Water Deprivation. International Journal of Molecular Sciences, 23(16), 9410. https://doi.org/10.3390/ijms23169410






